This chapter should be cited as follows:
Doiron T, Ghahremani T, et al., Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.419193
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 18
Ultrasound in obstetrics
Volume Editors:
Professor Caterina M (Katia) Bilardo, Amsterdam UMC, Amsterdam and University of Groningen, Groningen, The Netherlands
Dr Valentina Tsibizova, PREIS International School, Florence, Italy

Chapter
Amniotic Fluid Volume in Fetal Health and Disease
First published: August 2023
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
Amniotic fluid is the result of a delicate balance between maternal, fetal, and placental systems, and can provide an indirect measure of fetal health and disease. Amniotic fluid is usually sterile until the onset of labor and has bacteriostatic properties that are immuno-protective for the fetus.1 The amniotic fluid acts to protect the developing fetus and umbilical cord, allows for normal fetal movements, and is essential for fetal development.2,3 Additionally, it is essential for lung and gastrointestinal development in the fetus.3 Disorders of amniotic fluid volume (AFV) are associated with adverse maternal and fetal outcomes. For this reason, the assessment of AFV has become part of routine obstetric practice. This chapter will review the basics of amniotic fluid evolution and measurement as well as the diagnosis and management of AFV disorders.
DYNAMICS OF AMNIOTIC FLUID
Recognizing the dynamics of amniotic fluid management can lead to an understanding of the underlying pathophysiology of fetal health and disease. Normal AFV has been estimated to be between 500–1200 ml at term.2,3 The management of AFV is through an interchange between resorption and production mechanisms including fetal urine production, fetal swallowing, secretion of fluid from the fetal lungs through the oral–nasal cavity, the intramembranous and trans-membranous pathways, and movement of fluid across the fetal skin prior to keratinization.4 Disorders in these mechanisms can lead to oligohydramnios (low AFV) or polyhydramnios (high AFV).
In the first half of pregnancy, the formation of amniotic fluid is not well understood. It is hypothesized that, in early pregnancy, an active solute transport into the amniotic space occurs with water passively moving toward the higher osmolality gradient.2 There is also a transudate through the non-keratinized fetal skin prior to 22 weeks into the amniotic space. Starting around 8–11 weeks of gestation, the fetus’ renal system begins functioning and dilute urine becomes the main source of amniotic fluid in the second and third trimesters,5 which results in the amniotic fluid osmolality decreasing as the pregnancy progresses.6 The fetus is estimated to produce 7 to 70 ml/hour of urine by 3D bladder volume estimates.7 The fetus responds to changes in perfusion and will adjust urine production, thereby changing the AFV.2 As we see later in this review, disorders in utero placental perfusion can lead to oligohydramnios.
The resorption of amniotic fluid is mainly through fetal swallowing. The fetus swallows, on average, 210–760 ml per day.2 The swallowing is also important for gastrointestinal development.
Amniotic fluid measurement
Measurement of amniotic fluid can be performed directly, indirectly, or sonographically.8 Direct measurement is done at the time of cesarean delivery, quantifying the amniotic fluid present.9 Indirect measurement is carried out by amniocentesis using dye-dilution techniques.10 Usually, routine antenatal fetal surveillance includes an estimation of AFV by measurement of amniotic fluid using ultrasonography.11
Ultrasound techniques for measurement of AFV include amniotic fluid index (AFI) and single deepest pocket (SDP). AFI is a quantification of all four quadrants, whereas SDP represents the quadrant with the deepest pocket of fluid. Multiple studies have compared these two methods, and both have utility in obstetrics. Oligohydramnios is defined as amniotic fluid volume less than the fifth percentile for gestational age, AFI less than 5 cm or single deepest pocket less than 2 cm. The single deepest pocket is the preferred method for diagnosing oligohydramnios (Figure 1).12
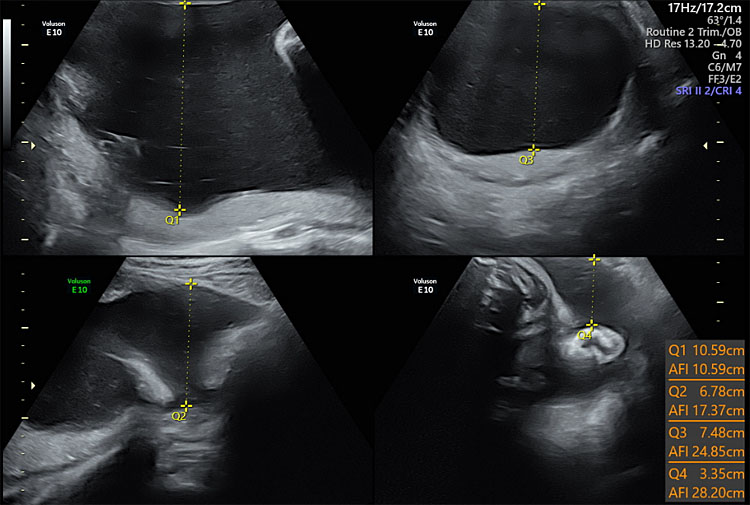
1
Ultrasound of amniotic fluid in four quadrants showing polyhydramnios.
