This chapter should be cited as follows:
Tyson NA, Labovsky MJ, et al., Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.418433
The Continuous Textbook of Women’s Medicine Series – Gynecology Module
Volume 2
Adolescent gynecology
Volume Editor: Professor Judith Simms-Cendan, University of Miami, USA

Chapter
Adolescent Contraception
First published: April 2023
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
https://drive.google.com/file/d/158gIjKNjZFbBz4wFYJS7BJ4bCgxsGyjh/view?usp=sharing.
The following material includes resources from published articles in medical journals, international and national committee guidelines, and expert opinion on adolescent reproductive health care. The authors of this chapter declare that they have no interests that conflict with the contents of this chapter.
INTRODUCTION
One of the major global public health issues that persists today is teenage pregnancy. Teen pregnancy, occurring in girls aged 13–19 years, accounts for nearly 13 million births per year, with the majority occurring in low and middle-income countries.1 According to the World Health Organization (WHO), seven countries account for half of all adolescent births: Bangladesh, Brazil, the Democratic Republic of the Congo, Ethiopia, India, Nigeria, and the United States of America. Complications during pregnancy and childbirth are the second leading cause of death among 15- to 19-year-old girls worldwide. Babies born to teenage mothers face a significantly higher risk of dying than those born to women between the ages of 20 and 24.1,2,3,4
A study conducted in developing countries identified the following reasons as contributing factors for teenage pregnancy:5,6
- Lack of sexuality education;
- Ineffective utilization of modern contraceptives;
- Cultural obedience;
- Socioeconomic dependence of females on males;
- Peer influence.
The age of first sexual intercourse impacts the choice of contraceptive method. The younger the age of initiation, the lower the probability of using a highly effective contraceptive method and the greater the risk of acquiring a sexually transmitted infection. By the age of 20, almost 75% of females have had sexual intercourse.1,2,3,4 Provision of contraception is an opportunity to guide adolescents in making healthy decisions about an intimate and complex aspect of their own life. Contraception consultation involves promoting a healthy, fulfilling, and safe sexual life, while minimizing risks. Additionally, effective, non-coercive contraception counseling is paramount to supporting adolescent decisions about family planning.
FACTORS AFFECTING ACCESS TO SEXUAL AND REPRODUCTIVE HEALTH IN ADOLESCENTS
Contraception in adolescents involves two key factors:
- Accessibility;
- Acceptability.
Accessibility and acceptability are region-dependent and potential barriers that affect the choice of contraceptive method and interferes with the proper use of contraception in this age group.
Adolescence is an important stage in life with biologic, psychologic, and social changes that ultimately lead to the development of an independent adult. Developmental growth includes establishing one’s identity and the achieving autonomy. Although the process is highly variable for each individual, adolescence typically involves a progressive pattern of three developmental phases: early, middle, and late adolescence. By considering the different stages of adolescence, we can ensure the information we provide aligns with their ability to assimilate and understand the information. Key components of these transitions are outlined in Table 1.7 Through provision of effective age-appropriate contraceptive counseling, the goal is to reduce unintended pregnancies among adolescents.
