This chapter should be cited as follows:
Brunelli E, Fiorentini M, et al., Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.419293
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 18
Ultrasound in obstetrics
Volume Editors:
Professor Caterina M (Katia) Bilardo, Amsterdam UMC, Amsterdam and University of Groningen, Groningen, The Netherlands
Dr Valentina Tsibizova, PREIS International School, Florence, Italy

Chapter
Ultrasound Evaluation of the Neural Tube
First published: August 2023
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
Ultrasound evaluation of the neural tube is part of routine mid-trimester screening ultrasound assessment. It includes evaluation of the fetal spine and central nervous system (CNS). Failure of neural tube closure during the third and fourth weeks after conception results in neural tube defects (NTDs). NTDs may involve any component of CNS and spine at any level. This, together with the extension of the lesion, will determine the fetal outcome. Notably, it has been shown that folic acid supplementation is able to prevent NTDs. Technological progress and ultrasound developing experience have made mid-trimester screening ultrasound the most useful NTD diagnostic tool, allowing individualized multidisciplinary prenatal counseling and planification of gestational and postnatal management.
In this chapter we will provide an overview of etiology, embryogenetic development, ultrasound features, prenatal counseling, gestational and postnatal management of the principal NTDs.
DEFINITION
Neural tube defects (NTDs) are a spectrum of congenital malformations caused by failure of the neural tube fusion during early embryogenesis.
EMBRYOLOGIC AND PATHOPHYSIOLOGIC EXPLANATION OF NEURAL TUBE DEFECTS
The central nervous system (CNS) in the embryo originates from the ectodermal neural plate, a flat sheet of neuroepithelial cells. The neural plate folds in the midline creating a U-shaped structure and then fuses posteriorly, forming a continuous closed cavity (neural tube) in a process called neurulation. Fusion of the neural folds begins on the 21st day after conception in the cervical region and proceeds both cranially and caudally. As a result, the neural tube cranial end closure (anterior neuropore) will be completed on the 25th day, while the caudal end closure (posterior neuropore) will be completed on the 29th day after conception.1,2 The neurulation occurs at the very beginning of pregnancy, in a period in which many women may not be aware of their state yet.3
The process requires extensive interactions between a variety of cellular and molecular actors, massive movements of the neural folds and strict temporo-spatial coordination between the neural ectoderm, mesenchyme, and surface ectoderm. Quite predictably, perturbations in this complex and highly orchestrated process, or mutations in any of the numerous genes involved may result in NTDs.4
Moreover, failure of the neural tube closure may occur at any level, explaining the wide variety of NTDs. For example, a failure of closure in the cranial portion of the neural tube will lead to anencephaly, a defect at the level of cervical and upper thoracic vertebrae will cause iniencephaly, while a failure of fusion at the caudal end will result in spina bifida.3 Each type of NTD will be discussed separately in the following paragraphs.
ETIOPATHOGENESIS OF NEURAL TUBE DEFECTS
Every year NTDs affect approximately 300,000 babies worldwide.5 CNS anomalies are among the most common congenital fetal malformations, with NTDs counting as the most frequent CNS anomalies. Isolated NTDs have generally multifactorial causes, due to both genetic and environmental factors.3
Trisomy 13, 18 and triploidy are associated with NTDs, together with some genetic syndromes like DiGeorge and Meckel-Gruber.6,7,8,9,10 Furthermore, families with a history of NTDs are at higher risk of developing another NTDs11,12 and ethnic background seems to play a role in the etiopathogenesis of NTDs, as the Hispanic population has a higher rate of NTDs compared to other ethnicities.13 Among environmental factors, some medications have been associated with NTDs, especially those that interfere with folic acid metabolism, such as antiepileptic drugs like carbamazepine and valproic acid12,14 and folate antagonists, such as aminopterin and methotrexate.12 Other environmental factors associated with NTDs are maternal hyperthermia, due to fever15,16 or exposure to heat early in pregnancy;17,18 pregestational diabetes,12,19,20 obesity,12,21,22 and maternal diet. Mediterranean dietary regimen and high intake of healthy food (e.g., fruits, vegetables, yogurt, reduced-fat milk, whole wheat bread, and fish) seem to be associated with reduction in the risk of offspring being affected by NTDs.23,24
NORMAL ASPECT OF THE FETAL SPINE AND CENTRAL NERVOUS SYSTEM DURING MID-TRIMESTER ULTRASOUND EXAMINATION
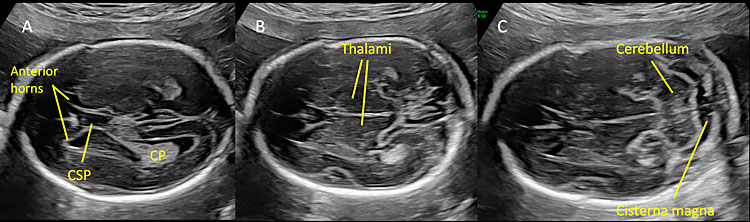
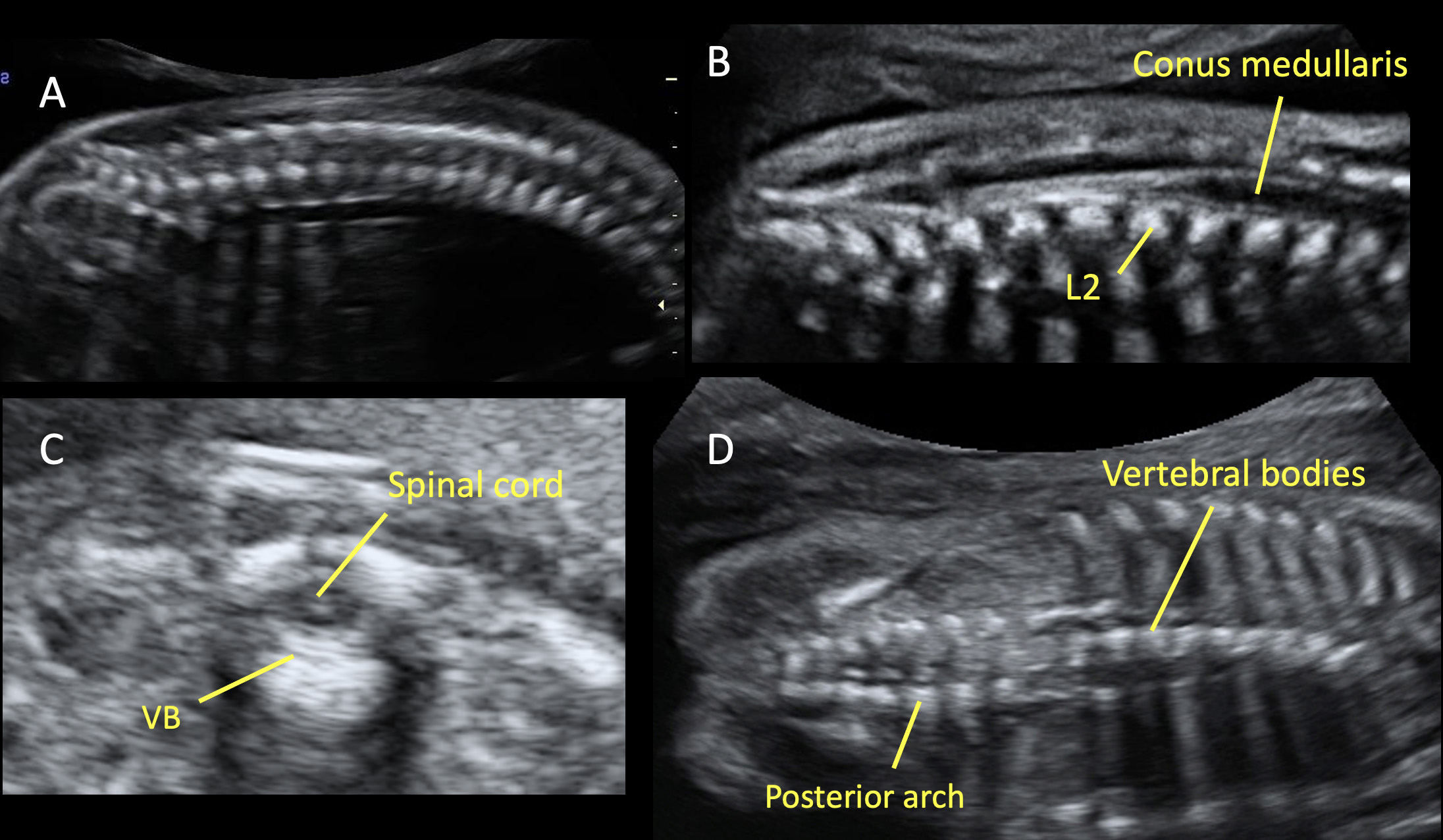
Most countries offer mid-trimester scan as part of standard prenatal care for detection of structural anomalies and as an important baseline to compare them with later scans.25 Recommended minimum requirements for basic mid-trimester fetal anatomy survey for the study of the neural tube includes the evaluation of fetal head and spine.25 In particular, the study of the fetal head includes both the skull and the brain. The fetal central nervous system screening evaluation includes three axial planes, generally detected transabdominally: transventricular, transthalamic, and transcerebellar planes (Figure 1). Abnormal intracranial anatomy detection in these planes represent an indication for a complete spinal evaluation and targeted fetal neurosonography (transabdominal and eventually transvaginal ultrasound, if the fetus is in cephalic presentation).26 As reported in the ISUOG guidelines, neurosonography represents a targeted examination of the fetal head and spine with a greater diagnostic potential with respect to the basic screening examination.27 The routine mid-trimester fetal spine examination should analyze systematically the whole spine. However, results are heavily dependent on fetal position.28 The sagittal section of the spine shows three ossification centers of the vertebrae surrounding the neural tube, as two parallel lines that converge in the sacrum. In addition, this view shows both the spinal canal and the spinal cord within it. The visualization and normal localization of the conus medullaris (should end approximately at L2 in the midtrimester) reinforces the diagnosis of normality. A detailed examination on axial view of each vertebra will offer the best visualization of ossification centers. Axial plane evaluation is performed dynamically by sweeping the probe along the whole fetal spine. Intact soft tissues covering the spine can be demonstrated both in sagittal and axial views.