Polyhydramnios or hydramnios, is defined as an AFI or SDP greater than the 95th percentile for gestational age. Some investigators have called for a more restrictive definition because only 1–2% of pregnancies have high AFVs. Polyhydramnios has been defined as a single deepest pocket greater than 8 cm or an amniotic fluid index greater than 24 or 25 cm.13 Polyhydramnios is caused by decreased absorption of amniotic fluid, or an increased production of amniotic fluid.13 The majority are idiopathic and this accounts for 65 percent of cases.14 Regardless of the gestational age at diagnosis, polyhydramnios is associated with poor pregnancy outcomes.3
OLIGOHYDRAMNIOS
Oligohydramnios is a fetal condition in which the AFV is low. While the average amount of AFV varies throughout the gestation, oligohydramnios has been associated with poor fetal, maternal, and neonatal outcomes, which are listed in Table 1.15,12 Several fetal conditions are known to be associated with oligohydramnios.
1
Fetal/neonatal risks associated with oligohydramnios.
NICU admission |
Cesarean delivery |
Meconium aspiration syndrome |
Low birth weight |
Stillbirth |
Neonatal death |
Congenital anomalies
Congenital anomalies comprise a subset of structural causes of oligohydramnios. One of the most lethal of these is bilateral renal agenesis in which the fetal kidneys are absent. The subsequent oligohydramnios or anhydramnios – the absence of fluid – leads to fetal pulmonary hypoplasia, which is the cause of death in most cases.16 Multicystic dysplastic kidney (MCDK) is the number one incidental cystic renal lesion found on prenatal ultrasound.17 If the bilateral kidneys are affected, oligohydramnios is likely. Prognosis is dependent on severity, whether unilateral or bilateral, and if other concurrent urinary tract anomalies are present.17 Autosomal recessive polycystic kidney disease (ARPKD) may cause fetal renal insufficiency and resulting oligohydramnios and pulmonary hypoplasia (Figure 2).18 Fetal bladder outlet obstruction is usually caused by posterior urethral valves, which obstruct fetal urination, resulting in oligohydramnios.19 Other causes of fetal bladder outlet obstruction include urethral atresia/stenosis20 and aphallia.20
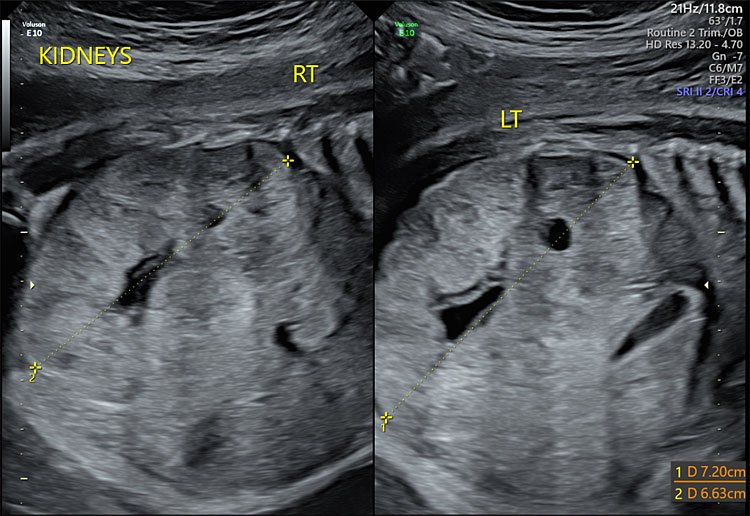
2
Ultrasound showing enlarged bilateral kidneys.
