This chapter should be cited as follows:
Doss GL, Daniels JL, et al., Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.418993
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 19
Pregnancy shortening: etiology, prediction and prevention
Volume Editors:
Professor Arri Coomarasamy, University of Birmingham, UK
Professor Gian Carlo Di Renzo, University of Perugia, Perugia, Italy
Professor Eduardo Fonseca, Federal University of Paraiba, Brazil

Chapter
Measuring the Length of Pregnancy and Identifying its Determinants
First published: December 2023
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
Gestational age has traditionally been assigned using the date of a pregnant person’s last menstrual period (LMP). This date is most precisely defined as the first day of bleeding in the last menstrual cycle prior to conception.1 However, when not solicited using a clear definition and with accurate recall, a pregnant person may offer other dates such as the start of spotting or last day of bleeding when asked to report LMP.
From an accurate LMP, the length of human pregnancy is conventionally estimated to be approximately 280 days (40 weeks). However, our understanding of gestational length and its variability in conceptions that occur outside of assisted reproductive technologies is limited because the exact timing of conception is unobservable.2,3
Pregnancy dating by LMP is prone to considerable error. Even when LMP is accurately recalled, the menstrual cycle is highly variable between different people, and often, between cycles within an individual.4 This variation in menstrual cycle length includes differences in the timing of ovulation and thus conception, resulting in error in gestational age estimation by LMP. Because of sources of error in LMP, the American College of Obstetricians and Gynecologists recommends that gestational age be established or confirmed in the first trimester by applying a standard growth model, which predicts the LMP-based gestational age of an embryo or fetus from ultrasound measurements.5 Assessment of gestational age by ultrasonography is also vulnerable to measurement error. In addition to variability in ultrasound equipment and ultrasound technicians, differences in fetal growth are associated with pregnancy and parental characteristics.6,7,8,9,10,11
While some difference in the length of pregnancy between individuals is due to measurement error and limitations in our assessment techniques, numerous genetic, environmental, and social factors are clearly associated with pregnancy duration. Our understanding of these factors continues to evolve. The length of pregnancy can further be influenced through medical intervention. This chapter takes an epidemiologic perspective to explore some of the assumptions made in the estimation of gestational age and provides a brief overview of many of the determinants of pregnancy length in humans.
USE OF LAST MENSTRUAL PERIOD (LMP) IN THE ASSESSMENT OF GESTATIONAL AGE
LMP is defined as the first day of bleeding in the last menstrual cycle before becoming pregnant.1 It is an important marker in pregnancy because its identification does not require technical equipment or advanced training. Unfortunately, using it to estimate gestational age is challenging for many reasons. For one, a pregnant person may not have prospectively recorded their menstrual cycle, particularly if they were not planning to become pregnant. In the United States, for example, 45% of pregnancies are unintended.12
Abnormal vaginal bleeding or spotting during pregnancy may also be mistaken for a menstrual period resulting in misidentification of LMP. In one study of early pregnancy, 9% of people had at least 1 day of bleeding during the first 8 weeks of pregnancy.13 If this bleeding were mistaken for a period, it would result in an underestimation of gestational age by the incorrectly identified LMP.
Even with accurate recall and without misidentification of menstrual history, LMP remains an imprecise estimate of gestational age due to differences in ovulation timing between menstrual cycles.14 In unassisted conceptions, fertilization occurs a maximum of 24 hours after ovulation, making ovulation a useful marker when considering the start of pregnancy.15 Unfortunately, LMP is an inaccurate indicator of the timing of ovulation. In a previous study of 202 menstrual cycles, it was shown that ovulation occurred with a right-skewed distribution around a median of cycle day 14 and more than 20% of cycles had a day of ovulation which was at least 1 week after this median.14 The result of this distribution is that conception occurs at different times relative to LMP in different pregnancies and often later in the menstrual cycle than commonly expected, potentially resulting in an overestimation of gestational age by LMP.16 Previous research has shown that, when compared to early ultrasound-based estimation of gestational age, LMP is more likely to result in an overestimation of gestational age at delivery and, consequently, an underestimation of the number of preterm births.17,18
When considering pregnancy length, both natural variability in the timing of ovulation and misidentification of LMP may cause preterm or postterm misclassification of gestational age at delivery. When LMP is accurately recalled and used alone to estimate gestational age, those who ovulate late in their menstrual cycles will have gestational ages overestimated by LMP and those who ovulate early will have gestational ages underestimated by LMP.