1
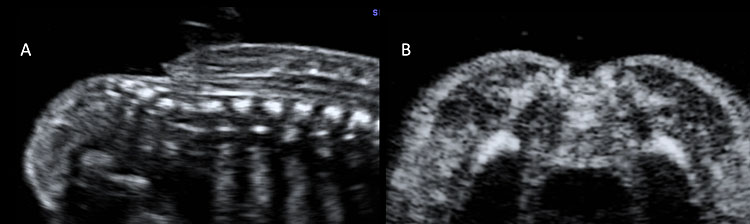
Normal mid-trimester scans of the fetal central nervous system. (A) Transventricular scan shows the anterior horns separated by the cavum septi pellucidi (CSP) and, posteriorly, the atrium and posterior horn of the lateral ventricle located distal to the transducer. CP indicates the choroid plexus located in the lateral ventricle. (B) Transthalamic scan represents the reference scan for fetal biometry (biparietal diameter and head circumference). In this plane anterior horns of the lateral ventricles, CSP and thalami are visible. (C) Transcerebellar plane shows the thalami, the cerebellum as a butterfly-shaped structure, and posteriorly the cisterna magna.
2
Various planes for studying the fetal spine during the mid-trimester ultrasound. (A) Sagittal plane, in which the spine is visible as two parallel echogenic lines (one for the vertebral bodies and one for the vertebral laminae). (B) This scan shows the conus medullaris, which ends at the level of the second lumbar vertebra (L2). This finding reinforces the diagnosis of normality. (C) Axial plane, in which three ossification centers are visible: the vertebral body (VB) in the center and laterally two masses, composed by the transverse, spinous, and the articular processes. In this plane is also visible the spinal cord, surrounded by the vertebra. In all planes (A, B, C) the overlying soft tissues cover the whole fetal spine; this is an indirect sign to detect the integrity of the spine. (D) Coronal plane allows to study the vertebral bodies and the posterior arch of the fetal spine.
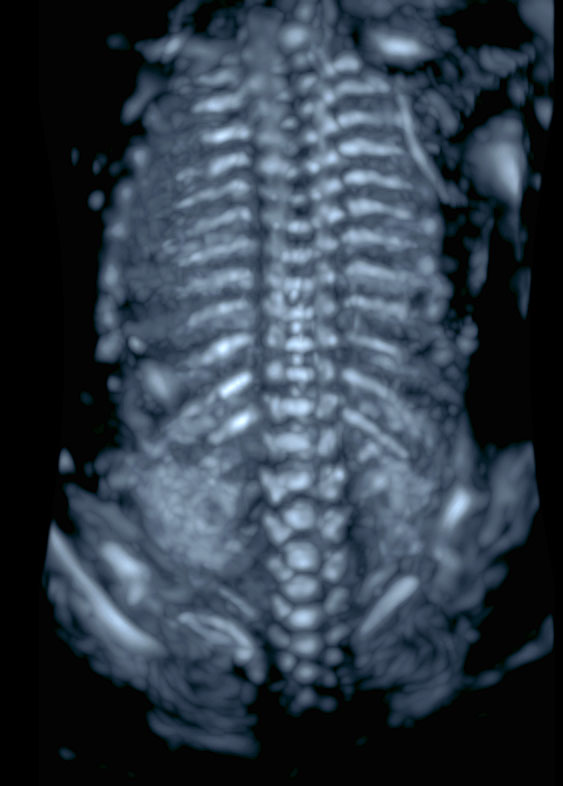
3
Three-dimensional ultrasound reconstruction of the fetal spine during in the mid-trimester. A render technique aiming to visualize the most echogenic structures should be used (e.g. maximum or skeleton mode)..
PRINCIPAL NEURAL TUBE DEFECTS
Anencephaly
Anencephaly is the most common NTD, affecting up to 3 per 10,000 births.30 Because its pathogenesis leads to a multifactorial and progressive damage of the central nervous system, it is commonly reported as a part of what is called “exencephaly – anencephaly sequence”. The malformation is also known as acrania. This sequence begins with a failure of closure of the cephalic portion of the neural tube. Consequently, the forming brain will lose the protection of the covering skull and meninges (exencephaly). The following mechanical and chemical insults of the amniotic fluid to the brain will cause the degeneration of the exposed neural tissue in a context of absent cranium (anencephaly).30
Exencephaly can be detected during first trimester ultrasound scan. The typical landmark is the absence of an echogenic calvarium covering the brain. As the bony structures of the base of the skull are still present, the cerebral hemispheres can be seen floating in the amniotic fluid above the skull base, resulting in a bilobed shaped head (“Mickey Mouse” sign). As the pregnancy progresses, only an irregular mass of vascular and brain tissues remains visible (area cerebrovasculosa). Because of the lack of echogenic calvarium and forehead, the eyes protrude (“frog’s face” sign).30,31 Figure 4 and Video 1 show the main sonography aspects of the exencephaly – anencephaly sequence.
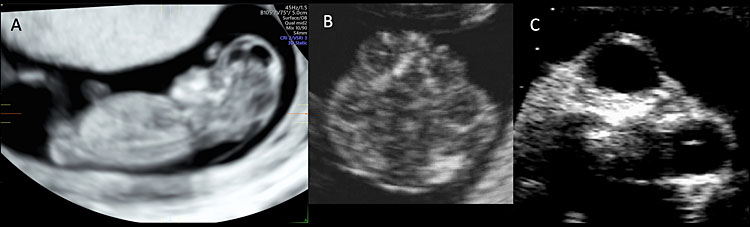
4
Ultrasound of a fetus affected by exencephaly-anencephaly sequence. (A) Sagittal plane of a 12 weeks’ fetus affected by exencephaly-anencephaly. The fetal head is flat and irregular and lacks a normal calvarium surrounding the brain. Figure (B) shows the abnormalities of the fetal head with uneven contour and disorganized structures at 12 weeks. Figure (C) shows the protrusion of the fetal orbits in the midtrimester scan (frog’s face sign).
