This chapter should be cited as follows:
Cruz Martínez R, Gil Pugliese S, Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.419263
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 18
Ultrasound in obstetrics
Volume Editors:
Professor Caterina M (Katia) Bilardo, Amsterdam UMC, Amsterdam and University of Groningen, Groningen, The Netherlands
Dr Valentina Tsibizova, PREIS International School, Florence, Italy

Chapter
Ultrasound Evaluation of the Fetal Thorax
First published: January 2024
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
According to the guidelines published by the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG), the ultrasonographic evaluation of the fetal thorax should be routinely performed as part of the second-trimester morphological scan.1 The thorax is mainly occupied by the lungs, which in fetal life have little functionality since the placenta is responsible for fetal blood oxygenation. However, normal pulmonary development during fetal life is crucial for extrauterine survival. A variety of conditions affecting fetal lung development can lead to increased neonatal morbidity and mortality secondary to pulmonary hypoplasia. Thus, prenatal diagnosis of lung anomalies is key to reducing adverse perinatal outcomes in affected babies. In this chapter, we will explain how to perform routine evaluation of the fetal thorax and provide technical tips for diagnosing the most common lung anomalies. We will also address all the existing fetal therapies for these conditions.
NORMAL FETAL THORAX
Routine evaluation of fetal thorax
A comprehensive routine fetal ultrasonography should include a checklist to ensure the correct evaluation of the fetal thorax, allocating sufficient time to examine the intrathoracic structures.2 On ultrasound, fetal lungs can be seen surrounding the heart and mediastinum and correlating posteriorly, laterally, and anteriorly with the spine, ribcage, and sternum, respectively, superiorly with the clavicles, and inferiorly with the diaphragm. Both fetal lungs present homogeneous echogenicity.3 According to international guidelines, three ultrasound views should be included: axial view, sagittal view, and coronal view.
Transverse or axial view of the fetal thorax
The transverse or axial view of the fetal thorax is the most important plane in the assessment of the lungs, allowing discrimination between normal and abnormal pulmonary morphology. In this view, both lungs should be seen in direct contact with the heart, mediastinum, and ribs, with no fluid occupying the pleural cavity (Figure 1). The heart should be located on the left side of the thorax with its apex pointing to the left (levocardia). Therefore, an abnormal position of the apex to the right side (dextrocardia) and/or a mediastinal shift with heart displacement to the right (dextroposition), should be a clue to identify left-side pulmonary anomalies. This view is also useful to assess lung size, the right lung should be bigger in size than the left lung as it is formed by three lobes (superior, middle, inferior) while the left lung presents only two lobes (superior and inferior) shearing the left hemithorax with most of the cardiac volume. Of note, the thin acoustic shadowing seen between the lungs and the ribs is considered a normal finding and should not be interpreted as fetal pleural effusions.
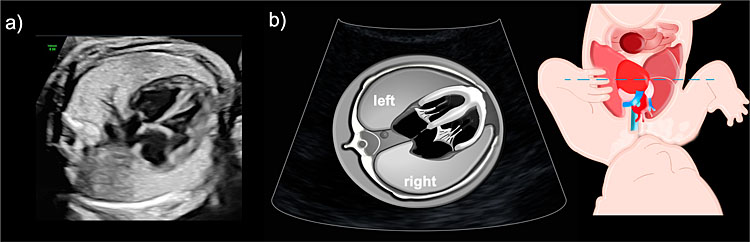
1
Fetal ultrasound image illustrating the axial view of the normal fetal thorax with normal size and echogenicity in both lungs.
Sagittal view of the fetal thorax
The left and right parasagittal views of the fetal thorax allow visualization of both diaphragms respectively. The normal diaphragm can be observed as a hypoechogenic line dividing the thorax from the abdominal cavity. Lung echogenicity is normally low in the first trimester of pregnancy but it increases throughout gestation. Previously, pulmonary echogenicity was incorrectly considered to be similar to hepatic echogenicity, but modern ultrasound technology with higher image quality, has shown that fetal lungs have higher echogenicity than fetal liver, and therefore, the right diaphragm can be easily observed separating the lung from the liver (Figure 2). Ideally, when the fetus lies with an anterior back (towards the maternal abdominal wall), the parasagittal view of the fetal thorax should be obtained on each side of the fetal spine avoiding its shadowing.
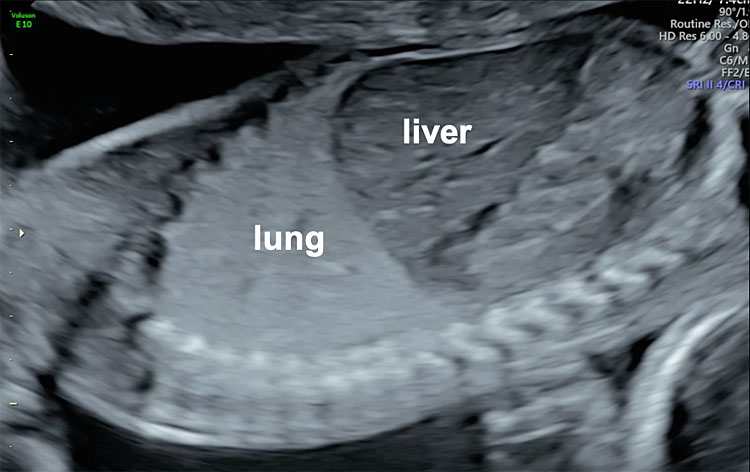
2
Sagittal view of the normal fetal thorax illustrating the convexity of the right diaphragm, highlighting the difference in echogenicity between the right lung and fetal liver.
Coronal view of the fetal thorax
The coronal view simultaneously depicts both diaphragms (Figure 3), allowing confirmation of their integrity and correct position in order to exclude lung anomalies caused by a congenital diaphragmatic hernia or diaphragm eventration.
3
Coronal view of the fetal thorax depicting the symmetry of the left and right diaphragm.