The ability of ovulation timing to impact the accuracy of the LMP-based estimate leads to another important consideration: when reading studies which aim to understand factors that influence pregnancy duration, any factor which affects the length of time between the first day of the menstrual period and ovulation will affect the LMP-based estimate of gestational age at delivery, even if it has no true impact on the time between conception and birth.
OVULATION-BASED ESTIMATION OF GESTATIONAL AGE
Fertilization is perhaps a more logical point to consider the start of pregnancy compared to LMP. After all, not only does the time between menses and ovulation vary between people, it varies with exposure to some known factors, including breastfeeding, psychological stress, heavy exercises, hypothyroidism, polycystic ovary syndrome, and certain medication use.19,20,21,22/a> For this reason, it cannot be assumed that ovulation occurs at the same time relative to LMP in different people and in different menstrual cycles. While conception cannot be observed directly, the fertile window extends, to at most 24 hours after the release of an egg.15 This makes ovulation a proxy for the time of fertilization and thus the start of pregnancy.
A study that reported the length and variation of human pregnancy in unassisted conceptions using ovulation to determine gestational age found that the median length of pregnancy from conception to birth was 268 days (38 weeks and 2 days).23 When comparing study participants’ ovulation and LMP-based measures of gestational age at birth, they found that the coefficient of variation (a statistical measure of the population standard deviation and mean) was reduced when ovulation-based gestational age was used. This indicated that ovulation was a more precise measure of length of gestation than LMP.
Ovulation can be detected using methods which include basal body temperature monitoring, cervical mucus monitoring, and ovulation tests which use urinary measurement of metabolites such as estrone-3-glucuronide (E3G) and luteinizing hormone (LH).24 Ovulation typically occurs 8–20 hours after the peak in luteinizing hormone, making detection of ovulation a potential tool to identify the start of pregnancy more accurately.25 However, because ovulation proceeds conception, information from ovulation detection can only be used to estimate gestational age when assessed prospectively. Typically, this occurs when couples are using ovulation tracking to identify the fertile window to either become pregnant or avoid pregnancy. Not all people who ovulate successfully detect their ovulation. Additionally, ovulation tests can be expensive, further limiting the number of people who use them. While this is one area of ongoing innovation in the estimation of pregnancy length, it is not widely used outside of research settings.
ULTRASONOGRAPHY-BASED ESTIMATION OF GESTATIONAL AGE
Considering the limitations of LMP described above, the American College of Obstetricians and Gynecologists (ACOG) recommends that ultrasound measurement of the embryo or fetus be used to establish or confirm gestational age.5 To assign gestational age in the first trimester, the length from the crown of the head to the rump of the embryo (crown-rump length or CRL) is commonly measured. A standard formula for early pregnancy growth is then applied. Numerous growth standards are in use globally, but what remains consistent is that the observations used to construct these growth standards were obtained from participants with regular menstrual cycles and known dates of LMP.26,27,28,29 In effect, ultrasound measurements attempt to estimate a pregnant person’s expected LMP under the circumstance of regular menstrual cycles (like the participants whose LMPs were used to construct the selected growth standard). This estimated LMP from the growth curve is used for gestational age assignment.
In 1975, Robinson and Fleming introduced a widely used standard for estimating embryonic age from ultrasound measurements of crown-rump length (n = 334). Their research was conducted with obstetric patients in Glasgow Scotland.27 In 1992, Hadlock et al. updated this standard with a study in a larger sample (n = 416) of US participants at the Baylor College of Medicine.26 Despite being developed over 30 years ago, this remains a widely used standard across the United States.
Ultrasonography methods have advanced considerably since it was first suggested that crown-rump length be measured as early as 6 completed weeks after LMP.30 In 2010, Pexsters et al. developed another curve using 3,710 singleton pregnancies with known LMP.28 The Pexsters et al. study was a retrospective database study of CRL. As with previous early growth standards, this was done in a predominately white population. Pexsters et al. found that their growth curve predicted smaller embryos than Hadlock et al. or Robinson and Fleming prior to 8 weeks' gestation but was consistent with predicted size after that point.
