This chapter should be cited as follows:
Mehdi M, Chandraharan E, Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.409603
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 13
Obstetric emergencies
Volume Editor: Dr María Fernanda Escobar Vidarte, Fundación Valle del Lili, Cali, Colombia

Chapter
Diagnosis and Management of Shock in Postpartum Hemorrhage
First published: February 2021
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
DEFINITION
Hemorrhagic shock refers to a decreased tissue perfusion, leading to insufficient nutrient and oxygen delivery that are required for the normal functioning of cells as a result of hypovolemia secondary to massive hemorrhage. Reduced tissue perfusion, which occurs when the demand for oxygen exceeds that of supply, may lead to the development of metabolic acidosis and multiorgan failure.
PATHOPHYSIOLOGY
Massive blood loss results in the loss of circulating volume, which may trigger an array of compensatory responses (Figure 1), which are important for systematically diverting the available oxygenated blood away from “non-essential” organs in order to conserve circulating volume for the vital organs. This ‘centralization’ is essential for survival. Acute hemorrhage results in reduced pulse pressure as a result of an intense peripheral vasoconstriction and reduced cardiac output, and these hemodynamic changes are detected by the volume receptors in the atrium and the baroreceptors located within the aortic arch. When the blood volume is reduced, neural reflexes bring about an increased sympathetic activation in the heart and the rest of the organs. This leads to an intense vasoconstriction, as well as redistribution of blood flow away from “non-essential” organs, such as the kidneys, gastrointestinal tract, and the skin to the central organs, coupled with an increase in the heart rate.1
Neural compensatory mechanisms are accompanied by endocrine responses to maintain circulating blood volume. Corticotropin-releasing hormone (CRH) is immediately released, and this activates the release of beta-endorphin and glucocorticoid. Vasopressin is also released from the posterior pituitary gland, leading to retention of water at the distal tubules. When mean arterial pressure is reduced, the renin–angiotensin–aldosterone system is activated, which ensures water retention, and sodium resorption. Hyperglycemia frequently develops in acute hemorrhage because growth hormone and glucagon increase the production of glucose through glycogenolysis and gluconeogenesis. In addition, circulating catecholamines slow down the release and activity of insulin and this causes a rise in the plasma glucose.1