Thoracic circumference
Measurement of the thoracic circumference is not routinely recommended, but it can be subjectively assessed on ultrasound, as it is slightly smaller (20%) when compared to the abdominal circumference. If fetal thoracic hypoplasia is suspected, a strict axial view at the level of the four-chamber view should be obtained and the circumference outlined by the outer limits of the bone structures (spine, ribs, and sternum) should be measured, excluding skin and subcutaneous tissue (Figure 4). Thoracic hypoplasia can be diagnosed when the abdominal circumference/thoracic circumference (AC/TC) ratio is above 0.8.4,5
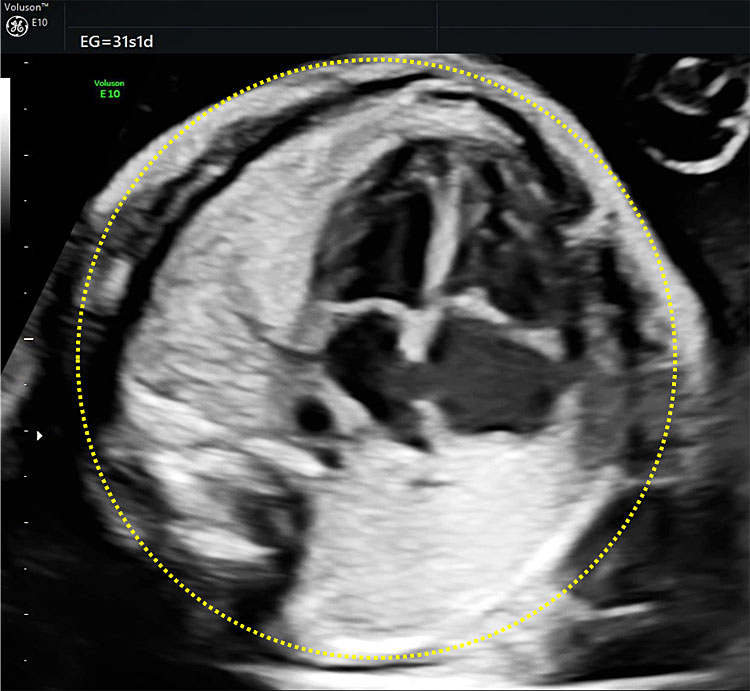
4
Ultrasonographic measurement of the thoracic circumference at 31 weeks of gestation.
Lung size assessment
The size of the lungs progressively increases throughout pregnancy and a sufficient amount of amniotic fluid is crucial to allow for normal lung growth.6 Normal reference ranges according to the gestational age and side should be used to confirm the normality of the size of the lungs.7
Stringent criteria should be used to assess fetal lung size:
- A strict transverse view of the fetal thorax at the level of the four-chamber view of the heart is needed. Therefore, it is mandatory to observe simultaneously only one rib on the right and the left hemithorax. An oblique section of the fetal thorax (more than one rib observed) may overestimate lung size. The lung under examination should be seen on the screen as close to the ultrasound probe as possible.
Lung area can be estimated by two different methods:
- The longest perpendicular diameter: multiplication of the longest lung diameter by its longest perpendicular (90°) diameter.
- Manual tracing: delineation of the limits of the lungs for automatic calculation of its area.
In order to correct for gestational age, the lung area should be divided by the head circumference to obtain the lung-to-head ratio (LHR). The obtained LHR value should be divided by the expected (normal) value according to gestational age to calculate the observed/expected lung-to-head ratio (O/E-LHR) and expressed as a percentage.8 The reproducibility of the O/E-LHR to estimate lung hypoplasia in fetuses affected with congenital diaphragmatic hernia (CDH) has been proven; it is therefore used to classify these cases according to their severity.9 On-line calculators for O/E-LHR are available (e.g., our USFetal mobile app.). Of note, we have demonstrated that correct measurement of the fetal lung is challenging and requires training to achieve competence, 70 consecutive measurements are needed to complete the learning curve.10 Although lung size estimation can also be performed by 3D ultrasound and fetal magnetic resonance imaging, these techniques are cumbersome and have not been shown significantly superior to 2D ultrasound.11
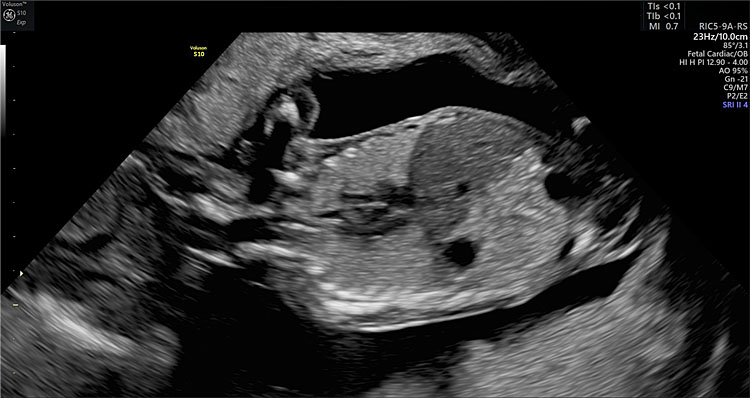
Intrapulmonary Doppler
Color Doppler ultrasound can be useful in the evaluation of the fetal thorax to assess the normal pulmonary venous drainage into the left atrium (Figure 5) to exclude abnormal venous drainage as seen in Scimitar syndrome, frequently associated with hypoplastic right lung. When investigating the blood flow of the pulmonary veins with spectral Doppler, a triphasic waveform should be seen, similar to that of the ductus venosus but presenting with lower systolic velocities (Figure 6). Regarding lung arterial circulation, each lung receives blood supply from one of the branches (left and right, respectively) of the main pulmonary artery. In a transverse view of the fetal thorax, the main branch of the right and left intrapulmonary artery can be observed with color Doppler. The spectral Doppler sample volume should be placed in the center of each pulmonary branch (right and left) close to its origin from the main pulmonary artery keeping an insonation angle (between the ultrasound beam and the direction of the blood flow) as close to 0° as possible (post-acquisition angle correction can be used to correct for up to 30°). The waveform of the intrapulmonary artery is unique, presenting with a spike of anterograde flow at the beginning of the systole, followed by a gradual deceleration, which is interrupted in most cases by a short early diastolic reverse flow (Figure 6).12 Routine evaluation using spectral Doppler ultrasound of the intrapulmonary arteries is not recommended in normal fetuses, but we have demonstrated that it can predict the risk of neonatal death in fetuses affected with severe CDH and it also has a potential utility in the evaluation of fetal arrhythmias.13,14
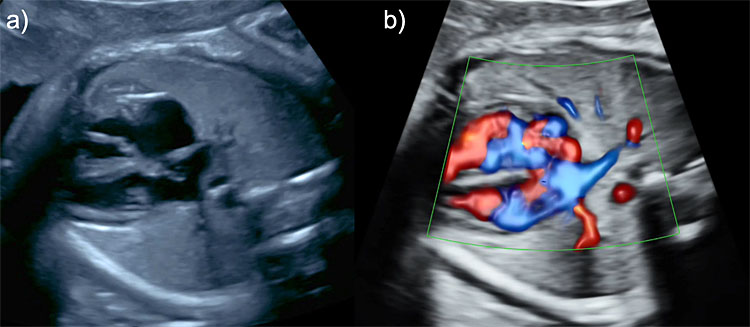
5
Color Doppler ultrasonographic evaluation of the fetal thorax. Two pulmonary veins draining from the left (a) and right lung (b), respectively, into the left atrium.