A newer and international standard for pregnancy dating is the International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st).29 This curve was the first combined, international prescriptive standard for fetal growth (though very early pregnancy measurements are still lacking). Intergrowth-21st is part of ongoing research which posits that there is a universal standard for healthy human growth when nutritional needs are met, though this remains a subject of debate.29 These are only a small sample of the growth standards which are in use today all over the world.
Every growth curve includes some variance from the expected value that is dependent on the gestational age at which CRL measurements are taken. This is due to a combination of measurement error and natural variability. Even under conditions of perfect recall, when gestational age is assessed by LMP, all CRLs measured at a given LMP will not truly be taken from the same point in development due to variability in the length of time between LMP and ovulation. Some natural variability in the size of the embryo or fetus at any given gestational age is also expected.
Differences in equipment, fetal positioning and operator training can introduce both random and systematic error into measurements of crown-rump length, which impact the assessment of gestational age by CRL. In a study of these potential effects, a decrease in first trimester CRL of 2 mm altered gestational age sufficiently to shift the estimated fetal weight at 20 week scan from the 10th to 20th percentile.31 Error may further be introduced from inherent differences in growth between healthy pregnancies as well as differences in growth caused by external factors.
Factors which cause adverse pregnancy outcomes may also slow the growth of a developing embryo early in pregnancy. In this way, if ultrasonography alone is used to estimate gestational age, exposures which impact growth may appear to be more strongly associated with gestational age at delivery. These characteristics may include maternal age, nutrition, parental height, maternal BMI, ethnicity, substance use, diabetes, and fetal chromosomal anomalies.6,7,8,9,10,11 As evidence of this, a greater underestimation of gestational age by ultrasound relative to LMP has been observed in tobacco smoke-exposed pregnancies, possibly suggesting that the growth restriction associated with smoking is sufficient to impact ultrasound-based dating.32
It is unknown how much of the variability in current growth standards can be attributed to differences in the timing of ovulation between these study participants, how much can be attributed to other sources of measurement error, and how much is the result of biological differences in growth. Despite this, the error in dating introduced by differences in first-trimester growth is assumed to be low because this time window is still early in development.
ASSESSMENT OF MEASUREMENT ERROR IN THE ESTIMATION OF GESTATIONAL AGE
Several previous studies have compared LMP and ultrasound-based estimates of gestational age at delivery and investigated how misclassification of gestational age could alter our estimates of the prevalence of preterm birth, postterm birth, and their associated severity.2,16,18,32,33,34
Because conception is not observed in unassisted pregnancies, one strategy to assess the accuracy of gestational age measures has been to assess how well each dating method’s classification of preterm birth aligns with the diagnosis of adverse neonatal outcomes. Following this strategy, the gestational age dating methods that are being compared are each used to identify preterm births. If one gestational age dating method results in better concordance of preterm birth and admission to the neonatal intensive care unit (NICU), it is deemed more accurate under the assumption that true preterm birth causes increased morbidity.35 However, it is important to consider that factors such as tobacco smoke exposure, are likely causes of both preterm birth and NICU admission through other pathology besides shortened pregnancy length (Figure 1). Thus, NICU admission alone does not confirm preterm birth.
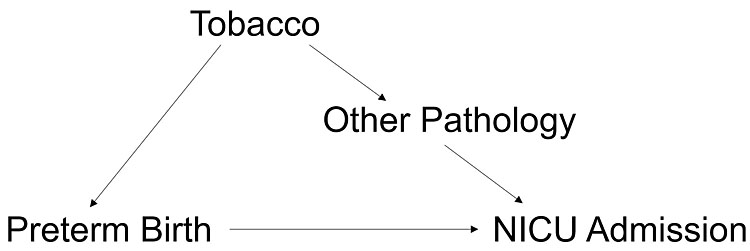
1
Preterm birth alone may not be the cause of increased NICU admissions in tobacco smoke exposed pregnancies.