Vesicoamniotic shunting may be helpful for selected cases of bladder outlet obstruction21 in order to correct the oligohydramnios and allow for fetal lung development. A persistent cloaca in the fetus is a rare anorectal/urogenital malformation that may be seen in association with oligohydramnios and other findings on prenatal ultrasound including fetal ascites, abdominal cystic mass, hydrocolpos, hydronephrosis, or hydroureter.22,23 Oligohydramnios in this setting independently increases the risk of irreversible renal dysfunction.23 Lastly, sirenomelia refers to congenital maldevelopment of the fetal caudal body.24 This diagnosis is associated with renal agenesis and absent urinary bladder, leading to oligohydramnios and pulmonary hypoplasia.24
Preterm pre-labor rupture of membranes
Preterm pre-labor rupture of membranes (PPROM) describes a disruption in the amniotic sac, resulting in amniotic fluid leakage, in the absence of labor prior to 37 weeks of gestation. The incidence is about 3% of pregnancies.25 Oligohydramnios following membrane rupture occurs in over one-third of cases.26 Prior to 24 weeks, oligohydramnios that is the result of premature rupture of membranes is associated with very poor perinatal outcomes. This is primarily due to pulmonary hypoplasia. The neonates who survive are those whose AFV was normal initially.25 After 24 weeks, oligohydramnios and PPROM may be associated with a shorter interval time to delivery, chorioamnionitis, neonatal respiratory distress syndrome, low APGAR scores, cesarean delivery, and neonatal death.26
Maternal medications
Maternal ingestion of certain medications may have an impact on AFV. The use of non-steroidal anti-inflammatory drugs (NSAIDs) in the third trimester of pregnancy have been associated with oligohydramnios as well as premature closure of the ductus arteriosus. The premature closure of the ductus is due to decreased synthesis of prostaglandins caused by the NSAIDs27 The mechanism for oligohydramnios is the development of abnormal fetal renal function leading to a reduced urine output, which may be transient or severe and potentially lethal.28 Both angiotensin-converting enzyme inhibitors (ACE-Is) and angiotensin receptor antagonists (ARBs) interfere with the renin-angiotensin system, leading to oligohydramnios.29
Placental insufficiency
Uteroplacental insufficiency results in decreased blood, nutrients, and oxygen to be delivered to the fetus during the pregnancy. The fetus then undergoes a survival mechanism, shunting blood to its brain, heart, and adrenal glands at the expense of other parts of its body. Decreased blood flow to the fetal kidneys results in decreased renal perfusion and resultant oligohydramnios. Placental insufficiency of the placenta may be caused by maternal conditions such as pre-eclampsia, or fetal conditions such as aneuploidy. The most likely cause of uteroplacental insufficiency is abnormal placentation and remodeling of the spiral arteries, which causes hypoperfusion of the placenta.30 This may result in fetal growth restriction and oligohydramnios. Placentas in these pregnancies show evidence of placental malperfusion, including smaller placentas by weight, meconium-stained membranes, and vascular abnormalities.31 A post-term pregnancy can also result in oligohydramnios, as the AFV declines by approximately 8% per week due to the normal calcification process of the placenta.3
Isolated oligohydramnios
Isolated oligohydramnios in an otherwise normal-term gestation is generally considered to be a benign finding. However, it may be associated with a small for gestational infant that was not detected prenatally.32 Some studies show isolated oligohydramnios at term/post-term pregnancies is associated with an increased risk for cesarean delivery for fetal distress, and low APGAR scores, while more recent studies agree with increased risk for obstetrical interventions, but do not show an increase in adverse neonatal outcomes.33
POLYHYDRAMNIOS
Fetal conditions causing polyhydramnios
Polyhydramnios can be caused by decreased absorption of amniotic fluid or increased production of amnionic fluid. When a fetus is diagnosed with polyhydramnios, it is important to evaluate the fetal anatomy to determine if the hydramnios is caused by a fetal condition.3 The most common causes of polyhydramnios are fetal anomalies or maternal diabetes.34 There are several fetal anatomic abnormalities that can interfere with the mechanism of fetal swallowing, such as abnormalities in the fetal digestive tract, neurologic pathway, or respiratory tract.3,13 Furthermore, there are several conditions that can cause an increased production of amniotic fluid, such as abnormalities in the placenta, fetal heart, or kidneys.35
Aneuploidy
The reported risk for fetal aneuploidy when a pregnancy has sonographic fetal abnormalities and polyhydramnios is as high as 10% compared with only 1% in pregnancies with no sonographic abnormalities.36 While genetic testing should be offered to all pregnant mothers, the finding of polyhydramnios and a fetal anomaly should be referred for genetic counseling and possibly evaluation for neurologic disorders.34
Impaired swallowing and GI obstruction
Digestive tract
Abnormalities in the fetal digestive tract at any portion, can result in polyhydramnios. Craniofacial anomalies including cleft lip and palate and micrognathia,36 esophageal and tracheal development disorders,37 duodenal atresia and obstructions more distal in the digestive tract are all associated with polyhydramnios due to impaired ingestion of amniotic fluid (Figure 3).38
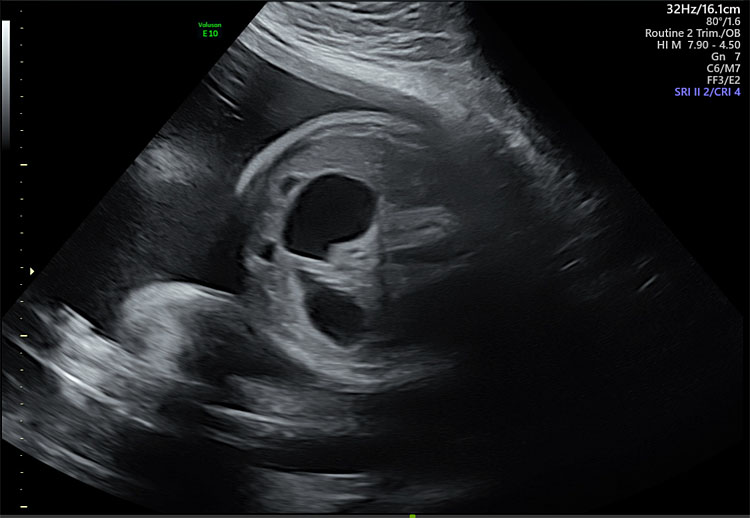
3
Same patient from Figure 1 with “double-bubble” sign indicative of duodenal atresia.