1
Ultrasound of a fetus affected by exencephaly-anencephaly sequence at 12 weeks’ gestation.
Chromosomal anomalies have been reported in association with NTDs,3 therefore genetic counseling is advised. Stillbirth occurs in most cases, either in utero or intrapartum, and liveborn neonates die within a few days of life. Therefore, as anencephaly is a lethal malformation, termination of pregnancy is an option that should be offered.30
Encephalocele
A cephalocele is defined as an herniation of meninges, brain or both from a cranial defect. When the herniation contains brain tissue the term used is encephalocele, while if no brain tissue is present in the sac it can be used the term meningocele.32,33 Cephaloceles are classified according to the site of the cranial defect, as they may involve occipital, frontal, parietal, or temporal region of the cranium.32 The incidence of cephalocele is estimated to be 0.8 per 10,000 births,32,34 with strong differences in incidence and form with ethnicity, sex, and geographic distribution.34
Cephaloceles are variable in size, shape, and skin cover, with a cystic or mixed solid-cystic consistency. Typically, the cephalocele is covered by intact skins.34 As herniation reduces intracranial volume, there is a tendency to microcephaly.
The diagnosis is usually made by ultrasound. In a posterior (occipital) encephalocele, the head circumference may be far smaller than expected for gestational age and a saclike structure connected to the fetal head is typically seen. The sac usually appears covered by skin and the brain content of the sac (usually occipital lobes) takes connection with the intracranial portions of the brain through the bone defect (Figure 5). Three-dimensional ultrasound may be useful to better evaluate the exact location of the defect and to study intracranial anatomy.32
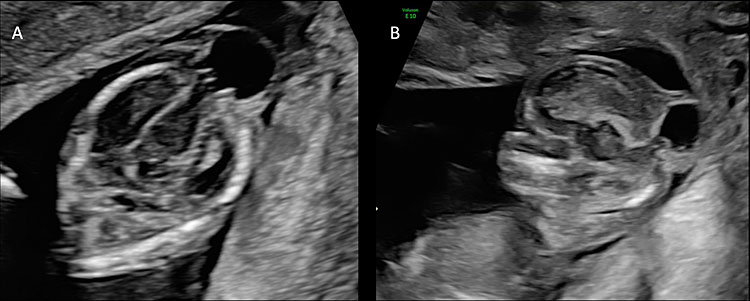
5
(A) The figure shows an axial scan of the fetal head of a fetus affected by posterior encephalocele at 14 weeks’ gestation. The head circumference is smaller than expected for gestational age and there is a saclike structure connected to the fetal head and covered by skin. The brain content of the sac is connected to the intracranial portions of the brain through the bone defect. (B) The sagittal plane shows the posterior encephalocele and the herniation of fetal brain through the cranial defect.
Association with chromosomal anomalies such as trisomies 13, 18, and 21 and with syndromes such as Meckel-Gruber, Walker-Warburg or Knobloch syndromes has been shown.35 Associated malformations have been reported as well, in particular microcephaly, ventriculomegaly, agenesis of the corpus callosum, lobar holoprosencephaly, cerebellar dysplasia, Dandy-Walker and Arnold-Chiari type II malformations, spina bifida, microphthalmia, cleft palate, tracheoesophageal and cardiac defects.32,34,36
When encephalocele is detected, genetic counseling and diagnostic testing for karyotype and array CGH should be proposed. Pregnancy termination is an option that can be offered. In case of continuation of pregnancy, a third trimester ultrasound and delivery in a tertiary care hospital with neonatal intensive care unit are recommended, but timing and mode of delivery should be individualized.32
Prognosis depends on the size, location, and content of the lesion together with the presence of associated malformations.37,38 Intrauterine demise and neonatal death in the first days of life are common and surviving children frequently suffer from physical and intellectual disabilities.37
Iniencephaly
Iniencephaly is a neural tube defect involving the occipital bone and the inion. It presents with rachischisis of the cervical and thoracic spine, which results in extreme fixed retroflexion of the head and an apparent absence of the neck.36,39 In comparison to other NTDs, iniencephaly is quite rare and the incidence is reported to be of 0.1 to 10 per 10,000 pregnancies. Associations with anomalies such as holoprosencephaly, cephalocele, spinal dysraphism, abdominal wall defects, diaphragmatic hernia, cleft lip and cleft palate, cardiovascular, urinary system, skeletal and gastrointestinal anomalies have been reported.36,40,41
As shown in Figure 6, sonographic findings include deformation and shortening of cervical and thoracic spine, alteration of intracranial anatomy and extreme retroflexion of the head.36,42 The fetus assumes therefore a typical position known as the “star-gazing appearance”.43
6
Image of a fetus with iniencephaly during the first trimester of pregnancy. Cervical and thoracic spine are shorter, and extreme retroflexion of the head is present. The fetus assumes a typical position known as the “star-gazing appearance”.
Iniencephaly is mostly lethal resulting in either stillbirth or neonatal death. In the few survivors, individualized surgical repair may be attempted.41 Therefore, early diagnosis is essential for adequate parents counseling and pregnancy management.39,41
Spina bifida
Failure of fusion at the caudal end on the neural tube results in spina bifida or spinal dysraphism, affecting 1 in 1000 newborns. Spinal dysraphism is classically divided into two types: open (OSD) and closed (CSD) spinal dysraphism. Both types are typically located at lumbar or lumbosacral level. In OSD a congenital bone defect causes the exposure of the meninges or exposure of the meninges together with nervous tissue to the environment. In contrast, in CSD the bone defect is covered by skin (this means there is no exposed nervous tissue), usually in association with dimples, dyschromic patches or a hairy spot.2 OSD and CSD main features will be described separately in the following paragraphs.
Open spina bifida (OSD)
OSD is the most common type of spina bifida, representing about 75% of cases.44,45,46 The malformation is characterized by a bony defect of the fetal spine with the exposure of neural tissue and/or meninges to the amniotic fluid, with no skin covering the lesion. Its most common forms are myelomeningocele (MMC) and myelocele (myeloschisis).47 As shown in Figure 7 and Video 2, in myelomeningocele, a dorsal bone defect allows the protrusion of the meninges together with neural tissue, which is therefore exposed. The expansion of the underlying subarachnoid space makes myelomeningocele elevated above the skin, differentiating it from the far less common myelocele, in which the neural tissue lies at the level of the skin surface (Figure 8).2,47 OSD is typically associated with a posterior fossa anomaly called Arnold-Chiari type II malformation, characterized by a continuous cerebrospinal fluid leakage through the NTD into the amniotic cavity, which causes caudal herniation of the cerebellar vermis and displacement of brain stem and fourth ventricle downward in the neural canal2,31 (Figure 9 and Video 3). Chiari II malformation almost always leads to hydrocephalus, resulting from obstruction of cerebrospinal fluid flow at the level of the fourth ventricle. As hydrocephalus stretches the white matter, a cortical reorganization and a reduction of fiber tracts occur, with direct effect on fetal cognitive and motor outcomes.48
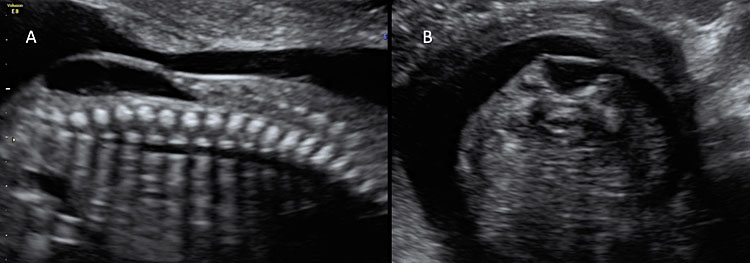
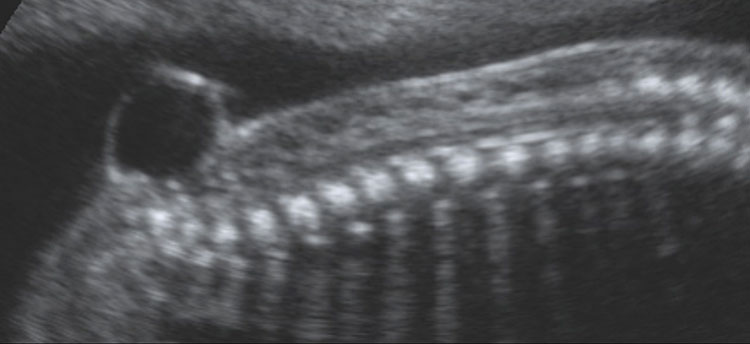
7
Ultrasound of a fetus with myelomeningocele at the mid-trimester scan. (A) On the sagittal plane, a bone defect can be found that allows the protrusion of meninges and neural tissue. This type of open spina bifida is highly recognizable for the cystic aspects of the lesion. (B) On axial plane, the cystic aspect of the lesion is also visible and easily recognizable.