To illustrate this, consider the example of a Danish study of pregnant participants who had a consistent cycle length (+/− 3 days). LMP was compared to an ultrasound-based measure of gestational age. A greater proportion of births were assigned preterm at delivery by ultrasonography than by LMP. However, this proportion was higher, and therefore the effect more pronounced, in female fetuses which are known to be smaller than male fetuses, and in pregnancies with tobacco smoke exposure. The authors concluded that smaller fetal size, such as in tobacco smoke exposed pregnancies, resulted in inaccurate estimation of gestational age by ultrasound.32 Exposure to tobacco smoke could also cause adverse neonatal outcomes, such as NICU admission. Thus, it is possible that tobacco smoke may decrease fetal size at ultrasound, which leads to an underestimate of the true gestational age at ultrasound, and correspondingly, reduces the estimated gestational age at delivery (Figure 2). Pregnancies that are small at ultrasound are more likely to appear preterm at delivery, regardless of whether they are, in truth, preterm deliveries.
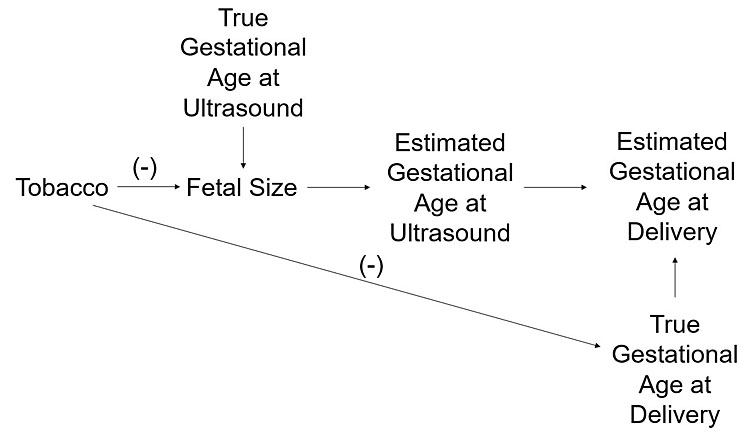
2
Diagram showing how tobacco smoke may appear to shorten pregnancy by both a true effect on gestational age and by adversely impacting growth, leading to an underestimate of gestational age at delivery.
When attempting to compare the accuracy of LMP and ultrasound in a population, multiple techniques can be considered together to provide a more complete picture. Consider another study that compared rates of preterm and various birth outcomes when gestational age was defined by first-trimester ultrasound versus LMP.18 When the authors compared LMP-based gestational age to first-trimester ultrasound-based gestational age, they found that when ultrasound-based gestational age estimated preterm birth, it was more likely to result in low birthweight rate than when using the LMP-based measure. The authors also noted that LMP estimated substantially more postterm deliveries than ultrasound (12.1% vs. 3.4%).18 These authors concluded that ultrasound was likely more accurate than LMP for gestational age dating. The concordance of low birthweight with preterm birth alone does not confirm that preterm birth was more accurately assessed. However, the authors also noted flaws in LMP such as a digit preference for reporting LMP on certain days of the month as well as certain days on the week, providing more evidence of the fallibility of LMP in their US-based study population.
In summary, when attempting to assess the validity of gestational age estimates, it is important to remember that a risk factor can simultaneously cause a fetus to be small at ultrasound, resulting in an underestimate of gestational age, and cause adverse health effects or preterm birth. An underestimate of gestational age will cause a newborn to appear younger at delivery than it truly is. While tobacco smoke exposure provides an example here, the same conceptual model can be applied to many other factors. Any exposure that reduces fetal size will appear to be associated with preterm birth when ultrasound alone is used to assess gestational age. Similarly, any factor that delays ovulation will appear to be associated with postterm birth when LMP alone is used to assess gestational age.