1
Key factors of adolescent changes. Reproduced with permission from Global Accelerated Action for the Health of Adolescents (AA-HA!).7
Physical, cognitive, social, emotional, and sexual development | Widening gap with transition to adulthood | Balancing protection and autonomy |
|
|
|
In addition to the complexities of adolescent development, contraception provision is complicated by an individual’s access to information and the availability of birth control, which is dependent on social, cultural, political, and religious factors. This is exemplified in the work of the Global Care Group Targeting Factors Change in Figure 1.8
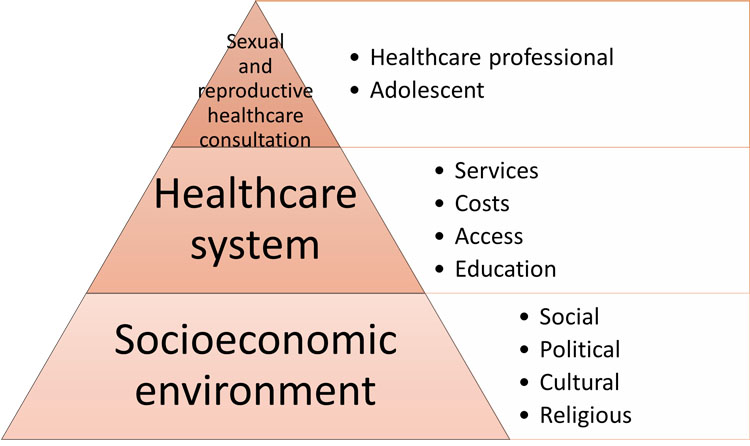
1
Factors influencing pregnancy risk in adolescence. Adapted from Bitzer et al. (2016).8
ADOLESCENT CONTRACEPTIVE COUNSELING (SEE PODCAST FOR HIGHLIGHTING KEY ADOLESCENT COUNSELING POINTS)
Contraception counseling is a care device that involves personalized advice carried out by trained personnel to accompany people in making autonomous decisions about their sexual and reproductive health. The objective is to provide quality information while promoting autonomy free from value judgments. Contraception counseling for adolescents particularly aims to provide a contraceptive method without delay.
The key information included in reproductive health counseling:
- Contraceptive methods;
- Negotiation of the use of condoms;
- Promoting healthy and fulfilling sexual experiences;
- Practices for the care and prevention of sexually transmitted infections (STIs), including HIV/AIDS.
Professionals must be mindful of the cultural and social context in which they are counseling and incorporate the reproductive justice framework by recognizing the following:9
- Mistreatment experienced by marginalized groups and the subsequent effects on their reproductive choices;
- Potential unconscious bias that may influence patient counseling;
- Prioritizing patient values, preferences, and experiences when choosing a contraceptive method.
Counseling should be done in friendly and private spaces, free of judgment, and be easily accessible to all adolescents. It should be comprehensive and include counseling on mental health disorders, nutrition, substance use, and chronic illness, in addition to sexual and reproductive health care.
First and foremost, contraceptive counseling includes obtaining a thorough personal and family history to examine potential contraindications, which could affect the safety of specific methods. Medical associations and governmental and non-governmental organization maintain evidence-based recommendations for use of contraceptive methods in the context of a range of medical conditions and personal characteristics. Common medical considerations include cardiovascular conditions (i.e., hypertension or history of venous thrombosis), a history of migraine with aura, and drug–drug interactions.10 Adolescents have no contraindications for the use of any method based solely on their age. Quick start methods should be routinely prescribed when possible. Contraceptive initiation can result in changes in menstrual cycles and irregular bleeding. Dissatisfaction with irregular bleeding patterns is a common reason for discontinuation in adolescents. Detailed counseling and anticipatory guidance about bleeding expectations is important at time of contraceptive method initiation to improve continuation. Dual method use should always be recommended, which entails using a condom in conjunction with a more effective method of contraception to prevent sexually transmitted infections.
Contraception counseling should be considered a flexible process that avoids rigid protocols. Adolescents often need more than one meeting to understand and assimilate information, make independent decisions, and carry these decisions forward. The process also allows time to build a trusting relationship between adolescents and their health care provider.
Confidentiality is an essential part of counseling; it is the first step in establishing trust between the adolescent and the health professional. Adolescents should have the opportunity to provide their personal history, express doubts and fears, and obtain quality information. The importance and limits of confidentiality should be discussed with the adolescent and their parents if present for the consultation.
Addressing adolescent behavior and development will help guide the adolescent to select an appropriate, effective, and acceptable contraceptive method (Table 2). After a contraceptive method is selected, attention should then be directed to ensuring correct method use, continued use, and reliable follow-up care.
2
Developmental and sexuality differences between early and middle adolescence.