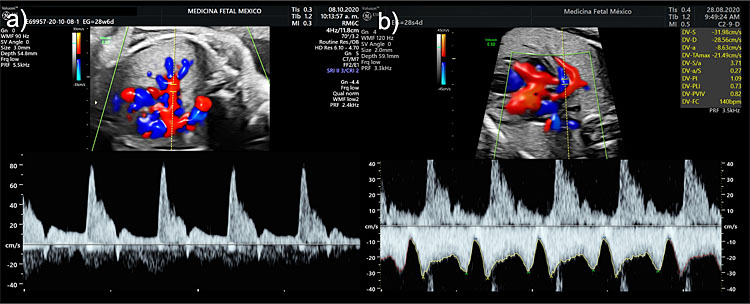
6
Spectral Doppler examination of the intrapulmonary (a) artery and (b) vein waveforms.
LUNG ANOMALIES
Congenital cystic adenomatoid malformation (CCAM)
CAM is the most common fetal hyperechogenic lung lesion. It is a developmental abnormality of the fetal lung that complicates about 1 in 3000 live births. CCAM is characterized by a lack of normal alveoli with excessive adenomatoid proliferation and cystic dilatation of terminal bronchioles (from 1 mm to over 10 cm in diameter).15
Diagnosis
Three types of CCAM can be differentiated according to the ultrasonographic appearance in the following:
- Microcystic CCAM: uniformly hyperechogenic mass;
- Macrocystic CCAM: echo-free cysts;
- Mixed CCAM: multicystic tumor with echogenic stroma.
Regardless of its type, CCAM is usually unilateral and involves one lobe of the lung. Its blood supply typically arises from the pulmonary artery.16
The prognosis in CCAM is good unless hydrops (at least two of the following: scalp/skin edema, ascites, pleural effusion, pericardial effusion) develops as a consequence of impaired cardiac function due to mediastinal shift and compression of systemic vessels.17,18
Thus, once a lung mass is identified, its location, size, echogenicity, and blood supply must be evaluated using 2D and spectral or power Doppler ultrasound.
The best prenatal predictors of prognosis are firstly, the relative size of the thoracic mass and secondly, the development of fetal pleural effusion or hydrops as a complication.16,19 In the presence of massive pleural effusion, and/or hydrops, the mortality rate increases to almost 100% primarily due to fetal heart failure and extremely severe pulmonary hypoplasia.
To detect fetuses at risk of adverse perinatal outcome, the CCAM volume (Figure 7) can be calculated with the formula:
| CCAM volume (cm2) = length (cm) × height (cm) × width (cm) × 0.52 |
CCAM volume-to-head ratio (CVR) can be obtained by dividing the CCAM volume by the head circumference
| CVR = | length (cm) × height (cm) × width (cm) × 0.52 | |
| Fetal head circumference |
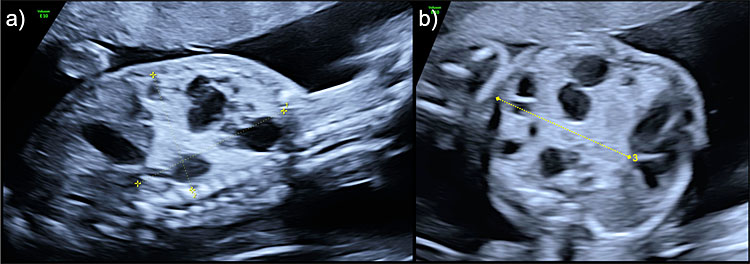
7
Congenital cystic airways malformation (CCAM) volume measurement of a mixed lesion in (a) sagittal view for longitudinal and anteroposterior diameters and (b) axial view for transverse diameter.
Fetuses with a CVR >1.6 show an increased risk of developing fetal hydrops.20,21 Similarly, cases with either CVR >1.0, O/E LHR below 45% or a mass-to-thorax ratio <0.51 are at a higher risk of adverse perinatal outcomes or need for fetal intervention21 Expectant management during pregnancy with continuous ultrasonographic follow-up and postnatal surgery seems to be a reasonable recommendation for low-risk cases. Postnatal thoracotomy with lobectomy for congenital lung lesions is often indicated depending on the size of the mass or in the case of respiratory distress, hydrothorax or pneumothorax. In addition, several studies have reported that 10% of CCAM could be associated with an increased risk of developing pleuropulmonary blastoma, which prompts timely surgical interventions, even in asymptomatic newborns, to prevent the potential risk of malignancy.
Treatment
For cases of macrocystic or mixed lung lesions with a dominant large cyst, major mediastinal shift, hydrops, and/or CVR >1.6 presenting before 34 weeks, ultrasound-guided thoraco-amniotic shunting for intrauterine cyst drainage is the first line of treatment. A recent case series and review of the literature analyzing 98 cases treated with thoraco-amniotic shunting for a large cystic lesion, reported 77% survival in cases presenting with hydrops and 90% survival in those treated before the appearance of hydrops. Therefore, it is reasonable to offer thoraco-amniotic shunting for macrocystic lesions presenting with a dominant cyst and a CVR >1.6.22,23
Cases presenting after 34 weeks could benefit from ultrasound-guided cyst aspiration and planned preterm cesarean delivery.
For cases of large microcystic CCAM presenting with at least one of the following ultrasonographic signs: skin/scalp edema, ascites, pleural effusion, pericardial effusion or a CVR >1.6, maternal steroid therapy with betamethasone 12 mg intramuscularly, two doses, 24 hours apart administered as a single course or in multiple courses for “not responders” (up to three courses 7 days apart), has shown to significantly reduce CCAM volume and CVR, reversing hydrops and increasing the survival rate to approximately 90%.24
Although different fetal interventions have been proposed to improve prognosis, none of them have shown better results than treatment with maternal betamethasone. Percutaneous intra-tumoral laser coagulation of the microcystic lesions in hydropic fetuses has been reported to achieve a modest survival of 29%.25,26,27 Likewise, percutaneous intratumoral sclerotherapy with ethanolamine or polidocanol28,29 has been proposed for the treatment of microcystic CCAM, although the evidence is scant. In a case series using this method, authors reported a reduction in the size of the lung mass in all but one case and resolution of the hydrops and delivery at term in all cases, with an overall survival rate of 83% (5/6).
Bronchopulmonary sequestration (BPS)
BPS is a solid lung lesion of nonfunctioning pulmonary tissue. A supernumerary lobe of the lung, with no connection to the tracheobronchial tree and perfused by an aberrant systemic feeding artery, originating usually from the descending aorta.