Neuromuscular impairment
Impaired swallowing can also be caused by central nervous system anatomic anomalies.34 Neuromuscular disorders are implicated in impaired swallowing due to poor motor function such as myotonic dystrophy and arthrogryposis.35 For example, polyhydramnios is the most common prenatal ultrasound finding for fetuses with congenital myotonic dystrophy.39
Respiratory tract
Tracheal compression, such as neck venolymphatic abnormalities and congenital high airway obstruction (CHAOS) can also prevent amniotic fluid consumption.40 CHAOS is a rare fetal malformation due to airway obstruction from a variety of etiologies, such as laryngeal atresia/webs/cysts, subglottic stenosis, or tracheal atresia.41 In most cases of CHAOS, polyhydramnios is due to esophageal compression causing difficulty swallowing.41 Thoracic or GI obstructions, such as diaphragmatic hernias, cystic adenomatoid malformations, pulmonary sequestrations can also impair the swallowing mechanism due to a compressive mediastinal shift and subsequent esophageal distortion.34
Overproduction
Polyhydramnios can also be caused by increased production of amniotic fluid. Increased production can arise from a variety of different etiologies, such as ureteropelvic junction obstruction, Bartter syndrome, maternal diabetes, and cardiac abnormalities.
Ureteropelvic junction obstruction
Paradoxical polyhydramnios can result from ureteropelvic junction obstruction.34 This concept seems counterintuitive because after the second trimester, fetal urinary excretion is the primary source of amniotic fluid. However, due to urinary tract obstruction and resulting hydronephrosis, it is speculated that impaired kidney function results in a higher volume of less concentrated urine resulting in polyhydramnios.42
4
Concept map.
Bartter syndrome is an autosomal recessive disorder caused by genetic mutations in sodium chloride reabsorption in the ascending loop of Henle. It is characterized by hypokalemic, hypochloremic metabolic acidosis and often presents prenatally with severe polyhydramnios.43 The polyhydramnios in fetuses with Bartter syndrome can be attributed to severe polyuria.44 Bartter syndrome is a clinical diagnosis made postnatally and confirmed with genetic testing. Therefore, this presents a challenge in antenatal diagnosis, and in patients with early onset severe polyhydramnios with no fetal anatomic abnormalities, Bartter syndrome should be considered.44
Cardiac output
Conditions in which cardiac output is increased may also increase the rate of polyhydramnios due to increased renal perfusion and increased urine output. Cardiac etiologies may also play a role in polyhydramnios; specifically, those resulting in a high-output cardiac state, such as fetal anemia, thyrotoxicosis, Ebstein anomaly, and Tetralogy of Fallot with an absent pulmonary valve.45 A functional cardiac etiology can also be the cause of polyhydramnios from a high-output state, such as cardiomyopathy and myocarditis.45 Cardiac arrhythmias, both tachyarrhythmias and bradyarrhythmias, are also a possible cardiac etiology of polyhydramnios.46 Other causes of high cardiac output, like neoplastic processes, such as sacrococcygeal teratomas, mesoblastic nephromas, and placental chorioangiomas, can all result in polyhydramnios.47,48 The etiology of polyhydramnios in these cases is due to vascular shunting from the fetus leading to a high cardiac output state, increased renal perfusion, and increased urine output. All of these cardiac conditions are also at risk for nonimmune hydrops fetalis, which explains why cases of hydrops can present with polyhydramnios.49
Infections
Isolated polyhydramnios is extremely nonspecific, especially in relation to TORCH infections.50 Polyhydramnios can be associated with CMV, toxoplasmosis, syphilis, and parvovirus infection. However, several studies both observational and retrospective suggest that polyhydramnios and infection are not directly associated and that testing for infection in patients with polyhydramnios alone may not be indicated.51,52
Twin–twin transfusion syndrome
Twin-to-twin transfusion syndrome presents in monochorionic twin pregnancies and is due to abnormal vascular connections in the shared placenta.53 The first presenting sign of TTTS is polyhydramnios in the recipient twin due to increased perfusion and presumed increased fetal urine production and oligohydramnios in the donor twin due to decreased renal perfusion.53
Maternal conditions resulting in polyhydramnios: diabetes
Maternal diabetes is a disease process that is believed to cause polyhydramnios through increased production of amniotic fluid. Roughly 15% of cases of polyhydramnios are amongst pregnant patients with diabetes.54 The pathophysiologic mechanism of this phenomenon is through increased maternal serum glucose causing increased amniotic fluid glucose concentration. Fetal hyperglycemia invokes osmotic diuresis in the fetal kidneys and polyuria with overall increased production of amniotic fluid.55 Screening for gestational diabetes in every pregnancy is recommended; however, repeat screening is not indicated based on polyhydramnios alone.34
Idiopathic polyhydramnios
The prevalence of polyhydramnios is 1–2% with 50–60% of those cases being idiopathic. Idiopathic polyhydramnios has been linked in a review of cases between 1950 and 2007 with macrosomia and 2–5-fold increase in the risk of perinatal mortality.56 A more recent systematic review and meta-analysis compared 2392 women with idiopathic polyhydramnios with 160,135 controls.57 The pregnancies with idiopathic polyhydramnios had higher odd ratios (OR) of neonatal death (OR 8.68; 95% CI 2.91–25.87), intrauterine fetal demise (OR 7.64; 95% CI 2.50–23.38), newborn intensive care unit admission (OR 1.94; 95% CI 1.45–2.59), 5 minute Apgar score <7 (OR 2.21; 95% CI 1.34–3.62), macrosomia (OR 2.93; 95%CI 2.39–3.59), and cesarean delivery (OR 2.31; 95% CI 1.79–2.99), as seen in Table 2.