Early adolescence | Middle adolescence | |
Physical, cognitive and social development | Initiation or early puberty Abundant physical changes Concrete thinking Reliance on family-parents, same sex peer relationships and few romantic relationships | Completing puberty Increasing ability for abstract reasoning Limited comprehensive of risk & consequence Peer influence, sexual exploration, differentiating from family |
Sexuality | Discussion of puberty development Masturbation Sexual relations at during this period should make us think about ruling out sexual abuse and/or coercion Assess partners age, if abuse and/or coercion are ruled out according to the laws of each country, provide counseling and contraception | Discuss sexual debut With whom and how they have protected themselves from pregnancy and STIs Sexual history includes assessing what sexual practices they have experienced (oral sex, anal, etc), whether their sexual experiences are pleasant or not, do they orgasm Be explicit to inquire if their sexual partner has a penis and testicles or a vulva, vagina and ovaries |
Some parents are actively involved when their teen chooses a contraceptive method. Parents often have more long-term concerns about fertility and cancer risks. In addition to dispelling these myths, it is important to acknowledge their concern, and address the potential non-contraceptive long-term benefits associated with hormonal contraception, including reduction in endometrial and ovarian cancer risk, avoidance of dysmenorrhea, and the many benefits pertaining to improving the quality of life.
NEXT STEPS?
Consideration should be made to provide adolescents contraceptive counseling from an interdisciplinary approach with teams of medical providers. Counseling should rely on real-time patient feedback, which can be made possible with today’s current technology and access to smart phones and mobile devices.
SPECIFIC CONTRACEPTIVE METHODS BY ORDER OF EFFICACY
Long-acting reversible contraception (LARC)
Since 2014, the American Academy of Pediatrics has endorsed long-acting reversible contraception (LARC), which includes the subdermal contraceptive implant (SCI) and the intrauterine device (IUD), as the recommended first-line contraceptive method for adolescents.11 The etonogestrel (ETG) SCI and intrauterine devices (IUD) are the most effective contraceptive methods available, with pregnancy rates in clinical studies of less than 1 pregnancy per 100 women each year and typical use pattern outcomes are equivalent to perfect use pattern outcomes.12 Pregnancy rate is estimated to be only 0.05% with typical use of the implant, 0.8% with the copper IUD, and 0.2% with the levonorgestrel (LNG) IUD, in comparison to 9% with typical use of short-acting reversible contraception (SARC).12 When studying the effectiveness of LARCs in the United States, unintended pregnancy was 22 times higher in patients who used SARC methods compared to LARCs.13 Within the group of adolescents under 21 years of age, that percentage doubled.13 LARCs do not depend on the behavior of the user, which mitigates the failure rate of typical use to that of perfect use (less than 1%).12 Another advantage of LARCs is their high continuity rate of over 80% at one year, in comparison to SARCs with a continuity rate of lower than 60% at one year.14 In patients under 20 years of age, continuity of LARCs was found to be similar to that of adults at 85% per year.14 Discontinuation of LARCs also requires a separate visit to a health center, which lends the opportunity for additional counseling that reinforces the benefits of this type of method, however studies are on their way evaluating self-removal of IUDs.
Findings from the Contraceptive CHOICE Project informed us that when the barriers of cost, access, and knowledge are removed, people choose the most effective and least user-dependent methods.15 In this landmark study, LARC methods were chosen the majority of time by adolescents with high continuation and satisfaction rates, analogous to the adult population. Among the young adolescents (age 14–17), 69% chose a LARC method, while older adolescents (age 18–20) chose a LARC method 61% of the time.16 One important difference was noted in the type of LARC method selected by young women: 14–17 year-old LARC users favored the contraceptive implant (63%), whereas the 18–20 year-old LARC users favored the IUD (71%).16 Adolescents who used LARC methods were more likely than non-LARC users to continue use at 12 and 24 months (86% vs. 55% at 12 months, 77% vs. 41% at 24 months).17
1. Subdermal implant
Norplant was the first widely used subdermal implant containing LNG and six silicone rods, but manufacturing was discontinued in 2009.18 Jadelle replaced Norplant, which also contains LNG, but only consists of two rods. Jadelle is not marketed in the United States.