Diagnosis
BPS is identified as a uniformly well-defined triangular echogenic lesion, usually unilateral, that affects the inferior lobes of the lungs (supradiaphragmatic). Its pathognomonic sign is the identification of a systemic arterial blood supply from an aberrant branch of the descending aorta that can be detected using spectral or power Doppler ultrasound (Figure 8).
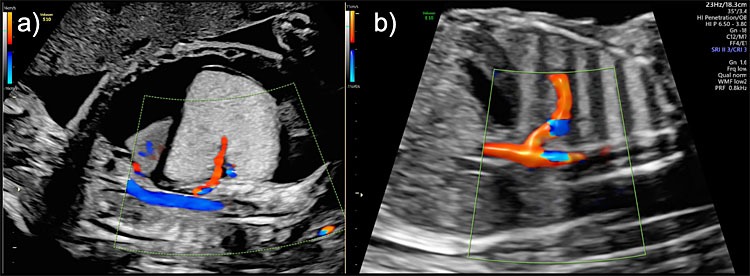
8
Bronchopulmonary sequestration (BPS) Hyperechogenic lesion with systemic arterial blood supply from an aberrant branch of the descending aorta seen on color Doppler in (a) a high-risk lesion presenting with hydrops and in (b) a low-risk lesion with no pleural effusion or ascites.
Although the vast majority of BPS present in the thorax, occasionally abdominal lesions can also be seen below the diaphragm.
Treatment
In BPS cases with pleural effusions and/or hydrops, laser ablation of the feeding artery under ultrasound guidance may improve prognosis.30,31,32,33,34
We have demonstrated that ultrasound-guided fetal laser ablation of the feeding artery (FLAFA) induces necrosis and regression of the lung mass, disappearance of fetal hydrops, and normalization of pulmonary growth,35 avoiding the need for neonatal surgery.34
Hybrid lesions
BPS are solid hyperechogenic lesions, therefore when a cystic tumor presents with systemic vascular supply from the aorta the lesion is defined as “hybrid” (both BPS and CCAM components are present).36
We have demonstrated that FLAFA is also of benefit in improving survival for cases with hybrid lesions, although it does not avoid the need for neonatal surgery in all cases, as the CCMA component may still need to be resected after birth.37 In any case, this therapeutic modality appears to be superior to other treatments.
Alternative management of pulmonary masses
Open fetal surgery
Preliminary studies using open fetal surgery (performing a hysterotomy) with resection of the lesion between 22 to 32 weeks of gestation, reported a survival rate of 50%.38
The ex-utero intrapartum treatment (EXIT) procedure
Consists of partial delivery of the baby by cesarean section under maternal general anesthesia and uterine relaxation to avoid placental detachment. This allows for maintaining placental circulation to secure fetal oxygenation, while thoracotomy and surgical resection of the pulmonary mass is performed before cord clamping.39,40 But many concerns regarding this technique have been raised, especially because of the need for general anesthesia to the mother, increased maternal blood loss during the procedure, wound infections, and severe insult to the uterus affecting future fertility in some cases.41 Considering the promising results seen with maternal steroid treatment for microcystic lesions, thoraco-amniotic shunting for macrocystic lesions and FLAFA for BSP and hybrid lesions, the applicability of open fetal surgery and surgical resection on EXIT is nowadays probably relegated to very specific cases of microcystic lesions not responding to steroids and treated in high-volume experienced centers. Modern minimally invasive techniques that are safer for the fetus and the mother will probably take these procedures to extinction.
Congenital high airway obstruction (CHAOS)
CHAOS is a rare congenital anomaly consisting of complete intrinsic obstruction of the fetal upper airway usually located at the level of the larynx or trachea that induces a progressive bilateral lung expansion with pulmonary developmental impairment and tracheobronchial dilatation secondary to retention of bronchial secretions. CHAOS can also be unilateral, obstructing only one main bronchus (bronchial atresia).
It is usually a lethal anomaly, especially in the presence of fetal hydrops secondary to heart compression.42
Diagnosis
In laryngeal or tracheal obstruction, fetal ultrasonography, the thoracic exploration shows hyperexpanded hyperechoic lungs, flattened diaphragms, intrapulmonary tracheobronchial dilatation, and mediastinal compression (Figure 9).
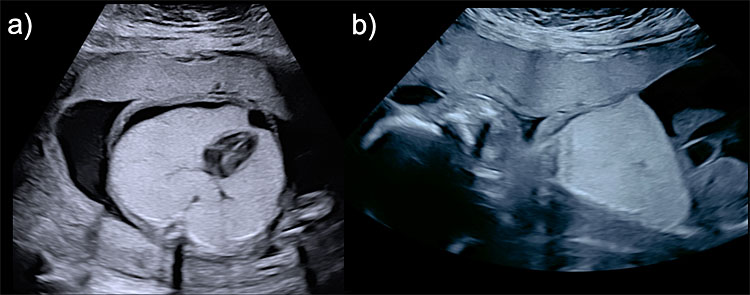
9
Congenital high airways obstruction (CHAOS) (a) axial view of the thorax showing bilateral hyperechogenic lungs compressing the mediastinum and displacing the heart to the center of the thorax and its apex to the midline (mesocardia) and presenting with right pleural effusion. (b) Sagittal view of the left hemithorax showing diaphragmatic eversion, and ascites.
In bronchial atresia, findings are a unilateral massively enlarged lung inducing an eversion of the diaphragmatic normal convexity, mediastinal shift with a displacement of the heart to the contralateral side of the thorax, and contralateral lung compression.43
Differential diagnosis
On ultrasonographic examination, microcystic CCAM and bronchial obstruction, both present as a hyperechogenic mass with variable degrees of mediastinal shift.
While CCMA usually affects only one lobe of the lung (a remnant of healthy pulmonary tissue can be seen), main bronchial atresia (MBA) affects every pulmonary lobe of the affected lung by preventing the physiological evacuation of lung secretions and causing flattening of the diaphragmatic dome. However, a giant microcystic CCAM can mimic MBA by collapsing the remnant normal pulmonary tissue and everting the diaphragm. In these cases, the differential diagnosis is based on the typical observation of a dilated hypoechogenic bronchial tree seen inside a hyperechogenic affected lung in MBA. Unfortunately, main bronchial atresia without bronchial tree dilatation has also been described, therefore, fetal bronchoscopy aids in the final diagnosis, identifying and treating MBA by fetoscopic laser recanalization of the fetal airway to improve survival.44
Treatment
Spontaneous lung decompression has been reported in CHAOS, leading to a progressive decrease in lung volume and disappearance of diaphragmatic eversion, possibly secondary to small fistulas or minor pharyngotracheal or laryngotracheal communications.45 Preliminary studies reported that hydropic fetuses with CHAOS can benefit from fetoscopic laser recanalization of the obstructed trachea or bronchi, resulting in an amelioration of the intrapulmonary expansion, mediastinal decompression, and an improvement in venous return to the heart.44,45,46,47,48,49
Alternative management of CHAOS
Ex-utero intrapartum treatment (EXIT)
The only available postnatal treatment option to improve the survival of fetuses with CHAOS is an EXIT-to-tracheostomy to secure the airways in placental circulation.50,51,52
Fetal hydrothorax
Fetal hydrothorax (FH) is a congenital condition with an estimated incidence of 1 per 15,000 newborns.53 Primary hydrothorax is caused by the accumulation of chyle in the thorax of a fetus with abnormal development of the lymphatic drainage.54 Secondary hydrothorax can be seen in a wide range of conditions, including genetic syndromes,55 fetal infections,56 and structural abnormalities.57,58 Chromosomal anomalies, mainly trisomy 21 (Down's syndrome) and monosomy X (Turner's syndrome) have been reported in up to 10–20% of the cases, particularly if the fetus is hydropic.