2
Fetal/neonatal risks associated with idiopathic polyhydramnios.
Intrauterine fetal demise |
Neonatal death |
NICU admission |
Cesarean delivery |
Macrosomia |
Malpresentation |
Undetected fetal anomaly |
Undetected fetal aneuploidy |
Another risk factor associated with idiopathic polyhydramnios is undetected fetal anomaly and/or aneuploidy. A study by Abele et al. examined 272 cases of unexplained polyhydramnios. In 154 cases, a specific cause of polyhydramnios was found either fetal or maternal in origin. However, in the remaining 118 patients, no direct cause was able to be identified. In 11 of these cases (~10%), a fetal anomaly was diagnosed after delivery.46 Similarly, Dashe et al. did a retrospective review of pregnancies with polyhydramnios and fetal anomalies. This study only removed patients with prenatally diagnosed anomalies, not diabetes or fetal infection, but there were still 16 undiagnosed fetal anomalies in a population of 611 patients (~2%).36 In addition to undiagnosed anomalies, idiopathic polyhydramnios is associated with fetal aneuploidy. A study by Brady et al. demonstrated a five-times increased risk of aneuploidy when compared to the risk of aneuploidy in the general population.58
Preterm birth has classically been associated with polyhydramnios given increased uterine distension and risk for prelabor rupture of membranes. However, this has not been demonstrated consistently in studies as an independent risk factor of polyhydramnios. A study by Many et al. found that idiopathic polyhydramnios was not associated with preterm birth.67 However, polyhydramnios related to fetal malformations or maternal diabetes was associated with preterm birth. Other studies by Khan and Donnelly as well as by Panting-Kemp et al. echoed this observation and found no statistical difference in the rate of preterm birth when examining idiopathic polyhydramnios alone.59,14
One specific group of cases at markedly increased risk are fetuses with ultrasound-assessed growth restriction with polyhydramnios. These pregnancies are at an incredibly high risk of adverse pregnancy outcomes. In these pregnancies, the perinatal death rate is approximately 60–65%. Most of these cases are associated with chromosomal and/or complex malformation syndromes, which accounts for the poor prognosis. All patients with this finding should be offered genetic testing and a detailed fetal ultrasound.60,61
Treatment of polyhydramnios
Treatment of polyhydramnios is not typically pursued. This may be related to a lack of treatment options that demonstrate long-term benefit and low risk. Amnioreduction is currently the only known way to acutely decrease the amount of amniotic fluid, but it is associated with risks of preterm labor, preterm delivery, placental abruption, preterm prelabor rupture of membranes, chorioamnionitis, and IUFD.62 Additionally, around 50% of patients need multiple treatments, potentially increasing these risks.62,63 Although, a recent study by Kleine et al. showed that these risks are not statistically increased from the baseline risks associated with polyhydramnios alone.64 Even so, amnioreduction is reserved for cases of polyhydramnios causing severe maternal discomfort from uterine distention or severe dyspnea related to upward pressure on the diaphragm.34 Historically, the only fetal indication for amnioreduction was for reduction of the donor twin’s sac in the setting of twin–twin transfusion syndrome in monochorionic, diamniotic twin pregnancies. However, this treatment modality is no longer widely accepted with the emergence of fetoscopic laser ablation as a more effective treatment option.65
Treatment with indomethacin has been proposed as a treatment for polyhydramnios as well. Indomethacin use is associated with decreased fetal and neonatal urine output, which may lead to a diminishment of amniotic fluid as less fluid is created.34 Kirshon et al. used indomethacin in conjunction with amnioreduction and showed that fewer patients required a repeat procedure.66 However, indomethacin has been shown to have several fetal and neonatal effects and is generally limited to 48 to 72 hours of therapy and less than 200 mg/day. Given these limitations, the current recommendation is against the use of indomethacin for the sole purpose of decreasing amniotic fluid.34
CONCLUSIONS
AFV has been described as the most important measure of fetal well-being in the biophysical profile. Understanding the mechanism of amniotic fluid regulation can assist with intrauterine fetal diagnosis in the setting of abnormal AFV.