2
Transvaginal ultrasound of a fetus affected by myelomeningocele. The lesion is well recognizable both in sagittal and axial planes thanks to the cystic aspect of the lesion.
8
Ultrasound scan of a fetus affected by myelocele at 20 weeks’ gestation. In this case the neural tissue lies at the level of the skin surface, without a posterior cyst, both in sagittal (A) and in axial planes (B).
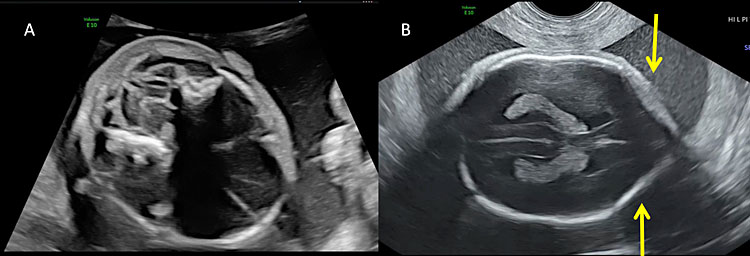
9
Most of the fetuses with open spina bifida present with alterations of intracranial anatomy (Arnold-Chiari type II malformation). (A) The downward displacement of the cerebellum due to the obliteration of cisterna magna secondary to the leakage of cerebro-spinal fluid and the depletion of the subarachnoid spaces result in the so-called “banana sign”. (B) The “lemon sign” describes the alteration of the calvarium shape on the transverse plane. The lemon sign is characterized by the concavity instead of normal convexity of the fetal frontal bone close to the coronal suture (arrows).
3
Intracranial ultrasound of a fetus affected by open spina bifida: the lemon sign and the banana sign.
Other typical anomalies associated with OSD are foot deformities, scoliosis, kyphosis and hip dislocation.48,49 It has been proposed that the muscle imbalance between antagonist muscle groups and reflex spasticity occurring in the intrauterine period may be the cause of foot deformities in spina bifida, however the pathologic explanation of this association has yet to be clarified.49
It has been demonstrated that indirect OSD cranial findings can be seen as early as in the first trimester. In particular, a reduced width of the cisterna magna and a dominance of the brainstem in the midsagittal view can be observed50 (Figure 10). In addition, a new sonographic sign was recently described in the axial plane, named the “crash sign”, which is characterized by the posterior displacement of the mesencephalon and deformation against the occipital bone.51
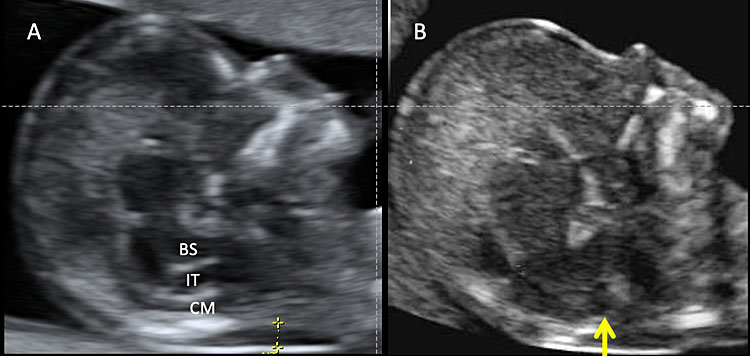
10
Transabdominal ultrasound of a normal fetus during nuchal translucency (NT) screening in the first trimester (A). Normally, three spaces can be detectable in the fetal head: brainstem (BS), fourth ventricle or intracranial translucency (IT), and cisterna magna (CM). Calipers indicate the nuchal translucency. (B) In some cases of open spina bifida there is an obliteration of IT (arrow).
To rule out OSD at mid-trimester ultrasound, a careful examination of the whole spine both transversely and longitudinally is mandatory. With OSD, the normal spine appearance in longitudinal view of two parallel lines with overlying uninterrupted skin line is disrupted at the level of the defect. In case of myelomeningocele, the defect is topped by a thin-walled cyst, often with thin septations. In transverse view, a spatial separation of the lateral processes of the affected vertebrae can be seen, as well as the absence of skin and muscles above the defect (Figure 7). Coronal plane examination may also be useful, as the normal spine architecture of three parallel lines gives way to the loss of the central line at the level of ODS .52,53 Three-dimensional (3D) ultrasound can be a powerful tool as well, as it provides excellent views of the spine and the spinal defect.33
As maternal habitus and unfavorable fetal position may further complicate an already challenging diagnosis, a valuable diagnostic aid is the presence of specific indirect cranial features, such as an edgy frontal bone (lemon sign) and a curved cerebellar shape with obliteration of cisterna magna (banana sign) (Figure 9). Ventriculomegaly of the posterior horn is also often present. These are easily attainable markers of OSD already visible in the standard view used to measure head biometry. If detected, their presence should urge a detailed spine examination.54,55
Closed spina bifida (CSD)
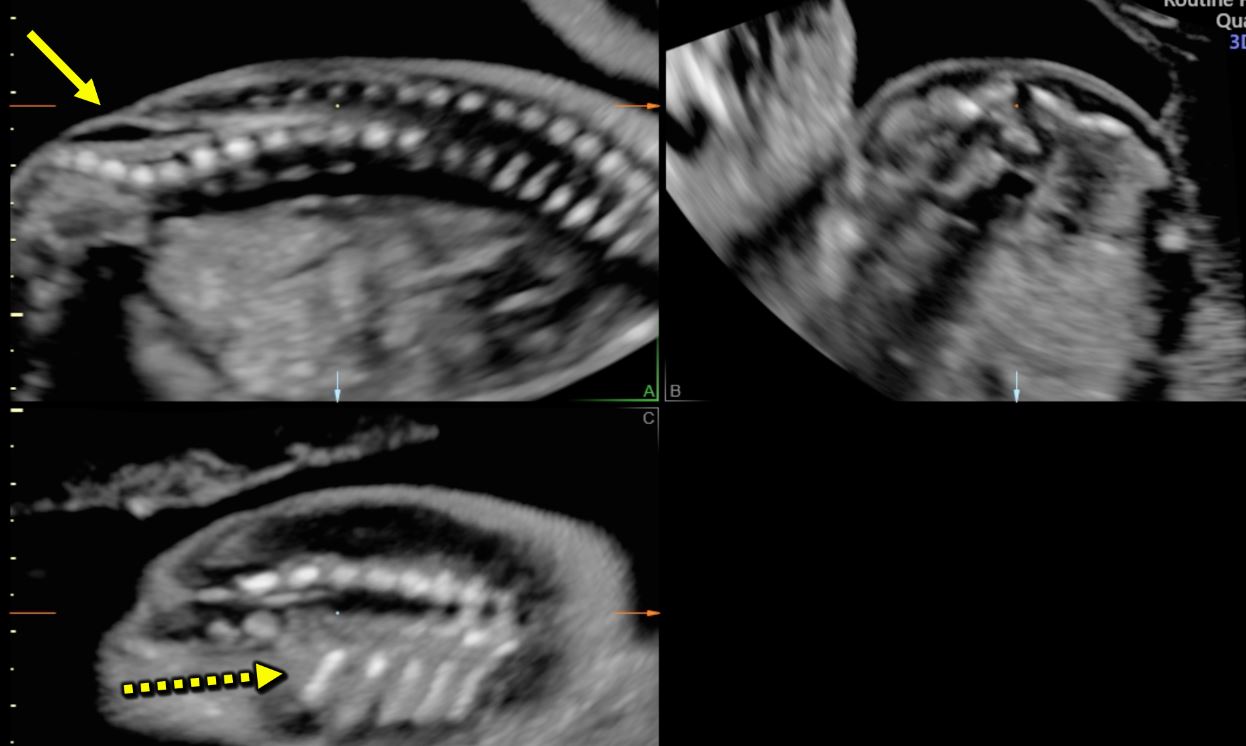
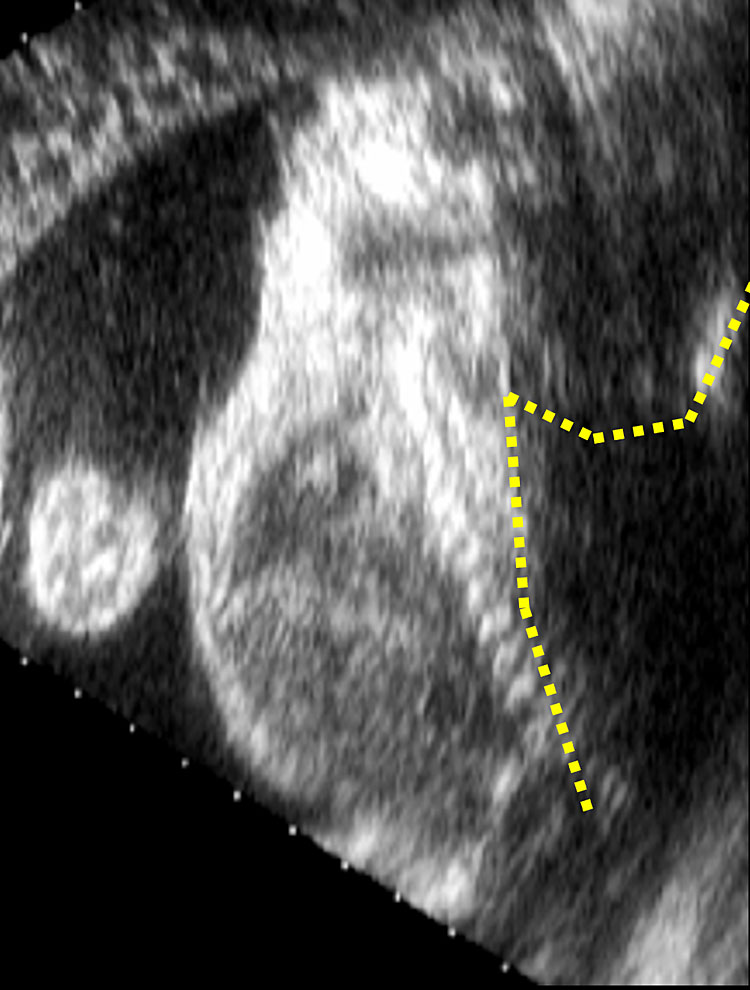
Closed spina bifida (CSD) is far less common than OSD. In CSD the neural tube defect is covered by skin. A subcutaneous mass may be present or not.31 As in CSD the skin covers the spinal defect, there is no cerebrospinal fluid leakage and therefore cranial indirect signs typical of OSD are not present.56 This makes CSD diagnosis difficult as it depends entirely upon the visualization of the spinal defect. As a consequence, CSD detection is often missed at prenatal ultrasound if there is no associated subcutaneous mass protruding.47 However, a clue for the diagnosis of CSD could be the visualization of an abnormally low position of the conus medullaris, which should arise suspicion of a tethered cord31 (Figure 11). At the beginning of the embryonic period, the spinal cord reaches a very low position in the spinal canal, but because of the continuous growth of the spine it progressively ascends during pregnancy until reaching the level of L1-L2.57,58 When the distal portion of the spinal cord fixates in a lower position by the terminal filum, a neurological disorder known as tethered cord syndrome (TCS) arises. TCS is characterized by progressive neurological, urological, and orthopedic dysfunctions. The true incidence is unknown. Differently form OSD, diagnosis of TCS is often made only with the onset of symptoms.59 Prenatal diagnosis is possible if the conus medullaris is visualized at a lower level than expected for gestational age.60 In particular, between 13 and 18 weeks’ gestation, the conus medullaris is most often situated lower than the fourth lumbar (L4) vertebral level, and between 19 and 36 weeks’ gestation, is at the level of third lumbar vertebra (L3) or higher. After 36 weeks, the conus medullaris level is most often higher than the second lumbar vertebra (L2). Subsequently, the diagnosis of fetal tethered cord can be made if the conus medullaris is visible lower than the L3–L4 levels after 18 weeks. As in TCS the neurological deterioration is progressive if untreated and deficit once developed are not reversible, a neurological consultation in early life is advisable.59 In order to assess the relationship between the level of the conus medullaris and the vertebrae two methods can be used. The first and simpler one is by using the midsagittal (median) view on which both the conus medullaris and all the vertebrae can be seen. By counting back from the lowest vertebra (S5), the operator can assess at which level the conus medullaris finishes. The second technique is by using 3D ultrasound. On the multiplanar mode (Figure 12), the operator can visualize both the sagittal (upper left) and coronal (lower left) and. The dot seen on all three planes corresponds to the same structure on all planes. On the coronal view the fetal ribs can be seen. The last fetal rib corresponds to T12. By counting down on the sagittal view (Figure 12 upper left), one can assess the relationship between the conus medullaris and the fetal vertebrae. Both techniques described above can also be used to define the level of spina bifida (closed or open), which is the most cranial vertebra involved in the defect.