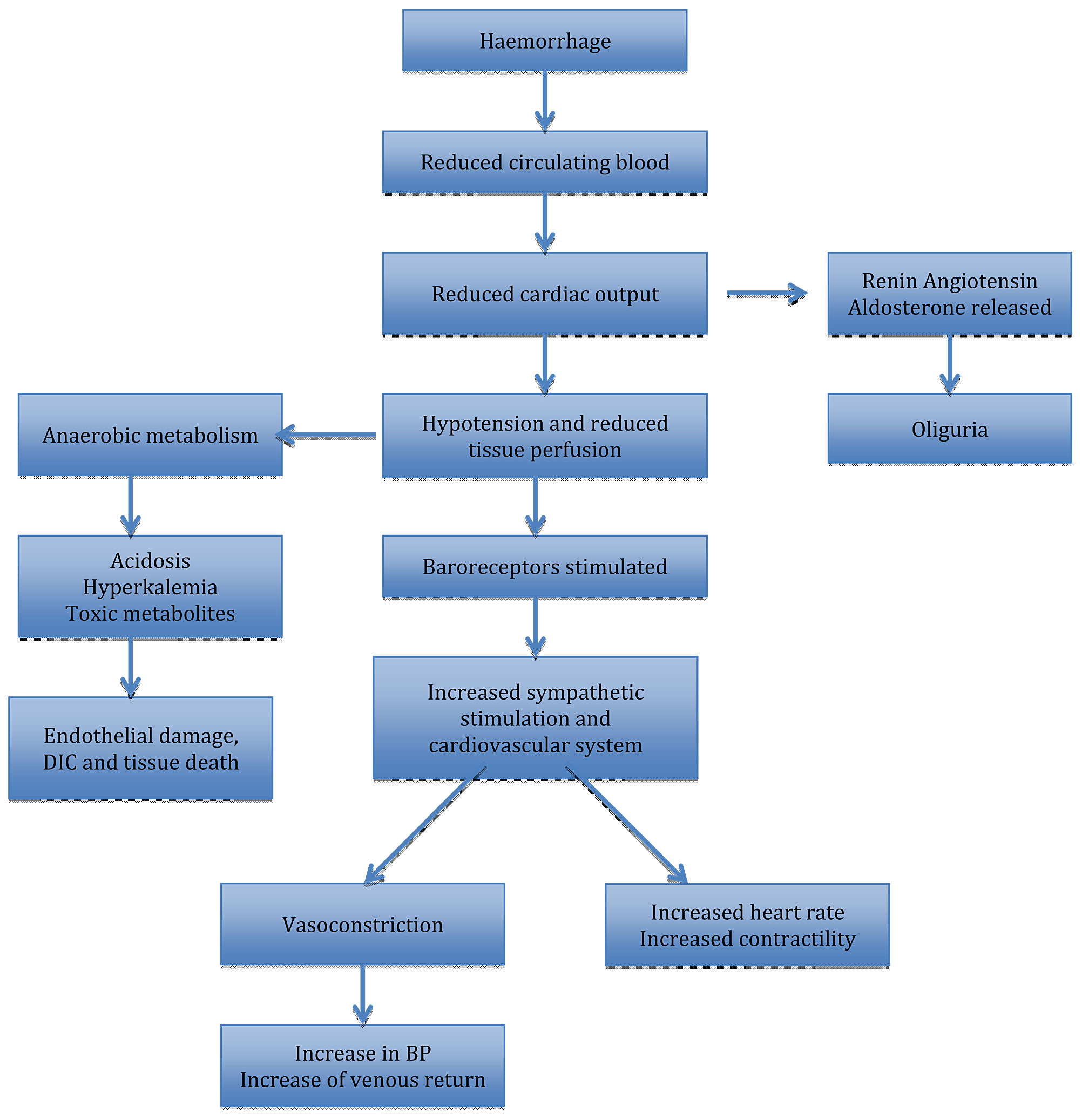
1
Compensatory mechanisms in massive hemorrhage.
Effective cerebral autoregulation ensures that the cerebral blood flow is kept constant despite the fall in the systemic mean arterial blood pressure. The kidneys are capable of tolerating up to a 90% drop in entire blood circulation for a short period of time. Intestinal blood flow is drastically reduced by splanchnic vasoconstriction after the circulatory volume is significantly decreased. Timely and effective resuscitation may prevent injury to organs because these adaptive, compensatory mechanisms are efficient in preserving the hemodynamic stability and perfusion to the vital organs, until decompensation sets in the loss of both oxygen-carrying capacity and circulating blood volume leading to the development of hemorrhagic shock. In obstetrics, massive antepartum hemorrhage (APH) and postpartum hemorrhage (PPH) may lead to hemorrhagic shock.
Figure 1 shows neural and endocrine compensatory mechanisms in massive hemorrhage.
DEFINITION OF MASSIVE PPH
The primary PPH refers to the blood loss of at least 500 ml, from the genital tract within 24 hours of delivery (more than 1000 ml in cesarean section). Secondary PPH refers to an excessive blood loss from 24 hours to 12 weeks after birth, while massive PPH is a blood loss of more than 2000 ml (>30% of the blood volume).
A more precise “clinical” definition of massive hemorrhage should be “any blood loss, which results in hemodynamic instability and endangers the life of a woman”. This is because in women with low BMI (body mass index) or women who are severely anemic, even the loss of 250 ml of blood may cause hemodynamic instability and rapid decompensation.2
RISK FACTORS AND CAUSES OF MASSIVE PPH
Table 1 illustrates the common causes of primary PPH.
Secondary PPH occurs after 24 h of delivery (usually after 5–6 days of birth) until 12 weeks, and is commonly caused by retained placenta and membranes with or without infection (endometritis).
1
Risk factors and causes of primary PPH.