PRACTICE RECOMMENDATIONS
- Amniotic fluid is a delicate balance of the production and resorption systems
- Amniotic fluid volume (AFV) may be measured by ultrasound and indicate fetal well-being or disease
- Disruptions in the amniotic fluid dynamics may lead to oligohydramnios or polyhydramnios
- Oligohydramnios is best defined by single deepest pocket of less than 2 cm polyhydramnios is best defined by amniotic fluid index of greater than 24 cm
- Oligohydramnios may be caused by disorders of production of amniotic fluid (structural blockage versus hypoperfusion), as well as ruptured membranes
- Polyhydramnios may be caused by increased production of amniotic fluid (gestational diabetes) or anomalous prevention of resorption
- Disorders of amniotic fluid can increase the risk of intrauterine fetal demise and increased fetal surveillance is often indicated in these cases
CONFLICTS OF INTEREST
Dr Everett Magann is the author of an UpToDate article on Assessment of Amniotic Fluid Volume. The remaining authors of this chapter declare that they have no interests that conflict with the contents of this chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Rehbinder EM, Lødrup Carlsen KC, Staff AC, et al. Is amniotic fluid of women with uncomplicated term pregnancies free of bacteria? Am J Obstet Gynecol 2018;219(3):289.e1–12. PMID 29852156. | |
Beall MH, van den Wijngaard JP, van Gemert MJ, et al. Amniotic fluid water dynamics. Placenta 2007;28(8–9):816–23. PMID 17254633. | |
Moore TR. Amniotic fluid dynamics reflect fetal and maternal health and disease. Obstet Gynecol 2010;116(3):759–65. PMID 20733463. | |
Modena AB, Fieni S. Amniotic fluid dynamics. Acta Biomed 2004;75(Suppl 1):11–3. PMID 15301282. | |
Beall MH, van den Wijngaard JP, van Gemert MJ, et al. Regulation of amniotic fluid volume. Placenta 2007;28(8–9):824–32. PMID 17303237. | |
Magann EF, Sandlin AT, Ounpraseuth ST. Amniotic fluid and the clinical relevance of the sonographically estimated amniotic fluid volume: oligohydramnios. J Ultrasound Med 2011;30(11):1573–85. PMID 22039031. | |
Hughes DS, Magann EF. Antenatal fetal surveillance "Assessment of the AFV". Best Pract Res Clin Obstet Gynaecol 2017;38:12–23. PMID 27756534. | |
Dubil EA, Magann EF. Amniotic fluid as a vital sign for fetal wellbeing. Australas J Ultrasound Med 2013;16(2):62–70. PMID 28191176. | |
Horsager R, Nathan L, Leveno KJ. Correlation of measured amniotic fluid volume and sonographic predictions of oligohydramnios. Obstet Gynecol 1994;83(6):955–8. PMID 8190439. | |
Magann EF, Whitworth NS, Files JC, et al. Dye-dilution techniques using aminohippurate sodium: do they accurately reflect amniotic fluid volume? J Matern Fetal Neonatal Med 2002;11(3):167–70. PMID 8190439. | |
Indications for Outpatient Antenatal Fetal Surveillance: ACOG Committee Opinion, Number 828. Obstet Gynecol 2021;137(6):e177–e97. PMID 34011892. | |
Rabie N, Magann E, Steelman S, et al. Oligohydramnios in complicated and uncomplicated pregnancy: a systematic review and meta-analysis. Ultrasound Obstet Gynecol 2017;49(4):442–9. PMID 27062200. | |
Whittington JR MP. Amniotic Fluid: Physiology and Assessment. In: Khalil A. (ed.) The Continuous Textbook of Women's Medicine – Obstetrics Module, 4: GLOWM, 2021. | |
Panting-Kemp A, Nguyen T, Chang E, et al. Idiopathic polyhydramnios and perinatal outcome. Am J Obstet Gynecol 1999;181(5 Pt 1):1079–82. PMID 10561621. | |
Figueroa L, McClure EM, Swanson J, et al. Oligohydramnios: a prospective study of fetal, neonatal and maternal outcomes in low-middle income countries. Reprod Health 2020;17(1):19. PMID 32000798. | |
Huber C, Shazly SA, Blumenfeld YJ, et al. Update on the Prenatal Diagnosis and Outcomes of Fetal Bilateral Renal Agenesis. Obstet Gynecol Surv 2019;74(5):298–302. PMID 31098643. | |
Ji H, Dong SZ. Magnetic resonance imaging for evaluation of foetal multicystic dysplastic kidney. Eur J Radiol 2018;108:128–32. PMID 30396644. | |
Büscher R, Büscher AK, Weber S, et al. Clinical manifestations of autosomal recessive polycystic kidney disease (ARPKD): kidney-related and non-kidney-related phenotypes. Pediatr Nephrol 2014;29(10):1915–25. PMID 24114580. | |
Farrugia MK. Fetal bladder outlet obstruction: Embryopathology, in utero intervention and outcome. J Pediatr Urol 2016;12(5):296–303. PMID 27570093. | |