11
Ultrasound scan of a fetus affected by close spina bifida at 20 weeks’ gestation. The diagnosis in utero can be challenging due to the lack of intracranial signs; however, the identification can be made easier if the lesion is associated with a meningocele.
12
Multiplanar view of a 3D ultrasound volume in a case of open spina bifida (confirmed by the presence of fetal intracranial signs). The solid arrow shows the defect. The three images represent the sagittal (A; upper left), transverse (B; upper right) and coronal (C; lower right) views. The dot on the three images corresponds always to the same structure, in this case T12 (last rib; dotted arrow).
Prenatal counseling and spinal dysraphism management
When a spinal dysraphism is diagnosed, genetic consultation and amniocentesis for array CGH are important, as associations with underlying genetic abnormalities have been established.31 Both open and closed spinal dysraphism can be related to VACTERL association, which refers to congenital anomalies of vertebrae, anorectal malformation, congenital cardiopathy, tracheoesophageal defects, renal anomalies and limb defects.61 In addition, patient counseling by a neurosurgeon should be considered to explore the available management options, both postnatal and prenatal ones, in case of possible access to the latter. This multidisciplinary approach can help the parents to have a complete idea on the prognosis and expected course of the pathology, and where available to decide on the possible termination pregnancy.62
The optimal mode of delivery in OSD is controversial, but infants delivered by cesarean section have shown improved motor function than those delivered vaginally.63 In the case of CSD, the delivery can occur vaginally and the obstetrics management should not be modified.53
In postnatal life, both OSD and CSD may present with neurological, urological, and motility symptoms.64 Patients affected may have difficulties with ambulation and mobility and may present with talipes and scoliosis, neurogenic sphincter dysfunctions and sexual dysfunctions.65 As a consequence, management of spinal dysraphism is multidisciplinary and includes neurosurgeons for the correction of the defect, orthopedics for mobility preservation, urologists to treat incontinence, and physiotherapists for long-term rehabilitation.64
Neurosurgical treatment: prenatal and postnatal options
In the case of OSD, neurosurgery is typically performed postnatally in the first 48 hours after birth and its purpose is not to prevent neurological damage but to avoid its progression. However, prenatal surgery performed between 19 and 25 weeks of gestational age is an option to minimize in-utero damage of the exposed cord from trauma and from contact with amniotic fluid.66 The Management of Myelomeningocele Study (MOMS)67 demonstrated that open prenatal surgery for myelomeningocele performed before 26 weeks of gestational age decreased the risk of death, improved mental and motor functions, reduced the need for shunting in the first year of life. These outcomes were probably due to improved cerebrospinal fluid flow and to the timing of repair, which may have improved prenatal nervous-system development. On the other hand, prenatal surgery was associated with increased risk of preterm delivery, uterine scar defects, and surgery complications.67 However, a recent systematic review and meta-analysis showed that neurodevelopmental outcome in children who had a prenatal repair surgery was similar to that of children who underwent postnatal repair, although the cases operated prenatally had a higher risk of prematurity.66
Fetal surgery is performed around the limits of fetal viability, has an associated risk of fetal death as a direct result of the procedure, offers no benefits to the mother but poses a risk for her health related to the procedure. In addition to that, it endangers both the remainder of the ongoing pregnancy and potentially future pregnancies, and it establishes the need for cesarean delivery for the current and for any future pregnancy due to the hysterotomy scar. For all these reasons, a comprehensive detailed counseling in a nondirective fashion regarding all management options is mandatory before proceeding with fetal surgery and this procedure should be offered only to carefully selected patients.65,68
Fetoscopic repair has been proposed alongside with open fetal surgery as a less invasive prenatal repair. However, as it seems to be associated with higher risk of prenatal rupture of membranes and prematurity, it cannot currently be recommended outside of an institutional review board approved investigational setting.68
Concerning CSD, indication for neurosurgical intervention of spinal cord untethering is new or progressive neurological, urological, or skeletal impairment. Conversely, surgery should be carefully considered in the case of asymptomatic patients and a conservative management may be prudent.69
SCREENING FOR NEURAL TUBE DEFECTS
Two-dimensional (2D) ultrasound plays a key role in the screening of neural tube defects. Intracranial translucency in the first trimester has been proposed as a screening tool for open spinal bifida;70 however, its accuracy for the detection of open spina bifida is currently limited.71 As previously reported, in open spina bifida many cases can be suspected and even diagnosed in the first trimester thanks to the presence of various ultrasonographic signs.51 In the other cases, the mid-trimester scan remains the referral screening tool.