Tone (commonest – up to 80% of primary PPH)
| Antepartum hemorrhage Prolonged labor Prolonged oxytocin use Induced labor Operative delivery Tocolytic drugs and general anesthesia Also uterine overdistension caused by multiple pregnancies, polyhydramnios, fetal macrosomia, uterine structural abnormalities, and fibroids |
Trauma
| Lacerations of perineum, vagina, cervix Cesarean sections, angle extensions, and episiotomy Spontaneous rupture of uterine or ovarian vessels and subcapsular liver rupture |
Tissue
| Retained placenta, membranes or clot Placenta accrete, increta, percreta |
Thrombin
| Pre-existing bleeding disorders Anticoagulant use Massive hemorrhage (<30% of blood volume) Sepsis and pre-eclampsia |
EVALUATING AND REPLENISHING LOST VOLUME
Shock refers to a reduction in tissue perfusion, which is insufficient to meet the metabolic requirements of tissues and organs. Insufficient blood flow may be clinically noticeable as the development of lactic acidosis, altered mental status, oliguria, pallor, tachycardia, or all these changes combination. Hypotension nearly always occurs in acute cases, although this sign may not occur in young, fit patients, until very late unless approximately 30% of blood volume has been lost. Therefore, it is a poor indicator of the volume of blood loss in early stages of massive hemorrhage. It is vital to appreciate the alterations in the cardiovascular physiology during pregnancy whilst managing patients with hemorrhagic shock.3
PHYSIOLOGY
Blood volume of a non-pregnant healthy adult is approximately 7.5% of body weight, or 70 ml/kg. Table 2 highlights the parameters in a non-pregnant and pregnant woman. The non-pregnant adult cardiac output is 4–6 l/min, and systemic vascular resistance is 10–5 mmHg/min. The blood volume of the mother increases to 40% more than before pregnancy by the 30th week of gestation, with an associated smaller (20–30%) rise in red cell volume. Cardiac output rises to 50% above the pre-pregnancy level at 24th week. There is more variation in systemic blood pressure for a healthy pregnancy, whereby it slightly drops in the first and second trimesters and resumes to pre-pregnancy levels by the last trimester. Resting heart rate escalates to 15–20 bpm more than the pre-pregnant state. In addition to these cardiovascular changes in pregnancy, there are other changes that take place in the circulation and the autoregulation of intravascular capacities. Both these changes influence the response of the body to blood loss. Examples comprise a diminished response to angiotensin II, which partially may be as a result of a high cardiac noradrenaline turnover, a low tolerance to postural changes and a high production of nitric oxide.3
Pregnancy-related diseases and their treatment affect the body’s ability to compensate for volume loss, the observed clinical signs of hypovolemia and the circulating volume, and this influence remains until the early postpartum period. When compared to the normal peripartum state, for instance, pre-eclampsia can cause a contracted arterial blood volume secondary to the loss of intravascular volume as a result of endothelial damage. The vascular response is impaired, and routinely administered drugs such as magnesium and antihypertensive drugs may compromise the capacity of the body to create compensatory vasoconstriction to combat hemorrhage. There is a failure to achieve a low systemic vascular resistance as well as in high plasma volume in pre-eclampsia because of insufficient trophoblastic invasion of the uterine spiral arteries. Therefore, women with pre-eclampsia have a high possibility of developing pulmonary edema during volume replacement because of left ventricular dysfunction, hypoalbuminemia, and high capillary permeability.
Normal expected blood loss after birth ranges from 750 to 1000 ml for a cesarean section and 300 to 500 ml for vaginal deliveries. Apart from blood loss from the body, a considerable volume of blood is transmitted into the systemic circulation immediately after birth, which is commonly called the “autotransfusion effect”. About 80% increase in cardiac output occurs as a result and this continues in healthy women during the postpartum period, progressively returning to non-pregnant levels by 2–3 weeks.4
2
Physiological changes during pregnancy.