Nexplanon is another widely used subdermal implant. It is a 4 cm long single rod subdermal implant containing 68 mg of etonogestrel (ETG) that releases small amounts of progestin daily. It was first marketed as Implanon, however, the inserter was modified and barium sulfate was added to make the rod radio-opaque on imaging. The Nexplanon has similar side effects to Norplant and Jadelle, but with less bleeding and a higher amenorrhea rate.18 It is approved for 3 years by the Food and Drug Administration; however, after 5 years of use in more than 300 users, no pregnancies were reported in the additional 2 years.19
The progestin containing subdermal implants distribute progestin systemically. It suppresses ovulation, thickens cervical mucous, and thins the endometrial lining. Within 8 hours of insertion, ETG levels are sufficient to suppress ovulation.18 Implants can cause irregular and unanticipated bleeding patterns especially in the first year of use. Implications of this side effect must be considered in a cultural and social context as some cultures restrict religious/household activities or sexual intercourse with menstruation.
2. Intrauterine devices (IUD)
There are two types of IUDs available on the market: LNG and copper-based IUDs. The main LNG IUDs include Mirena®, Liletta®, Skyla®, and Kyleena®. They are all T-shaped, release different amounts of hormones, and have slightly different dimensions (Table 3). The LNG IUDs are approved from 3 to 8 years depending on the type.18,20,21 Mirena and Liletta IUD contain the same 52 mg of LNG and are approved for 8 years.20,22,23 Kyleena and Skyla contain 19.5 and 13.5 mg of LNG are approved for 5 and 3 years, respectively.18 Although they are smaller IUDs and have been touted as a premier option for nulliparous patients, no studies have demonstrated that a smaller IUD frame increases ease of insertion or is associated with less pain with insertion. Kyleena and Skyla are associated with more irregular bleeding in comparison to Mirena and Liletta (Table 3).24 Because of the shorter duration of efficacy and side effect profile, Skyla or Kyleena are options in circumstances for teens who do not tolerate a 52 mg IUD insertion device or prefer lower hormones, and have been given anticipatory guidance regarding the bleeding side effect profile. The primary mechanism of action in preventing pregnancy is by thickening cervical mucous. It also creates an inhabitable environment for sperm within the endometrium and causes thinning of the endometrial lining.18 The LNG IUD comes with a number of non-contraceptive benefits, particularly for heavy uterine.
3
LNG IUD characteristics.
Mirena | Liletta | Kyleena | Skyla | |
LNG (mg) | 52 | 52 | 19.5 | 13.5 |
Dimensions (mm) | 32 × 32 | 32 × 32 | 28 × 30 | 28 × 30 |
Duration (years) | 8 | 8 | 5 | 3 |
Amenorrhea rate (%)* | 11 | 11 | 5 | 3 |
Irregular bleeding rate (%)* | 6 | 6 | 17 | 23 |
*Goldthwaite et al. (2019).24
The copper IUD, the T380A (Paragard) is hormonal free and contains a copper wire wrapper around the stem. It is approved for 10 years, however, studies have demonstrated effectiveness up to 12 years.18 It is slightly larger in size with a frame measuring 32 × 32 mm. Copper is spermicidal, but the copper IUD also alters cervical mucus/endometrial secretions and can impact the endometrium.18 The copper IUD has been associated with heavier periods and more dysmenorrhea particularly in the first 6 months of use. It is an ideal method for teens in whom progesterone is contraindicated, who are amenorrheic due to an eating disorder (to prevent masking resumption of menses), or who prefer a non-hormonal contraceptive option.
Other copper-based IUDs around the world include the following:
- TCu380Ag in Finland.
- TCu380 Slimline in Canada and the UK.
- Mona Lisa NT Mini IUD in Canada and Europe.
- Gynefix in Mexico, Mongolia, Vietnam, Bolivia, Europe, China, Kenya, and Indonesia.
- TCu220C, Nova-T, Multiload-375, Sof-T, Veracept IUD.
See Table 4 for evidence versus myths on the IUD.