Diagnosis
Prenatal diagnosis can be easily performed by identification of fluid effusion in the pleural space, seen as an anechoic image surrounding and compressing the fetal lungs (Figure 10). It is usually diagnosed on the four-chamber view of the fetal heart. FH can be unilateral or bilateral.
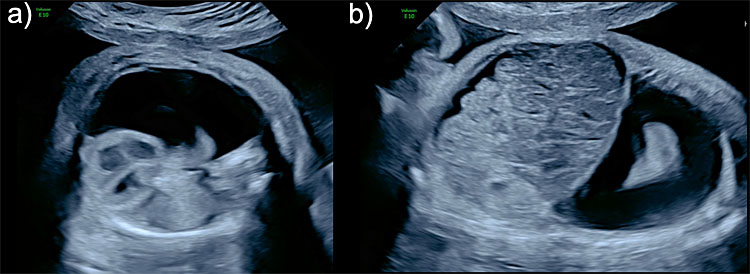
10
Fetal hydrothorax (FH). Unilateral right hydrothorax seen in (a) axial view with a severe deviation of the mediastinum and heart to the left (levoposition) and severe compression of the right lung, and (b) sagittal view showing ascites hydrothorax and ascites (hydropic fetus).
Treatment
Small effusions in the absence of significant mediastinal shift, hydrops or polyhydramnios can be managed expectantly during pregnancy with some of them showing in-utero spontaneous resolution during follow-up.
Fetuses with severe pleural effusions and hydrops are considered at the highest risk of either intrauterine fetal demise or neonatal death,53,55 and therefore, fetal therapy should be considered to improve survival. In hydropic fetuses with isolated FH (no associated anomalies or genetic syndrome), pleuro-amniotic shunt is the most effective fetal therapy to reverse fetal hydrops, improving survival by up to 80%.59,60,61 Shunt displacement or obstruction needing reintervention are not uncommon and may increase the risk of preterm premature ruptures of membranes.
Ultrasound-guided thoracocentesis to aspirate the pleural effusion has also been reported, but in the majority of cases, re-accumulation of the effusion takes place within 24–48 hours, requiring serial procedures. Therefore, it is only recommended at advanced gestation, prior to delivery to improve respiratory function in the neonate.
Congenital diaphragmatic hernia (CDH)
It is characterized by a defect in the diaphragm with protrusion of abdominal viscera into the thorax, leading to severe lung hypoplasia and/or pulmonary hypertension with elevated neonatal morbidity and mortality.62,63,64
Diagnosis
Early ultrasonographic prenatal diagnosis can be achieved. The axial view of the thorax, at the level of the four-chamber view of the heart, shows a mediastinal shift towards the contralateral side to the affected diaphragm, caused by the herniated abdominal viscera (Figure 11). Eighty-five percent of the lesions are left-sided, 13% right-sided and 2% bilateral. In left-sided CHD, the stomach can be observed as an anechoic image inside the fetal thorax, and in severe cases, a small portion of the fetal liver can also be observed inside the thoracic cavity. The spleen is usually located between the stomach and the contralateral lung and is discretely less echogenic than the lung parenchyma. In right-sided CDH, the liver is completely herniated into the fetal thorax, and the heart and mediastinum are usually shifted to the left side. Doppler identification of the hepatic vessels and the location of the gallbladder could help to refine its diagnosis. CDH may present in the context of a chromosomal or genetic abnormality in up to 20% of the cases and therefore, genetic invasive testing and genetic consultation are mandatory.
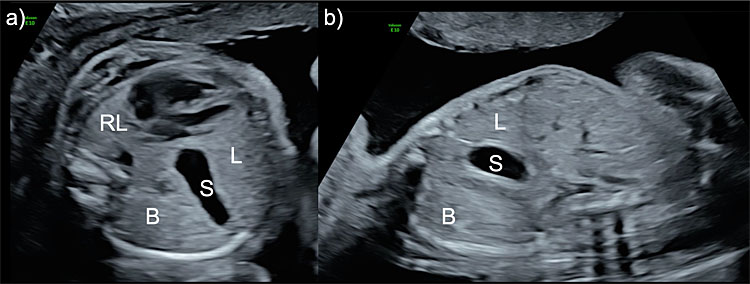
11
Ultrasound image of the axial (a) and sagittal (b) view of the fetal thorax in a case with left-sided congenital diaphragmatic hernia. The stomach (S), bowel (B) and a portion of the liver (L) are herniated into the thoracic cavity. The right lung (RL) presents with a reduced size.
Poor prognostic factors are right-sided CDH, hepatic herniation into the thorax (liver up), severe lung hypoplasia, low birth weight, and deficient neonatal care.8,65
Prenatal staging of left-sided CDH is based on the degree of pulmonary hypoplasia and the position of the liver (Table 1).
1
Estimated survival in fetuses with isolated left CDH based on prenatal severity staging according to the degree of lung hypoplasia and the position of the fetal liver.
Severity | Observed-to-expected lung-to-head ratio | Fetal liver | Survival |
Severe | <26% | Any | <15% |
Moderate | 26-35% 36-45% | Any Up | 50% 50% |
Mild | ≥36% ≥46% | Down Any | >75% >75% |
Fetal liver up: herniated into the thorax; Fetal liver down: not herniated into the thorax; Fetal liver any: either up or down.