Conditions | Change during pregnancy | Normal pregnancy values |
Cardiovascular Heart rate Cardiac output Mean arterial pressure Systematic vascular resistance | Increases 15–20 bpm Increases 30–50% Decreases 10 mmHg in midtrimester Decreases 10–15% | 75–95 bpm 4–6 l/min 80 mmHg 1200–1500 dynes/s/cm |
Respiratory Tidal volume Minute ventilation Expiratory reserve volume Functional residual capacity | Increased 40% Increased 40% Decreased 15–20% Decreased 20–25% | 700 ml 10.5 l/min 550 ml 1350 ml |
Hematologic Blood volume Erythrocyte volume Hematocrit White blood cell count Factor I, II, V, VII, VIII, IX, X and XII Fibrinogen | Increases 30–35% Increases 10–15% Decreased Increased Increased Increased | 4500 ml 32–34% 5000–15,000 mm More than 400 mg/dl |
Renal Renal blood flow Glomerular filtration rate Serum creatinine Serum urea nitrogen | Increased 50–60% Increased 60% Decreased Decreased | 700 ml/min 140 ml/min Less 0.8 mg/dl Less 13 mg/dl |
ASSESSMENT OF CIRCULATING BLOOD VOLUME
Young healthy individuals can compensate for the loss of significant volumes from the circulation with few visible clinical signs. This may pose a diagnostic dilemma and may result in a delay in diagnosis of massive obstetric hemorrhage. Symptoms frequently precede signs in massive obstetric hemorrhage. These comprise inexplicable restlessness and anxiety, the feeling of breathlessness (without or with a high respiratory rate), and a feeling of being cold or generally unwell. The essential cornerstone of management of massive PPH, involves prompt diagnosis and speedy replacement of lost blood volume as well as oxygen carrying capacity of blood, accompanied by instantaneous medical and surgical measures to address the underlying cause(s) to avert more loss.
Shock index (SI) together with rule of 30 are important tools that may aid clinicians in an emergency to determine the amount of blood loss and the degree of hemodynamic instability. SI refers to the ratio of pulse rate to systolic blood pressure (PR/SBP) and the normal value for a non-pregnant adult population has been reported as 0.5–0.7 prior to the fall in the systolic blood pressure, the heart rate rises to compensate for the blood loss. Therefore, the SI increases and it has been shown that the SI rises to 0.9–11.1 in acute hemorrhage conditions, and that an SI of over 0.9 may require an urgent intervention to restore hemodynamic stability.4
In a pregnant population, obstetric shock index (OSI) >1 (that is pulse rate > SBP) has been shown to increase the likelihood of blood transfusion. OSI may, therefore, act as an adjunct in minimizing errors as a result of visual estimation of blood loss.
Rule of 30 refers to a 30% fall in hematocrit, a 30 mmHg fall in systolic blood pressure, an increase by 30 beats/min of pulse rate, a 30% fall of hemoglobin (approximately 3 g/dl), and an approximate blood loss of 30% of normal (70 ml/kg in adults; 100 ml/kg throughout pregnancy).
If intravascular volume depletion is suspected, a rapid clinical assessment is required. This is because it is possible for the patient’s clinical condition to rapidly deteriorate leading to the development of hemorrhagic shock. Concurrently, clinical examination is carried out with history taking, replacement of intravascular volume as well as measures to control bleeding. Good history taking skills may highlight symptoms associated with shock such as pain and overt blood loss as well as general malaise, anxiety, and breathlessness. The physical examination is aimed to immediately assess the ABCDEs – conscious state and airway protection, oxygenation, and the adequacy of respiratory function, oxygenation and circulation.
The classification of hemorrhage into four classes has been derived according to clinical signs and percentage of blood loss. (Table 3).
3
Classification of hemorrhagic shock.
Class I | Class II | Class III | Class IV | |
Blood loss (ml) | <750 | 750–1500 | 1500–2000 | >2000 |
Blood loss (% blood volume) | <15 | 15–30 | 30–40 | >40 |
Heart rate | <100 | 100–120 | 120–140 | >140 |
Blood pressure | Normal | Normal | Decreased | Decreased |
Pulse pressure | Normal or increased | Decreased | Decreased | Decreased |
Respiratory rate | 14–20 | 20–30 | 30–40 | 35 |
Urine output (ml/hour) | More 30 | 20–30 | 5–15 | Negligible |
Mental status | Slightly anxious | Mildly anxious | Anxious, confused | Confused, lethargic |
Class I hemorrhage (loss of 0–15%)
Vital parameters may remain normal except for minimal tachycardia. Generally, no changes in respiratory rate, pulse pressure or blood pressure take place. A delay in capillary refill that exceeds 3 seconds corresponds to a volume loss of about 10%.
Class II hemorrhage (loss of 15–30%)
Clinical symptoms consist of tachypnea, slight anxiety, tachycardia (rate >100 beats/min), delayed capillary refill, cool clammy skin, and a decrease in pulse pressure. Due to the release of catecholamines and resultant intense peripheral vasoconstriction, the pulse pressure decreases secondary to the increase in the diastolic blood pressure.