DETERMINANTS OF PREGNANCY LENGTH
Factors that impact the true length of gestation have been extensively studied and yet, understanding these determinants remains an important and evolving area of scientific inquiry. Preterm birth (<37 weeks) is of particular concern as it is estimated to be the leading cause of neonatal death globally, with the most elevated risk of death among infants born extremely or very preterm (<32 weeks).36,37
A 2010 review of behavioral factors associated with preterm birth identified tobacco use as the factor most extensively studied, citing 58 publications, finding an association with preterm birth and in pregnancies exposed to secondhand smoke. Additionally, there were modest increases in risk with consumption of at least seven alcoholic drinks per week, consumption of at least three caffeinated drinks per day, any cannabis use, and any cocaine use.38
Numerous other factors have been identified as predictors of preterm birth, many of them non-modifiable. These factors include having a history of preterm birth in a previous pregnancy, maternal infection, high blood pressure, fetal anomalies, low and high body mass index, a history of spontaneous abortion, maternal age less than 18 years or over 40 years, diabetes, gestational diabetes, a history of fibroids, a time between pregnancies of less than 6 months, placenta previa, and multiple gestations.39,40,41,42,43,44 Both air and drinking water pollution, indoor mold, and ambient temperature have also been associated with preterm birth.45,46,47,48 In the United States, there is a marked racial disparity in the incidence of preterm birth between non-Hispanic white and non-Hispanic Black women with the increased risk of preterm birth among non-Hispanic Black women posited to be the result of numerous mechanisms of social inequity.49 A consensus statement released by the March of Dimes notes that most hypothesized causes of increased preterm birth in Black mothers, including socioeconomic factors and stress, are downstream effects of racism.50
Postterm pregnancy is often assumed to be the result of measurement error in the assessment of gestational age. However, it is also associated with an increased risk of neonatal mortality though its risk factors are less well understood.51 The risk of postterm pregnancy has been positively associated with history of prior postterm pregnancies, increased maternal age, first pregnancies, a male fetus, white race, and maternal obesity.52,53
Importantly, some of these previously identified factors may have been associated with misclassification of gestational age at birth rather than true changes in pregnancy duration or the magnitude of their effect may have been inaccurately estimated. Ovulation can be used to estimate gestational age as conception occurs close in time to ovulation due to the short lifespan of the ovulated egg. Factors found to be associated with longer pregnancy when measured from ovulation include, an early rapid progesterone rise, older maternal age, and a longer time from ovulation to implantation.54 Notably, in this study which measured pregnancy length from ovulation, an association was not seen with alcohol intake, parity, and fetal sex.3
Pregnancy length can also be shortened via induction when complications or preterm gestation threaten the health of the pregnant person or fetus.54 To lengthen pregnancy, vaginal progesterone and cervical cerclage are two interventions which have been implemented.55
The list of factors identified as important predictors of pregnancy length continues to grow and evolve with our understanding of biologic, social and environmental factors. However, just as important as the identification of these factors is an understanding of their relationships. Many of the factors above are co-associated such as increased maternal age and gestational diabetes. This makes careful consideration of confounding paramount before making statements of causality when assessing the determinants of pregnancy length.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Wilcox AJ. Fertility and pregnancy: an epidemiologic perspective. New York: Oxford University Press, 2010;xii:324. | |
Mongelli M, Wilcox M, Gardosi J. Estimating the date of confinement: ultrasonographic biometry versus certain menstrual dates. Am J Obstet Gynecol 1996;174(1 Pt 1):278–81. | |