PREGNANCY MANAGEMENT OF NEURAL TUBE DEFECTS
When a NTD is suspected at first or second trimester ultrasound screening evaluation, the patient should be referred to a tertiary center for specialized ultrasound examination and targeted neurosonography for confirmation of the lesion, evaluation of its size and location and detection of associated anomalies if present.3 Genetic evaluation and amniocentesis for karyotype and array CGH are recommended. When amniocentesis is performed for a suspicion of NTDs, information resulting from karyotype and array CGH can assist with the specific NTD diagnosis and guide the counseling about prognosis.62
The counseling with the parents must be comprehensive and individualized and it typically involves maternal fetal medicine specialists, geneticists, neonatologists and neurosurgeons.62 The counseling must consider the nature of the lesion, the possible outcomes and the expected natural history from childhood to adolescence to adulthood, which vary greatly depending on the type of lesion:3,62 while anencephaly is incompatible with survival, outcomes of spinal dysraphism vary greatly, therefore the counseling should be highly individualized and specific (see the previous paragraphs for more details). Management options to be offered are termination of pregnancy or expectant management, either with prenatal or postnatal surgery.3
THE ROLE OF FOLIC ACID IN NEURAL TUBE DEFECT PREVENTION
In 1991, the Medical Research Council Vitamin Study published a randomized double-blind multicentric prevention trial to determine whether supplementation with folic acid at the dosage of 4 mg per day or a mixture of other vitamins (A, D, B1, B2, B6, C, and nicotinamide) could prevent NTDs if taken periconceptionally. The results showed that folic acid administration had prevented 72% of NTDs recurrence in women with a previous affected pregnancy.72
An effort to reduce the rate of NTDs has been made in various countries over the last decades by introducing mandatory folic acid fortification of flour. This led to a decreased rate of NTDs,73,74 however it has been noted that this decline may as well be related to an increased use of screening tests and terminations of pregnancy following a NTDs' diagnosis.73
International guidelines now recommend assumption of at least 0.4 mg (400 mg) of folic acid in women of reproductive age with no risk factors for NTDs who could become pregnant, whether or not a pregnancy is contemplated, as many pregnancies are not planned.62,74,75 Supplementation should be started at least 1 month before conception and continued through the first 2 to 3 months of pregnancy,74 even though some recommend its continuation throughout the whole pregnancy and for the first 4 to 6 weeks postpartum.73
A higher dosage of folic acid is required if the following risk factors are present:62,73,74 a previous pregnancy affected by a NTDs, a family history of NTDs or other folic-acid-related congenital anomalies, pregestational diabetes, kidney dialysis, gastrointestinal malabsorption conditions, antiepileptic medications, advanced liver disease.62,73 The guidelines of the Society of Obstetricians and Gynaecologists of Canada propose three NTD risk groups (low, moderate, and high) of women requiring different amounts of folic acid supplementation, starting from 0.4 mg daily for low-risk group up to 5 mg daily for high-risk group.62,73 Similarly, many American associations recommend a supplementation of 4 mg of folic acid daily to women at high-risk for NTDs.74 This daily high-dosage supplement should be started at least 3 months before conception and continued until 12 weeks of gestational age.73,75
High-level folic acid supplementation is not believed to increase maternal or fetal risk,73,74 even though some state that it may potentially mask the symptoms of megaloblastic anemia due to vitamin B12 deficiency.75 However, it should be highlighted that, even though most over-the-counter multivitamin supplements contain 400 mg of folic acid, a higher level of folic acid supplementations should not be achieved by taking excessive multivitamins, but instead by taking specific additional folic acid supplements. This is because of the potential toxicity at high doses of some of the vitamins contained in multivitamins, especially vitamin A.3,73
PRACTICE RECOMMENDATIONS
- Ultrasound evaluation of the neural tube is part of routine mid-trimester screening ultrasound assessment. It includes evaluation of the fetal spine and central nervous system (CNS).
- In the case of suspicion of neural tube defect abnormality during the screening examination, women should undergo targeted neurosonography for a better characterization of the malformation.
- Exencephaly is a malformation characterized by the absence of a normal cranium. The brain is thus exposed to mechanical and chemical insults and progressively disintegrates, leading to anencephaly. Exencephaly-anencephaly sequence is a lethal malformation and is mostly diagnosed during first trimester ultrasound.
- Encephalocele is characterized by a herniation of meninges, brain or both from a cranial defect, and it is classified according to the site of cranial defect. Ultrasound hallmark is a saclike structure connected to the fetal head and cover by skin.
- Iniencephaly is a neural tube defect involving the occiput bone and the inion. It presents with rachischisis of the cervical and thoracic spine, which results in extreme fixed retroflexion of the head and an apparent absence of the neck.
- Open spina bifida is a malformation characterized by a bony defect of the fetal spine with the exposure of neural tissue and/or meninges to the amniotic fluid, with no skin covering the lesion. In myelomeningocele, the protrusion of the meninges and of the neural tissue have often the aspect of a cyst. In meningocele, the neural tissue lies at the level of the skin surface and is thus more difficult to visualize. Open spina bifida is typically associated with a posterior fossa anomaly called Arnold-Chiari type II malformation.
- Closed spina bifida is far less common than open spina bifida. The neural tube defect is covered by skin and a subcutaneous mass may be present or not. Cranial indirect signs of open spina bifida are not present in this case.