Class III hemorrhage (loss of 30–40%)
Patients by this point usually have noticeable tachycardia and tachypnea, oliguria, decreased systolic blood pressure as well as considerable alterations in mental status, for instance, agitation or confusion. For patients without fluid losses or other injuries, this is the minimum amount of blood loss that constantly leads to a decline in systolic blood pressure. The majority of these patients will need blood transfusions, though the decision of administering blood should be determined by the initial response to fluids.
Class IV hemorrhage (loss of >40%)
Loss of more than 40% of blood is immediately life-threatening. Patients present with symptoms and signs such as cold and pale skin, marked tachycardia, depressed mental status (or loss of consciousness), decreased systolic blood pressure, markedly decreased urinary output or oliguria, and narrowed pulse pressure (or immeasurable diastolic pressure).
MANAGEMENT
There are two main components in treatment of patients with PPH:
- Resuscitation and management of PPH and/or associated hemorrhagic shock.
- Identification and management of the underlying hemorrhage cause(s).
These components are separately discussed. It should be noted that successful PPH management necessitates that both components should be dealt with systematically and simultaneously.
After exhausting all the compensating mechanisms that are meant to maintain the blood pressure and tissue perfusion, there is often a dramatic fall in the systolic blood. Rapid replacement of blood volume through a large-bore peripheral access (i.e. 14 or 16 G cannula) as well as surgical intervention and medical therapies should be implemented.
The moment massive hemorrhage is diagnosed, replacement of the lost circulating blood volume has to begin, prior to the development of signs of considerable hypovolemia. Essential primary measures should include supplemental oxygen, at least 2 large-bore (14 G) peripheral intravenous access, and an early and thorough clinical assessment. Requesting timely help from senior members of the team, including the wider multi-disciplinary team comprising of hematology, anesthesia and intensive care is paramount.
Algorithms may assist in managing the PPH systematically in a sequential and logical manner. An example of such an algorithm is hemOSTASIS (illustrated in Table 4).5 It has been reported that using this algorithm ‘hemOSTASIS’ was associated with excellent outcomes as well as a low rate of Peripartum Hysterectomy.
The principal underlying aim of volume replacement during and following massive PPH is to restore and maintain tissue perfusion to all organs of the body with the aim of maintaining cellular viability and function. Despite the fact that the first focus has to be on restoration of the general clinical shock indicators, the clinician has to ensure that multiorgan failure is averted. Even though all the ‘clinical parameters’ are restored, a woman may still be in ‘shock’ on the basis of reduced perfusion to tissues and organs. The term often applied in describing the state where conventional hemodynamic features have been turned to normal is compensated shock. In spite of replacement of adequate volume, a patient might develop numerous organ dysfunctions, which leads to morbidity and mortality.
When controlling bleeding, replenishing of the lost blood volume has to go on simultaneously. Surgical and medical attempts to manage bleeding should not be hindered by extended volume resuscitation regardless of continuing blood loss. Volume resuscitation must be aimed at restoration of circulating blood volume as well as returning the hemostatic functions and oxygen-carrying capacity to an effective level, although subnormal.
Measuring the serum lactate and base deficit should assess tissues under perfusion, which may continue even after evident hemodynamic restoration. Efforts regarding measuring and enhancing tissue perfusion have to continue until all such strictures come back to normal.
For oxygen-carrying capacity as well as deliverance to the tissues, it is very important to maintain hemoglobin concentration. Titrating fluid as well as blood products to a precise level of hemoglobin in a rapidly bleeding patient is not easy. For those with ischemic heart disease, a hemoglobin level of 7–8 g/dl seems to be a suitable threshold in transfusing in the intensive care population, with achievable advantage for a higher level of 9 g/dl. It is reasonable to focus on the high end of the target range during resuscitation from hemorrhagic shock, since it can easily drift down. In the actively bleeding patient, a target of 10 g/dl has been recommended for a reasonable purpose. On the other hand, when it comes to PPH, the goal is a two-fold cessation of the bleeding in addition to restoring the level of hemoglobin. In women who refuse blood and blood products, an early recourse to a peripartum hysterectomy and/or interventional radiological procedures such as pelvic arterial embolization may help save lives.