4
IUD evidence versus myths. Adapted from Jensen and Creinin (2020).18
| IUD evidence vs myths | IUDs are not abortifacients | |
IUDs do not increase risk of PID | Can be inserted at time of STI screening | |
Prophylactic antiobiotics are not necessary for IUS insertion | ||
IUDs do not cause infertility | ||
IUDs do not increase risk of ectopic pregnancy | ||
IUDs can be placed in nulliparous and adolescent patients | ||
IUDs can be inserted immediately postpartum | ||
IUDs can be inserted in HIV-positive patients |
Quick start IUD
Quick start or same day IUD insertion is important to maximize pregnancy prevention in adolescents. The primary concern related to same day IUD insertion is placement at the time of an unrecognized early pregnancy that is not yet detectable by a pregnancy test. For initiation of IUDs, the Center of Disease Control has recommended six parameters to safely rule out pregnancy (Figure 2);25 however, a recent study demonstrated that these criteria may be too restrictive and may unnecessarily limit the provision of IUDs when patients most want or need them.26 This study demonstrated that there were significant missed opportunities (almost 40%) to place a desired IUD and that luteal phase pregnancies were rare. In adolescents who do not meet the checklist criteria, we encourage providers to engage in a shared decision-making discussion on the risks and benefits of immediate LNG-IUD placement and the utility of both LNG and copper IUDs as effective emergency contraceptive modalities.27

2
CDC’s guideline to determine pregnancy.
Breakthrough bleeding and spotting
Anticipatory guidance and addressing side effects of contraceptive methods is an important modality to promote use and enhance LARC retention. Unscheduled bleeding is a common and frequent side effect of LARCs. Menstrual periods may be heavier or lighter, and some may experience intermenstrual bleeding or amenorrhea. For many women, these bleeding patterns improve over time. Approximately 10 to 15% of patients have their implant with unscheduled bleeding as the most common reason.18 Irregular bleeding and amenorrhea do not indicate that the method is less effective. It is important to rule out pregnancy, particularly after mid-cycle insertions of LARCs, or in patients who also have pregnancy symptoms. Other causes of irregular bleeding include sexually transmitted infection, anatomical uterine conditions (e.g., fibroids or polyps), and interactions with other medications (e.g., anticonvulsants). There are options for short-term treatment of unscheduled bleeding with LARC use:28,29,30,31,32
- Nonsteroidal anti-inflammatory medication.
- Hormonal treatments (oral/transdermal estrogen, combination oral contraceptives).
- Doxycycline and Tranexamic acid (improves bleeding symptoms when compared to placebo, but symptoms tend to recur once treatment is stopped).
Short-acting reversible contraception-SARCs
1. Injectable contraceptive methods
Injectable contraception is widely available and ranges in frequency of administration from 1 to 3 months. Depo Provera, the most commonly used injectable contraceptive, is an injectable form of depot-medroxyprogesterone acetate (DMPA) that is dosed every 13 weeks intramuscularly and released slowly into the blood stream. It can safely be administered early and up to 15 weeks after prior dose without requiring additional contraceptive protection.25 Its efficacy is 0.2% with perfect use, whoever, with typical use, its effectiveness is 4%.18 Other injectable contraception methods include medroxyprogesterone acetate (MPA) administered subcutaneously every 3 months, norethindrone enanthate (NET-EN) administered intramuscularly every 2 months, or a combination of progestin and estradiol monthly.