Jukic AM, Baird DD, Weinberg CR, et al. Length of human pregnancy and contributors to its natural variation. Hum Reprod 2013;28(10):2848–55. | |
Chiazze L Jr, Brayer FT, Macisco JJ Jr, et al. The length and variability of the human menstrual cycle. JAMA 1968;203(6):377–80. | |
Committee Opinion No 700: Methods for Estimating the Due Date. Obstet Gynecol 2017;129(5):e150–e4. | |
Bottomley C, Daemen A, Mukri F, et al. Assessing first trimester growth: the influence of ethnic background and maternal age. Hum Reprod 2009;24(2):284–90. | |
Bahado-Singh RO, Lynch L, Deren O, et al. First-trimester growth restriction and fetal aneuploidy: the effect of type of aneuploidy and gestational age. Am J Obstet Gynecol 1997;176(5):976–80. | |
Hillman S, Peebles DM, Williams DJ. Paternal metabolic and cardiovascular risk factors for fetal growth restriction: a case-control study. Diabetes Care 2013;36(6):1675–80. | |
Janisse JJ, Bailey BA, Ager J, et al. Alcohol, Tobacco, Cocaine, and Marijuana Use: Relative Contributions to Preterm Delivery and Fetal Growth Restriction. Subst Abus 2014;35(1):60–7. | |
Mook-Kanamori DO, Steegers EA, Eilers PH, et al. Risk factors and outcomes associated with first-trimester fetal growth restriction. JAMA 2010;303(6):527–34. | |
Polzlberger E, Hartmann B, Hafner E, et al. Maternal Height and Pre-Pregnancy Weight Status Are Associated with Fetal Growth Patterns and Newborn Size. J Biosoc Sci 2017;49(3):392–407. | |
Finer LB, Zolna MR. Declines in Unintended Pregnancy in the United States, 2008–2011. N Engl J Med 2016;374(9):843–52. | |
Harville EW, Wilcox AJ, Baird DD, et al. Vaginal bleeding in very early pregnancy. Hum Reprod 2003;18(9):1944–7. | |
Baird DD, Mcconnaughey DR, Weinberg CR, et al. Application of a Method for Estimating Day of Ovulation Using Urinary Estrogen and Progesterone Metabolites. Epidemiology 1995;6(5):547–50. | |
Royston JP. Basal body temperature, ovulation and the risk of conception, with special reference to the lifetimes of sperm and egg. Biometrics 1982;38(2):397–406. | |
Kramer MS, McLean FH, Boyd ME, et al. The validity of gestational age estimation by menstrual dating in term, preterm, and postterm gestations. JAMA 1988;260(22):3306–8. | |
Blondel B, Morin I, Platt RW, et al. Algorithms for combining menstrual and ultrasound estimates of gestational age: consequences for rates of preterm and postterm birth. BJOG 2002;109(6):718–20. | |
Savitz DA, Terry JW Jr, Dole N, et al. Comparison of pregnancy dating by last menstrual period, ultrasound scanning, and their combination. Am J Obstet Gynecol 2002;187(6):1660–6. | |
Lewis PR, Brown JB, Renfree MB, et al. The resumption of ovulation and menstruation in a well-nourished population of women breastfeeding for an extended period of time. Fertil Steril 1991;55(3):529–36. | |
Peyser MR, Ayalon D, Harell A, et al. Stress induced delay of ovulation. Obstet Gynecol 1973;42(5):667–71. | |
Hakimi O, Cameron LC. Effect of Exercise on Ovulation: A Systematic Review. Sports Med 2017;47(8):1555–67. | |
Eldar-Geva T, Shoham M, Rosler A, et al. Subclinical hypothyroidism in infertile women: the importance of continuous monitoring and the role of the thyrotropin-releasing hormone stimulation test. Gynecol Endocrinol 2007;23(6):332–7. | |
Jukic AM, Baird DD, Weinberg CR, et al. Length of human pregnancy and contributors to its natural variation. Hum Reprod 2013;28(10):2848–55. | |
Su HW, Yi YC, Wei TY, et al. Detection of ovulation, a review of currently available methods. Bioeng Transl Med 2017;2(3):238–46. | |
Kerin J. Ovulation detection in the human. Clin Reprod Fertil 1982;1(1):27–54. | |
Hadlock FP, Shah YP, Kanon DJ, et al. Fetal crown-rump length: reevaluation of relation to menstrual age (5–18 weeks) with high-resolution real-time US. Radiology 1992;182(2):501–5. | |
Robinson HP, Fleming JE. A critical evaluation of sonar "crown-rump length" measurements. Br J Obstet Gynaecol 1975;82(9):702–10. | |
Pexsters A, Daemen A, Bottomley C, et al. New crown-rump length curve based on over 3500 pregnancies. Ultrasound Obstet Gynecol 2010;35(6):650–5. | |
Papageorghiou AT, Kennedy SH, Salomon LJ, et al. International standards for early fetal size and pregnancy dating based on ultrasound measurement of crown-rump length in the first trimester of pregnancy. Ultrasound Obstet Gynecol 2014;44(6):641–8. | |