In right-CDH, the liver is always herniated into the thorax and mild cases (O/E-LHR ≥50%) have an estimated survival rate of 60%, while severe cases (O/E-LHR <50%), have an estimated survival rate of 20%.66,67
Treatment
Clinical trials have demonstrated that, in isolated left severe CDH, fetal endoscopic tracheal occlusion (FETO) with an inflatable balloon can favor lung growth by preventing the expulsion of the physiological lung secretions. FETO is associated with an average increment of 30% in the neonatal survival rate when compared to expectantly managed controls.68,69 However, in moderate CDH, FETO has shown a more discrete increment in survival rates but a significantly lower neonatal respiratory morbidity.70,71
Additional ultrasound findings
Some indirect signs could be found in the ultrasonographic examination as a complication of lung anomalies. Large lung lesions, including masses, CHAOS, FH and CDH can induce mediastinal shift and/or esophageal compression. This can affect fetal swallowing resulting in polyhydramnios and a higher risk for preterm labor. In addition, displacement or compression of the heart and obstruction of its venous return can cause hydrops fetalis (≥2 of the following: scalp/skin edema, ascites, pleural effusion, pericardial effusions) an ominous sign that increases significantly the risk of stillbirth.
Additional examinations
Fetal echocardiography must be performed in every patient with a thoracic abnormality in order to screen for potential congenital heart malformations.
Isolated BPS are in general not associated with chromosomal abnormalities or genetic causes. However, in the presence of either CCAM, hydrothorax or CDH, the risk of chromosomal abnormalities increases and dictates the need for genetic consultation and testing.
Follow up
Depending on the type and size of the lung lesion, weekly scans should be performed to estimate lung mass volume, amniotic fluid volume, and cervical length via transvaginal scan. Additionally, ductus venosus (DV) and tricuspid valve Doppler assessment should also be performed to identify early signs of cardiac function impairment precluding hydrops (reverse a wave in DV and/or tricuspid regurgitation).
FINAL CONSIDERATIONS
Ultrasound examination of the fetal thorax is mandatory during the routine second-trimester scan and should confirm the following: normal lung morphology, normal echogenicity of the pulmonary parenchyma, normal mediastinal and heart position, absence of pleural effusions, normal shape of the diaphragms and normal pulmonary venous drainage. If any of these parameters is abnormal, the scan will allow discrimination of cases with solid or cystic lung masses, CHAOS, FH, CDH, thoracic hypoplasia, pulmonary hypoplasia, and abnormal pulmonary venous drainage. Accurate diagnosis and prompt referral to fetal surgery centers may improve neonatal survival and lower respiratory morbidity in these cases.
Due to the potential risks related to fetal interventions, especially preterm premature rupture of the membranes, and considering that a significant proportion of cases with lung masses and pleural effusion may resolve spontaneously and that mild CDH does not benefit from FETO; fetal intervention should be considered only for those cases with severe lung lesions at risk of perinatal death (i.e. those with severe pleural effusion or hydrops and/or severe lung hypoplasia).
PRACTICE RECOMMENDATIONS
- Include the axial, coronal, and parasagittal (left and right) views of the thorax in your routine anomaly scan.
- If a CCAM is detected, measure the CCAM volume and calculate CVR.
- If the mass has a dominant cyst and a CVR ≥1.6 or hydrops, the recommended treatment is thoraco-amniotic shunting.
- If the mass receives systemic arterial blood supply from an aberrant branch of the descending aorta and the fetus presents with hydrops, the recommended treatment is ultrasound-guided fetal laser ablation of the feeding artery.
- If the mass has a microcystic component and presents with hydrops or a CVR ≥1.6, the recommended treatment is maternal intramuscular betamethasone.
- In fetal hydrothorax rule out structural anomalies, genetic syndromes, and other causes of hydrops like fetal infections and anemia. If isolated and severe, thoraco-amniotic shunting is the recommended treatment
- In CHAOS affecting the larynx or trachea, both lungs will be hyperechogenic, the dilated bronchial tree will be visible and the diaphragms will be everted. These findings will be unilateral if the obstruction is at the level of a main bronchus.
- If a CDH is suspected the mediastinum and heart will be shifted to the contralateral side to the hernia and the abdominal viscera will be seen in the thorax. In the axial plane look behind the heart to find the contralateral lung to the hernia and measure O/E LHR, also check for intrathoracic herniation of the liver. The recommended treatment for severe lesions is fetoscopic tracheal occlusion.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Salomon LJ, Alfirevic Z, Berghella V, et al. Practice guidelines for performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet Gynecol 2011;37(1):116–26. | |
American Institute of Ultrasound in M. AIUM practice guideline for the performance of obstetric ultrasound examinations. J Ultrasound Med 2010;29(1):157–66. | |
Fried AM, Loh FK, Umer MA, et al. Echogenicity of fetal lung: relation to fetal age and maturity. AJR Am J Roentgenol 1985;145(3):591–4. | |
Simon NV, O'Connor TJ 3rd, Shearer DM. Detection of intrauterine fetal growth retardation with abdominal circumference and estimated fetal weight using cross-sectional growth curves. J Clin Ultrasound 1990;18(9):685–90. | |
Doulaveris G, Gallagher P, Romney E, et al. Fetal abdominal circumference in the second trimester and prediction of small for gestational age at birth. J Matern Fetal Neonatal Med 2020;33(14):2415–21. | |
Hubbard AM, Crombleholme TM. Anomalies and malformations affecting the fetal/neonatal chest. Semin Roentgenol 1998;33(2):117–25. | |
Britto IS, Araujo Júnior E, Sangi-Haghpeykar H, et al. Reference ranges for 2-dimensional sonographic lung measurements in healthy fetuses: a longitudinal study. J Ultrasound Med 2014;33(11):1917–238. | |