Injectable contraception is an ideal contraceptive method for patients who require a low maintenance method and desire menstrual suppression. The most significant advantage of injectable contraception over oral contraceptive pills (OCPs) is its longer duration of action with improved effectiveness. Disadvantages particularly associated with DMPA are the need to obtain injections every 3 months, irregular bleeding particularly after the first dose, and prolonged anovulation after discontinuation.18,25 There have been some concerns about weight gain associated with DMPA use. Observational studies have reported variable effects of DMPA on weight gain,33,34,35 but these studies have confounding factors, because individuals tend to gain weight over time irrespective of contraceptive use.36 Additionally, stopping and changing contraceptive methods over time, variability in study design, differing participant characteristic, and inconsistent endpoints make these observational studies inconclusive. It is advisable to counsel patients who tend to gain or struggle with weight that they may gain weight while using DMPA. In a prospective study of 450 adolescent (age 12–18) obese (BMI≥30) girls, those who used DMPA gained significantly more weight than did normal weight girls starting OCPs or placebo. At 18 months, mean weight gain was 9.4 kg for girls receiving DMPA, 0.2 kg for girls using OCPs, and 3.1 kg for control patients.33
In 2004, the Food and Drug Administration (FDA) issued a black box warning about prolonged DMPA use causing significant loss of bone density. This concern has largely dissipated as more recent studies have demonstrated that this decline in bone density is reversible after discontinuation.37,38 To date, there is no long-term data demonstrating negative sequelae of prolonged DMPA use. In 2006, the Society of Adolescent Health and Medicine (SAHM) published their position on DMPA, stating that DMPA can safely be prescribed to adolescents without a restricted duration of 2 years, and decisions regarding bone density monitoring should be individualized .39 The American College of Obstetricians and Gynecologists (ACOG) has since issued a similar recommendation.40
2. The contraceptive vaginal ring
NuvaRing, the original combined hormonal contraceptive ring, is a flexible polymer ring that contains ethinyl estradiol (EE) and ENG that is released in small amounts daily.18 Other bioequivalent forms include MyRing and Ornibel. Its intended use is for 21 days with a 7 day break, similar to OCPs, however, continuous use for menstrual suppression is safe and effective. Studies have demonstrated ovulation prevention for up to 6 weeks.41 For adolescents who prefer to have menstrual cycles, the ring can be removed for 4–7 days to allow for withdrawal bleeding. Its efficacy is 0.3% with perfect use, but with typical use, its effectiveness is 7%.18 The ring is usually placed by the patient in the vagina or can be guided by a health care provider with initial use.
The ring may be removed at the time of sexual intercourse; however, it should be replaced within 3 hours, otherwise, emergency contraception should be recommended. Counseling, initiation, and follow up is much like that of OCPs.
Annovera was approved in the United States in 2018. It is also a vaginal ring that contains EE and segesterone acetate (Nesterone). Segesterone acetate is a potent progestin that is not orally active. Annovera is approved for use over 13 consecutive cycles, with 21 day use and a 7 day break. It does not increase the risk of infection.18
3. Transdermal contraceptives
The transdermal contraceptive patch (Xulane) contains EE and norelgestromin, which is an active metabolite of norgestimate.18 The hormones are contained in its adhesive layer and are continuously absorbed through the skin. The patch is applied to the skin (buttock, abdomen, upper outer arm, upper torso) once a week for 3 weeks. On the fourth week, the patch is removed for 7 days. For patients who have difficulties remembering a daily birth control pill, this method may be a useful option. Its efficacy with perfect use is 0.3%, but with typical use, its effectiveness is 7%.18 Another available patch (Twirla) is used similarly, but contains less EE and LNG as its progestin.
If the patch detaches, patients should be counseled to reapply the patch. If it does not stay attached, or if the adhesive portion of the patch becomes wet, a new patch should be applied. The patch should also be applied to different areas each week to avoid skin irritation.
Those who weigh more than 90 kg (198 pounds) should be informed that their pregnancy risks may be higher on the patch compared people weighing less than 90 kg.42 Patients using the contraceptive patch have an increased exposure to estrogen when compared to those on most OCPs.43 This higher level of estrogen raised concern that there may be an elevated risk of venous thromboembolic events (VTE). A large post-marketing study did not show an increase in VTEs in women who used the contraceptive patch.44 The patch may be started using the QuickStart method much like OCPs. Many contraceptive experts dissuade from using the patch in an extended or continuous use fashion because of the concern for elevated and prolonged estrogen levels.
4. Combined oral contraceptive pills (COCPs)
Four out of five sexually active women in the United States have used the birth control pill and it continues to be one of the most widely used method across the globe.45,46 Most COCPs contain EE with a progestin component, which varies. The dose of EE ranges from 15 mcg to 35 mcg, all of which are considered a “low-dose” of estrogen.18 Its efficacy with perfect use is 0.2%, but with typical use, its effectiveness is 7%.18 The most common regimen is 21 days of hormonal pills followed by 7 days of placebo pills. There are multiple formulations of COCPs:
- Monophasic (all tablets have the same hormonal dose).
- Biphasic/multiphasic (variable doses of hormones).