Vaizer RP, Benton JZ, Morganstern BA. First Case of a Term Male Born with Aphallia and Complete Urethral Atresia. Urology 2021;156:e127–e30. PMID 34087315. | |
Jeong BD, Won HS, Lee MY. Perinatal Outcomes of Fetal Lower Urinary Tract Obstruction After Vesicoamniotic Shunting Using a Double-Basket Catheter. J Ultrasound Med 2018;37(9):2147–56. PMID 29498072. | |
Kawamura T, Kamo A, Nishiguchi T. Diagnosis of Persistent Cloaca by Ultrasonography and MRI: A Case Report. Am J Case Rep 2020;21:e921576. PMID 32381998. | |
Harumatsu T, Sugita K, Ieiri S, et al. Risk factor analysis of irreversible renal dysfunction based on fetal ultrasonographic findings in patients with persistent cloaca: Results from a nationwide survey in Japan. J Pediatr Surg 2022;57(2):229–34. PMID 34809962. | |
Ceylan Y, Doğan Y, Özkan Özdemir S, et al. Prenatal diagnosis of sirenomelia in the first trimester: A case report. Turk J Obstet Gynecol 2016;13(1):50–2. PMID 28913090. | |
Pylypjuk C, Majeau L. Perinatal Outcomes and Influence of Amniotic Fluid Volume Following Previable, Preterm Prelabor Rupture of Membranes (pPPROM): A Historical Cohort Study. Int J Womens Health 2021;13:627–37. PMID 34234574. | |
Ekin A, Gezer C, Taner CE, et al. Perinatal outcomes in pregnancies with oligohydramnios after preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2015;28(16):1918–22. PMID 25283853. | |
Antonucci R, Zaffanello M, Puxeddu E, et al. Use of non-steroidal anti-inflammatory drugs in pregnancy: impact on the fetus and newborn. Curr Drug Metab 2012;13(4):474–90. PMID 22299823. | |
Bloor M, Paech M. Nonsteroidal anti-inflammatory drugs during pregnancy and the initiation of lactation. Anesth Analg 2013;116(5):1063–75. PMID 23558845. | |
Bullo M, Tschumi S, Bucher BS, et al. Pregnancy outcome following exposure to angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists: a systematic review. Hypertension 2012;60(2):444–50. PMID 22753220. | |
Miremberg H, Grinstein E, Herman HG, et al. The association between isolated oligohydramnios at term and placental pathology in correlation with pregnancy outcomes. Placenta 2020;90:37–41. PMID 32056549. | |
Spinillo A, Cesari S, Bariselli S, et al. Placental lesions associated with oligohydramnios in fetal growth restricted (FGR) pregnancies. Placenta 2015;36(5):538–44. PMID 25735841. | |
Naveiro-Fuentes M, Puertas Prieto A, Ruíz RS, et al. Perinatal outcomes with isolated oligohydramnios at term pregnancy. J Perinat Med 2016;44(7):793–8. PMID 26506098. | |
Rossi AC, Prefumo F. Perinatal outcomes of isolated oligohydramnios at term and post-term pregnancy: a systematic review of literature with meta-analysis. Eur J Obstet Gynecol Reprod Biol 2013;169(2):149–54. PMID 23561019. | |
Dashe JS, Pressman EK, Hibbard JU. SMFM Consult Series #46: Evaluation and management of polyhydramnios. Am J Obstet Gynecol 2018;219(4):B2–8. PMID 30048635. | |
Hamza A, Herr D, Solomayer EF, et al. Polyhydramnios: Causes, Diagnosis and Therapy. Geburtshilfe Frauenheilkd 2013;73(12):1241–6. PMID 24771905. | |
Dashe JS, McIntire DD, Ramus RM, et al. Hydramnios: anomaly prevalence and sonographic detection. Obstet Gynecol 2002;100(1):134–9. PMID 12100815. | |
Katz JM, Malik A, Basit H. Embryology, Esophagus. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC, 2022. PMID 31194444. | |
Sigmon DF, Eovaldi BJ, Cohen HL. Duodenal Atresia And Stenosis. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC, 2022. PMID 29261981. | |
Rogers R, Moyer K, Moise KJ, et al. Congenital myotonic dystrophy: An overlooked diagnosis not amenable to detection by sequencing. Prenat Diagn 2022;42(2):233–5. PMID 35083764. | |
García-Díaz L, Chimenea A, de Agustín JC, et al. Ex-Utero Intrapartum Treatment (EXIT): indications and outcome in fetal cervical and oropharyngeal masses. BMC Pregnancy Childbirth 2020;20(1):598. PMID 33028259. | |
Hamid-Sowinska A, Ropacka-Lesiak M, Breborowicz GH. Congenital high airway obstruction syndrome. Neuro Endocrinol Lett 2011;32(5):623–6. PMID 22167132. | |
Kleiner B, Callen PW, Filly RA. Sonographic analysis of the fetus with ureteropelvic junction obstruction. AJR Am J Roentgenol 1987;148(2):359–63. PMID 3541550. | |
London S, Levine MA, Li D, et al. Hypocalcemia as the Initial Presentation of Type 2 Bartter Syndrome: A Family Report. J Clin Endocrinol Metab 2022;107(4):e1679–e88. PMID 34751387. | |