4
Management algorithm “hemostasis” for PPH.
H | Ask for Help and hand on uterus (uterine massage) |
A | Assess (ABC) and resuscitate (crystalloids 2 L, colloids 1 L, oxygen by mask (15 l/min) |
E | Establish etiology (atonic, traumatic, coagulopathy or trauma), ensure availability of blood and administer ecbolics (drugs that contract the uterus – oxytocin, ergometrine or syntometrine intramuscularly) |
M | Massage uterus |
O | Oxytocin infusion/prostaglandins- IV/IM/per rectal (second-line medications to contract the uterus) |
S | Shift to theatre – aortic pressure or anti-shock garment/bimanual compression as appropriate |
T | Tamponade balloon/uterine packing- after exclusion of tissue and trauma |
A | Apply compression sutures (B-Lynch/modified) |
S | Systematic pelvic devascularization-uterine/ovarian/quadruple/internal iliac |
I | Interventional radiology and, if appropriate uterine artery embolisation |
S | Subtotal/total abdominal hysterectomy |
Coagulation disorders are mutually consequences of and predisposing factors for massive PPH. Bleeding diatheses from platelet dysfunction, a coagulopathy or thrombocytopenia may be caused by pre-existing disease, a pregnancy-related disorder, like eclampsia, or treatment like aspirin. There is also immense blood loss, both thrombocytopenia and coagulopathy through consumption (i.e. the “washout phenomenon”) and dilution (i.e. “dilutional coagulopathy”).
Proposed algorithm “SHOCK” for the management of hemorrhagic shock (see Table 5).
5
Management algorithm “SHOCK” for hemorrhagic shock.
S | Seek HELP (senior obstetric and midwifery/nursing input, anesthetists, intensive care physicians and hematologists) Secure ABC and resuscitate with intravenous fluids (crystalloids 2 L and /or colloids) and send urgent blood investigations including full blood count (FBC), urea and electrolytes, coagulation profile and blood lactate, urgently Shock Index – accurately estimate the degree of visible and invisible (i.e. intra-peritoneal or bleeding into paravaginal tissue) blood loss |
H | Correct Hypervolemia – rapid infusion of intravenous fluids and monitor urine output Correct Hypoxia – 10–15 L/min of oxygen by mask immediately and ensure urgent blood transfusion (consider O negative uncross-matched blood) and monitor oxygen zaturation and vital signs Correct Hypo/hyperkalemia and other metabolic dysfunction – check electrolytes and blood sugar Avoid and Correct Hypothermia – warm blankets |
O | Organ support – ensures adequate perfusion and oxygenation to vital organs and shift to intensive care unit if multi-organ support is needed. Observations – continuously monitor vital signs and consider arterial line for blood pressure and CVP line |
C | Identify and correct the Cause of hemorrhagic shock (4 ‘T’s) concurrently as hemorrhagic shock is managed. Administer tranexamic acid 1 g IV Correct Coagulation – avoid dilutional coagulopathy and the ‘washout phenomenon’ Diagnose and manage Complications such as Sheehan’s syndrome and acute renal failure |
K | Keep clear and legible Records and Keep the woman and her family informed. Keep re-assessing the vital signs and response to treatment |
THE PRIMARY CAUSES OF PPH AND THEIR MANAGEMENT
Initial assessment
The risk factors of a patient as well as the events that lead to the diagnosis of PPH might explain a primary etiology. However, the understanding that many cases are as a result of uterine atony, and the necessity of being systematic contends for a stepwise, and logical approach to assessment and management. The patient’s status, the response to first management steps and the severity of ongoing bleeding severity determine the activation of the massive PPH protocol.