- Folic acid supplementation can prevent neural tube defects, and it is thus recommended oral assumption of at least 0.4 mg of folic acid in women who could be pregnant.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Gardner WJ. Myelomeningocele, the Result of Rupture of the Embryonic Neural Tube. Cleveland Clinic Journal of Medicine 1960;27:88–100. DOI: 10.3949/ccjm.27.2.88. | |
Tortori-Donati P, Rossi A, Cama A. Spinal dysraphism: a review of neuroradiological features with embryological correlations and proposal for a new classification. Neuroradiology 2000;42:471–91. DOI: 10.1007/s002340000325. | |
Practice Bulletin No. 187: Neural Tube Defects. Obstetrics and Gynecology 2017;130:e279–90. DOI: 10.1097/AOG.0000000000002412. | |
Wilde JJ, Petersen JR, Niswander L. Genetic, epigenetic, and environmental contributions to neural tube closure. Annu Rev Genet 2014;48:583–611. DOI: 10.1146/annurev-genet-120213-092208. | |
Christianson A, Howson CP. March of Dimes global report on birth defect: the hidden toll of dying and disabled children, 2006. | |
Flannery DB, Kahler SG. Neural tube defects in trisomy 18. Prenat Diagn 1986;6:97–9. DOI: 10.1002/pd.1970060204. | |
Chen C-P. Chromosomal abnormalities associated with neural tube defects (I): full aneuploidy. Taiwan J Obstet Gynecol 2007;46:325–35. DOI: 10.1016/S1028-4559(08)60002-9. | |
Chen C-P. Syndromes, disorders and maternal risk factors associated with neural tube defects (III). Taiwan J Obstet Gynecol 2008;47:131–40. DOI: 10.1016/S1028-4559(08)60070-4. | |
Chen C-P. Prenatal sonographic features of fetuses in trisomy 13 pregnancies (II). Taiwan J Obstet Gynecol 2009;48:218–24. DOI: 10.1016/S1028-4559(09)60293-X. | |
Leoni C, Stevenson DA, Geiersbach KB, et al. Neural tube defects and atypical deletion on 22q11.2. Am J Med Genet A 2014;164A:2701–6. DOI: 10.1002/ajmg.a.36701. | |
Chen C-P. Prenatal diagnosis, fetal surgery, recurrence risk and differential diagnosis of neural tube defects. Taiwan J Obstet Gynecol 2008;47:283–90. DOI: 10.1016/S1028-4559(08)60125-4. | |
Mitchell LE, Adzick NS, Melchionne J, et al. Spina bifida. Lancet 2004;364:1885–95. DOI: 10.1016/S0140-6736(04)17445-X. | |
Williams J, Mai CT, Mulinare J, et al. Updated estimates of neural tube defects prevented by mandatory folic Acid fortification – United States, 1995–2011. MMWR Morb Mortal Wkly Rep 2015;64:1–5. | |
Hill DS, Wlodarczyk BJ, Palacios AM, et al. Teratogenic effects of antiepileptic drugs. Expert Rev Neurother 2010;10:943–59. DOI: 10.1586/ern.10.57. | |
Moretti ME, Bar-Oz B, Fried S, et al. Maternal Hyperthermia and the Risk for Neural Tube Defects in Offspring: Systematic Review and Meta-Analysis. Epidemiology 2005;16:216–9. DOI: 10.1097/01.ede.0000152903.55579.15. | |
Dreier JW, Andersen A-MN, Berg-Beckhoff G. Systematic review and meta-analyses: fever in pregnancy and health impacts in the offspring. Pediatrics 2014;133:e674–88. DOI: 10.1542/peds.2013-3205. | |
Auger N, Fraser WD, Arbour L, et al. Elevated ambient temperatures and risk of neural tube defects. Occup Environ Med 2017;74:315–20. DOI: 10.1136/oemed-2016-103956. | |
Haghighi MM, Wright CY, Ayer J, et al. Impacts of High Environmental Temperatures on Congenital Anomalies: A Systematic Review. Int J Environ Res Public Health 2021;18:4910. DOI: 10.3390/ijerph18094910. | |
American College of O and Gynecologists' Committee on Practice BO. ACOG Practice Bulletin No. 201: Pregestational Diabetes Mellitus. Obstetrics and Gynecology 2018;132:e228–48. DOI: 10.1097/AOG.0000000000002960. | |
Lemire GT, Beauregard-Lacroix É, Campeau PM, et al. Retrospective analysis of fetal vertebral defects: Associated anomalies, etiologies, and outcome. Am J Med Genet A 2020;182:664–72. DOI: 10.1002/ajmg.a.61468. | |
ACOG Practice Bulletin No 156: Obesity in Pregnancy. Obstetrics and Gynecology 2015;126:e112–26. DOI: 10.1097/AOG.0000000000001211. | |
Tinker SC, Gilboa SM, Moore CA, et al. Modification of the association between diabetes and birth defects by obesity, National Birth Defects Prevention Study, 1997–2011. Birth Defects Res 2021;113:1084–97. DOI: 10.1002/bdr2.1900. | |
Sotres-Alvarez D, Siega-Riz AM, Herring AH, et al. Maternal dietary patterns are associated with risk of neural tube and congenital heart defects. Am J Epidemiol 2013;177:1279–88. 2013/05/04. DOI: 10.1093/aje/kws349. | |
Vujkovic M, Steegers EA, Looman CW, et al. The maternal Mediterranean dietary pattern is associated with a reduced risk of spina bifida in the offspring. BJOG 2009;116:408–15. 2009/02/04. DOI: 10.1111/j.1471-0528.2008.01963.x. | |
Salomon LJ, Alfirevic Z, Berghella V, et al. ISUOG Practice Guidelines (updated): performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet Gynecol 2022;59:840–56. 2022/05/21. DOI: 10.1002/uog.24888. | |
Malinger G, Paladini D, Haratz KK, et al. ISUOG Practice Guidelines (updated): sonographic examination of the fetal central nervous system. Part 1: performance of screening examination and indications for targeted neurosonography. Ultrasound Obstet Gynecol 2020;56:476–84. DOI: 10.1002/uog.22145. | |
Paladini D, Malinger G, Birnbaum R, et al. ISUOG Practice Guidelines (updated): sonographic examination of the fetal central nervous system. Part 2: performance of targeted neurosonography. Ultrasound Obstet Gynecol 2021;57:661–71. 2021/03/19. DOI: 10.1002/uog.23616. | |
Salomon LJ, Alfirevic Z, Berghella V, et al. ISUOG Practice Guidelines (updated): performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet Gynecol 2022;59:840–56. DOI: 10.1002/uog.24888. | |
Paladini D, Malinger G, Birnbaum R, et al. ISUOG Practice Guidelines (updated): sonographic examination of the fetal central nervous system. Part 2: performance of targeted neurosonography. Ultrasound Obstet Gynecol 2021;57:661–71. DOI: 10.1002/uog.23616. | |
Monteagudo A. Exencephaly-anencephaly Sequence. American Journal of Obstetrics and Gynecology 2020;223:B5–8. DOI: 10.1016/j.ajog.2020.08.176. | |
Leibovitz Z, Lerman-Sagie T, Haddad L. Fetal Brain Development: Regulating Processes and Related Malformations. Life (Basel) 2022;12:809. DOI: 10.3390/life12060809. | |
Monteagudo A. Posterior Encephalocele. American Journal of Obstetrics and Gynecology 2020;223:B9–12. DOI: 10.1016/j.ajog.2020.08.177. | |
Cameron M, Moran P. Prenatal screening and diagnosis of neural tube defects. Prenat Diagn 2009;29:402–11. DOI: 10.1002/pd.2250. | |
Naidich TP, Altman NR, Braffman BH, et al. Cephaloceles and related malformations. AJNR Am J Neuroradiol 1992;13:655–90. | |
Wen S, Ethen M, Langlois PH, et al. Prevalence of encephalocele in Texas, 1999–2002. Am J Med Genet A 2007;143A:2150–5. DOI: 10.1002/ajmg.a.31907. | |
Rădulescu M, Ulmeanu EC, Nedelea M, et al. Prenatal ultrasound diagnosis of neural tube defects. Pictorial essay. Med Ultrason 2012;14:147–53. | |
Siffel C, Wong L-YC, Olney RS, et al. Survival of infants diagnosed with encephalocele in Atlanta, 1979–98. Paediatr Perinat Epidemiol 2003;17:40–8. DOI: 10.1046/j.1365-3016.2003.00471.x. | |