Goldstein SR. Embryonic ultrasonographic measurements: crown-rump length revisited. Am J Obstet Gynecol 1991;165(3):497–501. | |
Gadsboll K, Wright A, Kristensen SE, et al. Crown-rump length measurement error: impact on assessment of growth. Ultrasound Obstet Gynecol 2021;58(3):354–9. | |
Henriksen TB, Wilcox AJ, Hedegaard M, et al. Bias in studies of preterm and postterm delivery due to ultrasound assessment of gestational age. Epidemiology 1995;6(5):533–7. | |
Morrison JC. Preterm birth: a puzzle worth solving. Obstet Gynecol 1990;76(Suppl 1):5S-12S. | |
Balchin I, Whittaker JC, Steer PJ, et al. Are reported preterm birth rates reliable? An analysis of interhospital differences in the calculation of the weeks of gestation at delivery and preterm birth rate. BJOG 2004;111(2):160–3. | |
Bibby E, Stewart A. The epidemiology of preterm birth. Neuro Endocrinol Lett 2004;25(Suppl 1):43–7. | |
Lehtonen L, Gimeno A, Parra-Llorca A, et al. Early neonatal death: A challenge worldwide. Semin Fetal Neonatal Med 2017;22(3):153–60. | |
Oza S, Lawn JE, Hogan DR, et al. Neonatal cause-of-death estimates for the early and late neonatal periods for 194 countries: 2000–2013. Bull World Health Organ 2015;93(1):19–28. | |
Savitz DA, Murnane P. Behavioral influences on preterm birth: a review. Epidemiology 2010;21(3):291–9. | |
Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet 2008;371(9606):75–84. | |
Yost NP, Cox SM. Infection and preterm labor. Clin Obstet Gynecol 2000;43(4):759–67. | |
Ekwo EE, Gosselink CA, Moawad A. Unfavorable outcome in penultimate pregnancy and premature rupture of membranes in successive pregnancy. Obstet Gynecol 1992;80(2):166–72. | |
Honein MA, Kirby RS, Meyer RE, et al. The association between major birth defects and preterm birth. Matern Child Health J 2009;13(2):164–75. | |
Oskovi Kaplan ZA, Ozgu-Erdinc AS. Prediction of Preterm Birth: Maternal Characteristics, Ultrasound Markers, and Biomarkers: An Updated Overview. J Pregnancy 2018;2018:8367571. | |
Fuchs F, Monet B, Ducruet T, et al. Effect of maternal age on the risk of preterm birth: A large cohort study. PLoS One 2018;13(1):e0191002. | |
Padula AM, Huang H, Baer RJ, et al. Environmental pollution and social factors as contributors to preterm birth in Fresno County. Environ Health 2018;17(1):70. | |
Gat R, Kachko E, Kloog I, et al. Differences in environmental factors contributing to preterm labor and PPROM – Population based study. Environ Res 2021;196:110894. | |
Patel CJ, Yang T, Hu Z, et al. Investigation of maternal environmental exposures in association with self-reported preterm birth. Reprod Toxicol 2014;45:1–7. | |
Lu C, Cao L, Norback D, et al. Combined effects of traffic air pollution and home environmental factors on preterm birth in China. Ecotoxicol Environ Saf 2019;184:109639. | |
Culhane JF, Goldenberg RL. Racial disparities in preterm birth. Semin Perinatol 2011;35(4):234–9. | |
Braveman P, Dominguez TP, Burke W, et al. Explaining the Black-White Disparity in Preterm Birth: A Consensus Statement From a Multi-Disciplinary Scientific Work Group Convened by the March of Dimes. Front Reprod Health 2021;3. | |
Galal M, Symonds I, Murray H, et al. Postterm pregnancy. Facts Views Vis Obgyn 2012;4(3):175–87. | |
Caughey AB, Stotland NE, Washington AE, et al. Who is at risk for prolonged and postterm pregnancy? Am J Obstet Gynecol 2009;200(6):683 e1–5. | |
Roos N, Sahlin L, Ekman-Ordeberg G, et al. Maternal risk factors for postterm pregnancy and cesarean delivery following labor induction. Acta Obstet Gynecol Scand 2010;89(8):1003–10. | |
Gill P, Lende MN, Van Hook JW. Induction of Labor. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Michelle Lende declares no relevant financial relationships with ineligible companies. Disclosure: James Van Hook declares no relevant financial relationships with ineligible companies, 2023. | |
Updated Clinical Guidance for the Use of Progesterone Supplementation for the Prevention of Recurrent Preterm Birth [Practice Advisory]. The American College of Obstetricians and Gynecologists, 2023 [updated April, 2023]. Available from: https://www.acog.org/en/clinical/clinical-guidance/practice-advisory/articles/2023/04/updated-guidance-use-of-progesterone-supplementation-for-prevention-of-recurrent-preterm-birth. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards programme CLICK HERE)