Uterine atony
Uterine tone and size should be assessed by massaging the uterus and placing a hand on the uterine fundus, so that any clots that might have been collected in the vagina and uterus are expelled. If the uterus is not well contracted and boggy, strong uterine massage and oxytocin should be started. Usually 20 IU in 500 ml of crystalloid solution (intravenously) is used, with the rate of dosage adjusted to the response (usual rate 250 ml/h). Intramuscular administration of 10 IU brings about a slower onset of action (3–7 min), although with an effect that is more long lasting (up to 60 min). Emptying the bladder is recommended for the facilitation of uterine contraction and further therapeutic management.3
In a situation where the uterus is still atonic, bimanual massage should be commenced. This stimulates uterine contractions and brings about mechanical constriction of myometrial blood vessels. The following are steps of uterine massage: first, a gloved hand is inserted into vagina, then it is pushed up against the uterus’ body; second, the other hand is placed above the uterine fundus on the abdomen, and then the posterior uterine wall should be compress against the hand in vagina. Likewise, bimanual compression assists in reducing the ongoing bleeding, and as a result facilitates rapid resuscitation. For this to be operational, the compression has to go on for approximately 10 minutes that would enable the blood clot in the uterine vessels.
In case the uterus is still atonic regardless of oxytocin and bimanual massage, other uterotonic agents such as Syntometrine and carbetocin should be used. Syntometrine can be given intravenously or intramuscularly (1 ampule – 5 IU of syntocinon and 500 mg of ergometrine). In women with hypertension and cardiac disease, syntometrine is contraindicated. Carbetocin is synthetic oxytocin receptors in the myometrium that is similar to oxytocin. The major benefit of Carbetocin over oxytocin is that it has a four-fold longer uterotonic activity, which lasts for 2 hours.3
In the treatment of PPH, misoprostol may be used, especially in a low resource setting: 400 μg in powdered form sublingually or three 20-μg tablets are orally administered. In a dose of 250 μg, prostaglandin F2a is administered IM; the highest number of doses is eight (15 min apart) (2 mg). In women with cardiac disease and asthma, it is contraindicated.3
Tranexamic acid 1–2 g hourly (intravenously) will reduce fibrinolysis as well as stabilizing the clot in the uterus vessels. It is best applied when prostaglandin is required and in a situation when the bleeding persist regardless of oxytocin.
When uterotonic medications (prostaglandin, oxytocin and ergometrine) and uterine massage have all been unsuccessful in controling PPH, recombinant factor VIIa (rFVIIa) is applied, although it is very expensive. The dose that is recommended is 40–6-mg/kg, which is administered intravenously. This drug is clinically used as a last resort owing to its expense and side-effects, especially stroke and myocardial infarction.
For women who have failed to respond to treatment with uterotonics, or in a situation where uterotonics are not available, using an intrauterine balloon is recommended in the treatment of PPH as a result of uterine atony. Uterine balloon tamponade may be a life-saving procedure and the reported complications such as uterine perforation and infection are rare. Balloon tamponade should be used subsequent to an atonic PPH and vaginal delivery that is not responding to uterotonics, preceding surgical interventions or interventional radiology procedures, e.g. B-Lynch suture, hysterectomy or iliac artery ligation or uterine artery embolization. It can be inserted after or during cesarean section as well as in a woman with vaginal birth after earlier cesarean section with PPH and after exclusion of rupture.6
When the tamponade test fails to arrest hemorrhage, laparotomy should be considered. Patient’s parity, amount of blood loss, hemodynamic status and continuing bleeding have to be taken into consideration before trying any conservative surgical measures. It is essential to avoid “too little being done too late”. Conservative surgical measures involve compression sutures, which consist of classical B-Lynch, or horizontal or vertical brace sutures with the use of absorbable suture material.4
Systematic pelvic revascularization
In case of failure of the compression sutures, ligation of blood vessels that supply the uterus has to be attempted. This entails ligation of the two uterine arteries, and then tubal branches of the two ovarian arteries proximal to the ovarian ligament (quadruple ligation). The moment the bladder is reflected down and the uterovesical fold of peritoneum is incised, uterine artery ligation is straightforward. In the broad ligament just lateral to the uterine vessels, a window is made and the needle is passed via this opening. The needle is medially passed via the lower uterine myometrium, approximately 2 cm from the lateral margin, therefore getting a decent “bite” before tying the sutures. On the other side, a similar procedure is performed.
If bleeding persists, internal iliac artery ligation is optional. This necessitates a surgeon who is experienced and well knowledgeable of the lateral pelvic wall anatomy. It has been found that bilateral internal artery ligation reduces the pulse pressure by up to 85% in arteries distal to the ligation.