Da Silva SL, Jeelani Y, Dang H, et al. Risk factors for hydrocephalus and neurological deficit in children born with an encephalocele. J Neurosurg Pediatr 2015;15:392–8. DOI: 10.3171/2014.10.PEDS14192. | |
Holmes LB, Toufaily MH, Westgate M-N. Iniencephaly. Birth Defects Res 2018;110:128–33. DOI: 10.1002/bdr2.1082. | |
Sahid S, Sepulveda W, Dezerega V, et al. Iniencephaly: prenatal diagnosis and management. Prenat Diagn 2000;20:202–5. | |
Khatri D, Gosal JS, Joseph J, et al. Surgical Outcome in an Iniencephaly Survivor: Case Report and Review of the Literature. World Neurosurg 2019;129:105–9. DOI: 10.1016/j.wneu.2019.05.202. | |
Chen C-P. Prenatal diagnosis of iniencephaly. Taiwan J Obstet Gynecol 2007;46:199–208. DOI: 10.1016/S1028-4559(08)60021-2. | |
Pungavkar SA, Sainani NI, Karnik AS, et al. Antenatal diagnosis of iniencephaly: sonographic and MR correlation: a case report. Korean J Radiol 2007;8:351–5. DOI: 10.3348/kjr.2007.8.4.351. | |
Atta CAM, Fiest KM, Frolkis AD, et al. Global Birth Prevalence of Spina Bifida by Folic Acid Fortification Status: A Systematic Review and Meta-Analysis. Am J Public Health 2016;106:e24–34. DOI: 10.2105/AJPH.2015.302902. | |
Thibadeau J. The National Spina Bifida Patient Registry: Past, present, and future. J Pediatr Rehabil Med 2017;10:205–10. DOI: 10.3233/PRM-170463. | |
Castillo J, Lupo PJ, Tu DD, et al. The National Spina Bifida Patient Registry: A Decade's journey. Birth Defects Res 2019;111:947–57. DOI: 10.1002/bdr2.1407. | |
Ben-Sira L, Garel C, Malinger G, et al. Prenatal diagnosis of spinal dysraphism. Childs Nerv Syst 2013;29:1541–52. DOI: 10.1007/s00381-013-2178-5. | |
Copp AJ, Adzick NS, Chitty LS, et al. Spina bifida. Nat Rev Dis Primers 2015;1:15007. DOI: 10.1038/nrdp.2015.7. | |
Gunay H, Sozbilen MC, Gurbuz Y, et al. Incidence and type of foot deformities in patients with spina bifida according to level of lesion. Childs Nerv Syst 2016;32:315–9. DOI: 10.1007/s00381-015-2944-7. | |
Volpe N, Dall'Asta A, Di Pasquo E, et al. First-trimester fetal neurosonography: technique and diagnostic potential. Ultrasound Obstet Gynecol 2021;57:204–14. DOI: 10.1002/uog.23149. | |
Ushakov F, Sacco A, Andreeva E, et al. Crash sign: new first-trimester sonographic marker of spina bifida. Ultrasound Obstet Gynecol 2019;54:740–5. DOI: 10.1002/uog.20285. | |
Coleman BG, Langer JE, Horii SC. The diagnostic features of spina bifida: the role of ultrasound. Fetal Diagn Ther 2015;37:179–96. DOI: 10.1159/000364806. | |
Montaguti EPGYABS. Spina Bifida, Visual Encyclopedia of Ultrasound in Obstetrics and Gynecology. wwwisuogorg, October 2018. | |
Nicolaides KH, Campbell S, Gabbe SG, et al. Ultrasound screening for spina bifida: cranial and cerebellar signs. Lancet 1986;2:72–4. DOI: 10.1016/s0140-6736(86)91610-7. | |
Van den Hof MC, Nicolaides KH, Campbell J, et al. Evaluation of the lemon and banana signs in one hundred thirty fetuses with open spina bifida. American Journal of Obstetrics and Gynecology 1990;162:322–7. DOI: 10.1016/0002-9378(90)90378-k. | |
Ghi T, Pilu G, Falco P, et al. Prenatal diagnosis of open and closed spina bifida. Ultrasound Obstet Gynecol 2006;28:899–903. DOI: 10.1002/uog.3865. | |
Zalel Y, Lehavi O, Aizenstein O, et al. Development of the fetal spinal cord: time of ascendance of the normal conus medullaris as detected by sonography. J Ultrasound Med 2006;25:1397–401; quiz 1402–3. DOI: 10.7863/jum.2006.25.11.1397. | |
Arthurs OJ, Thayyil S, Wade A, et al. Normal ascent of the conus medullaris: a post-mortem foetal MRI study. J Matern Fetal Neonat 2013;26:697–702. DOI: 10.3109/14767058.2012.746307. | |
Bui CJ, Tubbs RS, Oakes WJ. Tethered cord syndrome in children: a review. Neurosurg Focus 2007;23:E2. DOI: 10.3171/foc.2007.23.2.2. | |
Sohaey R, Oh KY, Kennedy AM, et al. Prenatal diagnosis of tethered spinal cord. Ultrasound Q 2009;25:83–7; quiz 93–5. DOI: 10.1097/RUQ.0b013e3181a32218. | |
Amelot A, Cretolle C, de Saint Denis T, et al. Spinal dysraphism as a new entity in V.A.C.TE.R.L syndrome, resulting in a novel acronym V.A.C.TE.R.L.S. Eur J Pediatr 2020;179:1121–9. DOI: 10.1007/s00431-020-03609-4. | |
Douglas Wilson R, Van Mieghem T, Langlois S, et al. Guideline No. 410: Prevention, Screening, Diagnosis and Pregnancy Management for Fetal Neural Tube Defects. J Obstet Gynaecol Can 2021;43:124–39 e128. 20201117. DOI: 10.1016/j.jogc.2020.11.003. | |
Luthy DA, Wardinsky T, Shurtleff DB, et al. Cesarean section before the onset of labor and subsequent motor function in infants with meningomyelocele diagnosed antenatally. N Engl J Med 1991;324:662–6. DOI: 10.1056/NEJM199103073241004. | |
Reghunath A, Ghasi RG, Aggarwal A. Unveiling the tale of the tail: an illustration of spinal dysraphisms. Neurosurg Rev 2021;44:97–114. DOI: 10.1007/s10143-019-01215-z. | |
Sacco A, Ushakov F, Thompson D, et al. Fetal surgery for open spina bifida. Obstet Gynaecol 2019;21:271–82. DOI: 10.1111/tog.12603. | |
Inversetti A, Van der Veeken L, Thompson D, et al. Neurodevelopmental outcome of children with spina bifida aperta repaired prenatally vs. postnatally: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2019;53:293–301. DOI: 10.1002/uog.20188. | |
Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med 2011;364:993–1004. DOI: 10.1056/NEJMoa1014379. | |
Committee Opinion No. 720: Maternal-Fetal Surgery for Myelomeningocele. Obstetrics and Gynecology 2017;130:e164–7. DOI: 10.1097/AOG.0000000000002303. | |
Tuite GF, Thompson DNP, Austin PF, et al. Evaluation and management of tethered cord syndrome in occult spinal dysraphism: Recommendations from the international children's continence society. Neurourol Urodyn 2018;37:890–903. DOI: 10.1002/nau.23382. | |
Chaoui R, Benoit B, Mitkowska-Wozniak H, et al. Assessment of intracranial translucency (IT) in the detection of spina bifida at the 11–13-week scan. Ultrasound Obstet Gynecol 2009;34:249–52. DOI: 10.1002/uog.7329. | |
Fong KW, Toi A, Okun N, et al. Retrospective review of diagnostic performance of intracranial translucency in detection of open spina bifida at the 11–13-week scan. Ultrasound Obstet Gynecol 2011;38:630–4. DOI: 10.1002/uog.8994. | |
Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet 1991;338:131–7. | |
Wilson RD, Genetics C, Wilson RD, et al. Pre-conception Folic Acid and Multivitamin Supplementation for the Primary and Secondary Prevention of Neural Tube Defects and Other Folic Acid-Sensitive Congenital Anomalies. J Obstet Gynaecol Can 2015;37:534–52. DOI: 10.1016/s1701-2163(15)30230-9. | |
Force USPST, Bibbins-Domingo K, Grossman DC, et al. Folic Acid Supplementation for the Prevention of Neural Tube Defects: US Preventive Services Task Force Recommendation Statement. JAMA 2017;317:183–9. DOI: 10.1001/jama.2016.19438. | |
Toriello HV, Policy and Practice Guideline Committee of the American College of Medical G. Policy statement on folic acid and neural tube defects. Genet Med 2011;13:593–6. DOI: 10.1097/GIM.0b013e31821d4188. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards programme CLICK HERE)