Nam G, Cho A, Park MH. A Rare Cause of Refractory Severe Polyhydramnios: Antenatal Bartter Syndrome. Medicina (Kaunas) 2021;57(3). PMID 33809664. | |
Desmedt EJ, Henry OA, Beischer NA. Polyhydramnios and associated maternal and fetal complications in singleton pregnancies. Br J Obstet Gynaecol 1990;97(12):1115–22. PMID 2279018. | |
Abele H, Starz S, Hoopmann M, et al. Idiopathic polyhydramnios and postnatal abnormalities. Fetal Diagn Ther 2012;32(4):251–5. PMID 22760013. | |
Wohlmuth C, Bergh E, Bell C, et al. Clinical Monitoring of Sacrococcygeal Teratoma. Fetal Diagn Ther 2019;46(5):333–40. PMID 30893693. | |
Ziemann M, Apostolidou S, Dum D, et al. [Chorangioma of the Placenta – A Rare Placental Cause of Fetal High Output Cardiac Failure]. Z Geburtshilfe Neonatol 2020;224(2):103–6. PMID 31559610. | |
Berger VK, Sparks TN, Jelin AC, et al. Non-Immune Hydrops Fetalis: Do Placentomegaly and Polyhydramnios Matter? J Ultrasound Med 2018;37(5):1185–91. PMID 29076544. | |
Fitzpatrick D, Holmes NE, Hui L. A systematic review of maternal TORCH serology as a screen for suspected fetal infection. Prenat Diagn 2022;42(1):87–96. PMID 34893980. | |
Yefet E, Ben Shmuel Y, Nachum Z. The association between polyhydramnios and CMV infection – retrospective cohort study. J Matern Fetal Neonatal Med 2021;34(22):3716–22. PMID 31698981. | |
Fayyaz H, Rafi J. TORCH screening in polyhydramnios: an observational study. J Matern Fetal Neonatal Med 2012;25(7):1069–72. PMID 21923307. | |
Solorio C, Guenther JS, Chon AH, et al. Twin-twin transfusion syndrome and the definition of recipient polyhydramnios. Am J Obstet Gynecol 2021;225(6):683.e1–8. PMID 34186067. | |
Khanduri S, Chawla H, Khan A, et al. Association and Correlation Between Amniotic Fluid Index and Glucose Concentration. Cureus 2022;14(6):e25973. PMID 35855256. | |
Sandlin AT, Chauhan SP, Magann EF. Clinical relevance of sonographically estimated amniotic fluid volume: polyhydramnios. J Ultrasound Med 2013;32(5):851–63. PMID 23620328. | |
Magann EF, Chauhan SP, Doherty DA, et al. A review of idiopathic hydramnios and pregnancy outcomes. Obstet Gynecol Surv 2007;62(12):795–802. PMID 18005456. | |
Pagan M, Magann EF, Rabie N, et al. Idiopathic polyhydramnios and pregnancy outcomes: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2022. PMID 35723677. | |
Brady K, Polzin WJ, Kopelman JN, et al. Risk of chromosomal abnormalities in patients with idiopathic polyhydramnios. Obstet Gynecol 1992;79(2):234–8. PMID 1731291. | |
Khan S, Donnelly J. Outcome of pregnancy in women diagnosed with idiopathic polyhydramnios. Aust N Z J Obstet Gynaecol 2017;57(1):57–62. PMID 28251633. | |
Sickler GK, Nyberg DA, Sohaey R, et al. Polyhydramnios and fetal intrauterine growth restriction: ominous combination. J Ultrasound Med 1997;16(9):609–14. PMID 9321781. | |
Walter A, Calite E, Berg C, et al. Prenatal diagnosis of fetal growth restriction with polyhydramnios, etiology and impact on postnatal outcome. Sci Rep 2022;12(1):415. PMID 35013541. | |
Dickinson JE, Tjioe YY, Jude E, et al. Amnioreduction in the management of polyhydramnios complicating singleton pregnancies. Am J Obstet Gynecol 2014;211(4):434.e1–7. PMID 24881825. | |
Erfani H, Diaz-Rodriguez GE, Aalipour S, et al. Amnioreduction in cases of polyhydramnios: Indications and outcomes in singleton pregnancies without fetal interventions. Eur J Obstet Gynecol Reprod Biol 2019;241:126–8. PMID 31160132. | |
Kleine RT, Bernardes LS, Carvalho MA, et al. Pregnancy outcomes in severe polyhydramnios: no increase in risk in patients needing amnioreduction for maternal pain or respiratory distress. J Matern Fetal Neonatal Med 2016;29(24):4031–4. PMID 26948899. | |
Bamberg C, Hecher K. Update on twin-to-twin transfusion syndrome. Best Pract Res Clin Obstet Gynaecol 2019;58:55–65. PMID 30850326. | |
Kirshon B, Mari G, Moise KJ, et al. Indomethacin therapy in the treatment of symptomatic polyhydramnios. Obstet Gynecol 1990;75(2):202–5. PMID 2405320. | |
Many A, Hill LM, Lazebnik N, Martin JG. The association between polyhydramnios and preterm delivery. Obstet Gynecol. 1995 Sep;86(3):389-91. doi: 10.1016/0029-7844(95)00179-U. PMID: 7651648. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards programme CLICK HERE)