Interventional radiology
Interventional radiology can be undertaken for women who are not intensely compromised or severely bleeding. The rates of success may be as high as 85–95% and the whole procedure may take approximately 1 hour. Uterine artery embolization assists in preserving future fertility and evading radical procedures
Total or subtotal hysterectomy
Peripartum hysterectomy should be considered when uterine atony is not responding to uterotonics as well as where embolization facilities are not available and/or the obstetrician is not sufficiently competent with the technique of iliac artery ligation or conservative surgical procedures. Subtotal hysterectomy is a relatively quicker procedure as compared to a total hysterectomy and may be useful in atonic or traumatic hemorrhage involving the upper segment of the uterus. Total hysterectomy may be necessary in cases of accreta, uterine rupture including the lower segment and bleeding secondary to a placenta previa.
SUMMARY
Management of massive obstetric hemorrhage and resultant hemorrhagic shock involves timely recognition and appropriate management. Algorithms such as ‘hemOSTASIS’ and ‘SHOCK’ may help clinicians to ensure a systematic, logical and stepwise approach in managing this acute obstetric emergency. Multidisciplinary input and continuous case reviews and learning from adverse events as well as morbidity and mortality reports are essential to avoid serious maternal morbidity and mortality.
PRACTICE RECOMMENDATIONS
- A more precise “clinical” definition of massive hemorrhage should be “any blood loss, which results in hemodynamic instability and endangers the life of a woman”.
- Shock refers to a reduction in tissue perfusion, which is insufficient to meet the metabolic requirements of tissues and organs. Insufficient blood flow may be clinically noticeable as the development of lactic acidosis, altered mental status, oliguria, pallor, tachycardia, or all these changes combination.
- Young healthy individuals can compensate for the loss of significant volumes from the circulation with few visible clinical signs. This may pose a diagnostic dilemma and may result in a delay in diagnosis of massive obstetric hemorrhage.
- In a pregnant population, obstetric shock index (OSI) >1 (that is pulse rate > systolic blood pressure) has been shown to increase the likelihood of blood transfusion. OSI may, therefore, act as an adjunct in minimizing errors as a result of visual estimation of blood loss.
- The use of logical, stepwise algorithms such hemOSTASIS (Table 4) have been reported to be associated with excellent outcomes as well as a low rate of peripartum hysterectomy.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Hemorrhagic shock, 27 March 2015, Author: John Udeani, Medscape. | |
Higgins S. Obstetric haemorrhage. Emerg Med (Fremantle), 2003. | |
Arulkumaran S, Karoshi M, Keith LG, et al. A Comprehensive Textbook of Postpartum Hemorrhage: An Essential Clinical Reference for Effective Management, 2012. | |
Chandraharan E, Arulkumaran S. Obstetric and Intrapartum Emergencies. A practical guide to management 2012. | |
Chandraharan E, Arulkumaran S. Management algorithm for atonic postpartum haemorrhage. J Paediatr Obstet Gynaecol 2005. | |
Condous GS, Arulkumaran S, Symonds I, et al. The tamponade test for massive postpartum haemorrhage. Obstet Gynecol 2003;104. |
FURTHER READING
Chandraharan E, Arulkumaran S. Surgical aspects of postpartum haemorrhage. Review Article. Best Pract Res Clin Obstet Gynaecol 2008;22(6):1089–102. | |
Krishna A, Chandraharan E. Management of Massive Obstetric Haemorrhage. Invited Article. Women’s Health Reviews. Current Women's Health Reviews 2011;7(2):136–142(7). | |
Varatharajan L, Chandraharan E, Sutton J, et al. Outcome of the management of massive postpartum hemorrhage using the algorithm "HEMOSTASIS". Int J Gynaecol Obstet 2011;113(2):152–4. | |
Le Bas A, Chandraharan E, Addei A, et al. Use of the "obstetric shock index" as an adjunct in identifying significant blood loss in patients with massive postpartum hemorrhage. Int J Gynaecol Obstet 2014;124(3):253–5. | |
Pinas Carillo A, Chandraharan E. Management of massive postpartum hemorrhage and management of coagulopathy. Review Article. Obstet Gynecol Reprod Med 2014;24(10):291–5. | |
Chandraharan E, Krishna A. Diagnosis and management of postpartum haemorrhage. BMJ 2017;358:j3875. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards programme CLICK HERE)