Jani J, Nicolaides KH, Keller RL, et al. Observed to expected lung area to head circumference ratio in the prediction of survival in fetuses with isolated diaphragmatic hernia. Ultrasound Obstet Gynecol 2007;30(1):67–71. | |
Ba'ath ME, Jesudason EC, Losty PD. How useful is the lung-to-head ratio in predicting outcome in the fetus with congenital diaphragmatic hernia? A systematic review and meta-analysis. Ultrasound Obstet Gynecol 2007;30(6):897–906. | |
Cruz-Martinez R, Figueras F, Moreno-Alvarez O, et al. Learning curve for lung area to head circumference ratio measurement in fetuses with congenital diaphragmatic hernia. Ultrasound Obstet Gynecol 2010;36(1):32–6. | |
Gerards FA, Engels MA, Twisk JW, et al. Normal fetal lung volume measured with three-dimensional ultrasound. Ultrasound Obstet Gynecol 2006;27(2):134–44. | |
Laudy JA. Doppler ultrasonography of the human fetal pulmonary circulation. Eur J Obstet Gynecol Reprod Biol 2001;99(1):3–5. | |
Cruz-Martinez R, Hernandez-Andrade E, Moreno-Alvarez O, et al. Prognostic value of pulmonary Doppler to predict response to tracheal occlusion in fetuses with congenital diaphragmatic hernia. Fetal Diagn Ther 2011;29(1):18–24. | |
Cruz-Martinez R, Castanon M, Moreno-Alvarez O, et al. Usefulness of lung-to-head ratio and intrapulmonary arterial Doppler in predicting neonatal morbidity in fetuses with congenital diaphragmatic hernia treated with fetoscopic tracheal occlusion. Ultrasound Obstet Gynecol 2013;41(1):59–65. | |
Stocker JT, Madewell JE, Drake RM. Congenital cystic adenomatoid malformation of the lung. Classification and morphologic spectrum. Hum Pathol 1977;8(2):155–71. | |
Adzick NS. Management of fetal lung lesions. Clin Perinatol 2009;36(2):363–76. | |
Adzick NS, Harrison MR, Glick PL. Fetal cystic adenomatoid malformation: prenatal diagnosis and natural history. J Pediatr Surg 1985;20:483–8. | |
Laberge JM, Flageole H, Pugash D, et al. Outcome of the prenatally diagnosed congenital cystic adenomatoid lung malformation: a Canadian experience. Fetal Diagn Ther 2001;16:178–86. | |
Hegde BN, Tsao K, Hirose S. Management of Congenital Lung Malformations. Clin Perinatol 2022;49(4):907–26. | |
Crombleholme TM, Coleman B, Hedrick H, et al. Cystic adenomatoid malformation volume ratio predicts outcome in prenatally diagnosed cystic adenomatoid malformation of the lung. J Pediatr Surg 2002;37(3):331–8. | |
Hellmund A, Berg C, Geipel A, et al. Prenatal Diagnosis and Evaluation of Sonographic Predictors for Intervention and Adverse Outcome in Congenital Pulmonary Airway Malformation. PLoS One 2016;11(3):e0150474. | |
Litwińska M, Litwińska E, Janiak K, et al. Thoracoamniotic Shunts in Macrocystic Lung Lesions: Case Series and Review of the Literature. Fetal Diagn Ther 2017;41(3):179–83. | |
Wilson RD, Hedrick HL, Liechty KW, et al. Cystic adenomatoid malformation of the lung: review of genetics, prenatal diagnosis, and in utero treatment. Am J Med Genet A 2006;140(2):151–5. | |
Peranteau WH, Boelig MM, Khalek N, et al. Effect of single and multiple courses of maternal betamethasone on prenatal congenital lung lesion growth and fetal survival. J Pediatr Surg 2016;51(1):28–32. | |
Ruano R, da Silva MM, Salustiano EM, et al. Percutaneous laser ablation under ultrasound guidance for fetal hyperechogenic microcystic lung lesions with hydrops: a single center cohort and a literature review. Prenat Diagn 2012;32(12):1127–32. | |
Bruner JP, Jarnagin BK, Reinisch L. Percutaneous laser ablation of fetal congenital cystic adenomatoid malformation: too little, too late? Fetal Diagn Ther 2000;15(6):359–63. | |
Ong SS, Chan SY, Ewer AK, et al. Laser ablation of foetal microcystic lung lesion: successful outcome and rationale for its use. Fetal Diagn Ther 2006;21(5):471–4. | |
Bermudez C, Perez-Wulff J, Arcadipane M, et al. Percutaneous fetal sclerotherapy for congenital cystic adenomatoid malformation of the lung. Fetal Diagn Ther 2008;24(3):237–40. | |
Lee FL, Said N, Grikscheit TC, et al. Treatment of congenital pulmonary airway malformation induced hydrops fetalis via percutaneous sclerotherapy. Fetal Diagn Ther 2012;31(4):264–8. | |
Oepkes D, Devlieger R, Lopriore E, et al. Successful ultrasound-guided laser treatment of fetal hydrops caused by pulmonary sequestration. Ultrasound Obstet Gynecol 2007;29(4):457–9. | |
Cavoretto P, Molina F, Poggi S, et al. Prenatal diagnosis and outcome of echogenic fetal lung lesions. Ultrasound Obstet Gynecol 2008;32(6):769–83. | |
Witlox RS, Lopriore E, Walther FJ, et al. Single-needle laser treatment with drainage of hydrothorax in fetal bronchopulmonary sequestration with hydrops. Ultrasound Obstet Gynecol 2009;34(3):355–7. | |
Mallmann MR, Geipel A, Bludau M, et al. Bronchopulmonary sequestration with massive pleural effusion: pleuroamniotic shunting vs. intrafetal vascular laser ablation. Ultrasound Obstet Gynecol 2014;44(4):441–6. | |
Cruz-Martinez R, Mendez A, Duenas-Riano J, et al. Fetal laser surgery prevents fetal death and avoids the need for neonatal sequestrectomy in cases with bronchopulmonary sequestration. Ultrasound Obstet Gynecol 2015;46(5):627–8. | |
Cruz-Martinez R, Nieto-Castro B, Martinez-Rodriguez M, et al. Thoracic changes after full laser ablation of the feeding artery in fetuses with bronchopulmonary sequestration. Fetal Diagn Ther 2017;in press. | |
Hutchin P, Friedman PJ, Saltzstein SL. Congenital cystic adenomatoid malformation with anomalous blood supply. J Thorac Cardiovasc Surg 1971;62(2):220–5. | |
Cruz-Martinez R, Martinez-Rodriguez M, Bermudez-Rojas M, et al. Fetal surgery by full laser ablation of the feeding artery for cystic lung lesions with systemic arterial blood supply (hybrid lung lesions). Ultrasound Obstet Gynecol 2016. | |
Grethel EJ, Wagner AJ, Clifton MS, et al. Fetal intervention for mass lesions and hydrops improves outcome: a 15-year experience. J Pediatr Surg 2007;42(1):117–23. | |
Hedrick HL, Flake AW, Crombleholme TM, et al. The ex utero intrapartum therapy procedure for high-risk fetal lung lesions. J Pediatr Surg 2005;40(6):1038–43. | |
Mychaliska GB, Bealer JF, Graf JL, et al. Operating on placental support: the ex utero intrapartum treatment procedure. J Pediatr Surg 1997;32(2):227–30. | |
Shamshirsaz AA, Aalipour S, Erfani H, et al. Obstetric outcomes of ex-utero intrapartum treatment (EXIT). Prenat Diagn 2019;39(8):643–646. | |
Lim FY, Crombleholme TM, Hedrick HL, et al. Congenital high airway obstruction syndrome: natural history and management. J Pediatr Surg 2003;38(6):940–5. | |
Onderoglu L, Saygan Karamursel B, Bulun A, et al. Prenatal diagnosis of laryngeal atresia. Prenat Diagn 2003;23(4):277–80. | |
Cruz-Martinez R, Mendez A, Perez-Garcilita O, et al. Fetal bronchoscopy as a useful procedure in a case with prenatal diagnosis of congenital microcystic adenomatoid malformation. Fetal Diagn Ther 2015;37(1):75–80. | |
Vidaeff AC, Szmuk P, Mastrobattista JM, et al. More or less CHAOS: case report and literature review suggesting the existence of a distinct subtype of congenital high airway obstruction syndrome. Ultrasound Obstet Gynecol 2007;30(1):114–7. | |
Paek BW, Callen PW, Kitterman J, et al. Successful fetal intervention for congenital high airway obstruction syndrome. Fetal Diagn Ther 2002;17(5):272–6. | |
Kohl T, Van de Vondel P, Stressig R, et al. Percutaneous fetoscopic laser decompression of congenital high airway obstruction syndrome (CHAOS) from laryngeal atresia via a single trocar–current technical constraints and potential solutions for future interventions. Fetal Diagn Ther 2009;25(1):67–71. | |
Martinez JM, Castanon M, Gomez O, et al. Evaluation of fetal vocal cords to select candidates for successful fetoscopic treatment of congenital high airway obstruction syndrome: preliminary case series. Fetal Diagn Ther 2013;34(2):77–84. | |
Martinez JM, Prat J, Gomez O, et al. Decompression through tracheobronchial endoscopy of bronchial atresia presenting as massive pulmonary tumor: a new indication for fetoscopic surgery. Fetal Diagn Ther 2013;33(1):69–74. | |
DeCou JM, Jones DC, Jacobs HD, et al. Successful ex utero intrapartum treatment (EXIT) procedure for congenital high airway obstruction syndrome (CHAOS) owing to laryngeal atresia. J Pediatr Surg 1998;33(10):1563–5. | |
Bui TH, Grunewald C, Frenckner B, et al. Successful EXIT (ex utero intrapartum treatment) procedure in a fetus diagnosed prenatally with congenital high-airway obstruction syndrome due to laryngeal atresia. Eur J Pediatr Surg 2000;10(5):328–33. | |
Crombleholme TM, Sylvester K, Flake AW, et al. Salvage of a fetus with congenital high airway obstruction syndrome by ex utero intrapartum treatment (EXIT) procedure. Fetal Diagn Ther 2000;15(5):280–2. | |
Longaker MT, Laberge JM, Dansereau J, et al. Primary fetal hydrothorax: natural history and management. J Pediatr Surg 1989;24(6):573–6. | |
Attar MA, Donn SM. Congenital chylothorax. Semin Fetal Neonatal Med 2017;22(4):234–39. | |
Ruano R, Ramalho AS, Cardoso AK, et al. Prenatal diagnosis and natural history of fetuses presenting with pleural effusion. Prenat Diagn 2011;31(5):496–9. | |
Puccetti C, Contoli M, Bonvicini F, et al. Parvovirus B19 in pregnancy: possible consequences of vertical transmission. Prenat Diagn 2012;32(9):897–902. | |
Nicolaides KH, Azar GB. Thoraco-amniotic shunting. Fetal Diagn Ther 1990;5(3–4):153–64. | |
Van Mieghem T, Cruz-Martinez R, Allegaert K, et al. Outcome of fetuses with congenital diaphragmatic hernia and associated intrafetal fluid effusions managed in the era of fetal surgery. Ultrasound Obstet Gynecol 2012;39(1):50–5. | |
Deurloo KL, Devlieger R, Lopriore E, et al. Isolated fetal hydrothorax with hydrops: a systematic review of prenatal treatment options. Prenat Diagn 2007;27(10):893–9. | |
Wada S, Jwa SC, Yumoto Y, et al. The prognostic factors and outcomes of primary fetal hydrothorax with the effects of fetal intervention. Prenat Diagn 2017;37(2):184–92. | |
Cruz-Martinez R, Sosa Sosa C, Martinez-Rodriguez M, et al. Single Uterine Access for Bilateral Pleuroamniotic Shunting in Fetuses with Severe Hydrothorax by an Internal Rotational Maneuver: Feasibility and Outcomes between Successful and Failed Procedures. Fetal Diagn Ther 2021;48(3):209–16. | |
Hedrick HL. Evaluation and management of congenital diaphragmatic hernia. Pediatr Case Rev 2001;1(1):25–36. | |
Stege G, Fenton A, Jaffray B. Nihilism in the 1990s: the true mortality of congenital diaphragmatic hernia. Pediatrics 2003;112(3 Pt 1):532–5. | |
Gallot D, Boda C, Ughetto S, et al. Prenatal detection and outcome of congenital diaphragmatic hernia: a French registry-based study. Ultrasound Obstet Gynecol 2007;29(3):276–83. | |
Cruz-Martinez R, Etchegaray A, Molina-Giraldo S, et al. A multicentre study to predict neonatal survival according to lung-to-head ratio and liver herniation in fetuses with left congenital diaphragmatic hernia (CDH): Hidden mortality from the Latin American CDH Study Group Registry. Prenat Diagn 2019;39(7):519–26. | |
Russo FM, Cordier AG, Basurto D, et al. Fetal endoscopic tracheal occlusion reverses the natural history of right-sided congenital diaphragmatic hernia: European multicenter experience. Ultrasound Obstet Gynecol 2021;57(3):378–85. | |
Cruz-Martínez R, Molina-Giraldo S, Etchegaray A, et al. Prediction of neonatal survival according to lung-to-head ratio in fetuses with right congenital diaphragmatic hernia (CDH): A multicentre study from the Latin American CDH Study Group registry. Prenat Diagn 2022;42(3):357–63. | |
Cruz-Martinez R, Martinez-Rodriguez M, Gamez-Varela A, et al. Survival outcome in severe left-sided congenital diaphragmatic hernia with and without fetal endoscopic tracheal occlusion in a country with suboptimal neonatal management. Ultrasound Obstet Gynecol 2020;56(4):516–21. | |
Deprest JA, Nicolaides KH, Benachi A, et al. Randomized Trial of Fetal Surgery for Severe Left Diaphragmatic Hernia. N Engl J Med 2021;385(2):107–18. | |
Deprest JA, Benachi A, Gratacos E, et al. Randomized Trial of Fetal Surgery for Moderate Left Diaphragmatic Hernia. N Engl J Med 2021;385(2):119–29. | |
Cruz-Martinez R, Shazly S, Martinez-Rodriguez M, et al. Impact of fetal endoscopic tracheal occlusion in fetuses with congenital diaphragmatic hernia and moderate lung hypoplasia. Prenat Diagn 2022;42(3):310–17. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards programme CLICK HERE)