- Extended regimens (24 days of hormonal pills, 4 day break).
- Extended cycles (tricyclic cycles, continuous cycles).
Because COCPs were initially developed to mimic the normal menstrual cycle, its traditional use (monthly menstrual cycles) is frequently associated with negative hormone-withdrawal symptoms such as bleeding, pain, cramping, mood swings, and headache. Studies have demonstrated numerous benefits of extended and continuous use of OCP regimens.47 These benefits include reduced frequency of premenstrual symptoms, diminished bleeding, improved quality of life, increased contraceptive efficacy due to regimen simplification, reduced costs of sanitary products, and reduced risk for anemia. Continuous use is beneficial in the treatment of abnormal uterine bleeding, dysmenorrhea, endometriosis, heavy menses, suppression of menses, and menstrual associated migraines. Additional benefits of COCP includes treatment for acne and PCOS, and a lower incidence of functional ovarian cysts, benign breast disease, and colorectal cancer.47,48
Emergency contraception
Emergency contraception (EC) is an important component of contraception counseling. A number of EC methods, including hormonal and non-hormonal options, are available on the market (Table 5). EC involves administration within 120 hours after unprotected or under-protected intercourse. Indications for emergency contraception include any situation in which sexual intercourse is unprotected, including reproductive coercion, sexual assault, or contraceptive method failure. Mechanisms are thought to inhibit or delay ovulation, interfere with tubal transport and fertilization, or prevent implantation.
5
Emergency contraception options. Adapted from Jensen and Creinin (2020).18
Plan B | Ulipristal | Cu IUD | LNG IUD | COCPs | |
Effectiveness (%) | 50 | 67 | 99 | Non inferior to Cu IUD* | |
Timing (h) | 120** | 120 | 120 | 120 | 72, with repeat at 12 h |
*Turok et al.
**FDA approved in United States for up to 72 h only.
LNG is a progestin-only EC that is commonly known as Plan B. Its single 1.5 mg dose formulation is taken within a 72 hour window following sexual intercourse per FDA guidelines in the United States, although some studies suggest moderate efficacy up to 120 hours.25,49,50 It works to delay follicular development if given before the onset of the luteinizing hormone (LH) surge. It has a pregnancy rate of 2.2%.51 Studies have shown a decreased effectiveness in a patients with a BMI of over 25.52 Contraception can be safely used immediately after taking LNG as EC.51
A newer and more effective EC called ulipristal acetate (UPA) was approved by the FDA in 2010 and can be taken up to 120 hours after intercourse.25 It is an selective progesterone receptor modulator and works by delaying follicular rupture even if given after onset of LH surge.51 It has a pregnancy rate of 1.4%. Unlike LNG EC, UPA is not affected by elevated BMI and may be a superior choice for patients with an elevated BMI.53 Patients using UPA as EC are advised to wait 5 days after use before starting an ongoing progestin-containing hormonal contraceptive method;51 however, a recent study comparing people who delayed hormonal contraception after UPA intake were more likely to ovulate during the following 5 days in comparison to people who started hormonal contraception immediately.54
The most effective form of EC is the copper IUD. It should be inserted within 5 days after unprotected intercourse, however, studies suggest that it can be effective with insertion for up to 14 days after intercourse.55 In addition to being the most effective EC method with a pregnancy rate of 0–2%,51 the copper IUD can be retained for effective long-term contraception. Its efficacy is not affected by BMI as it acts locally to inhibit sperm motility and it creates an inflammatory environment to sperm, ova, and implantation.56 One limitation is that the use of the copper IUD does require a timely office visit and a provider to insert the device.
A recent randomized study demonstrated that the 52 mg LNG IUD is noninferior to the copper IUD as effective EC.27 This provides an additional option that functions as both EC and effective long-term contraception with retention.
Postpartum and postabortion contraception
After childbirth, ACOG recommends an interval of at least 18 months before a repeat pregnancy.57 Despite this recommendation, 33% of unintended pregnancies are within 18 months of a previous live birth, which is considered a short interval pregnancy.58 Short interval pregnancies are associated with adverse outcomes, including low birth weight infant, preterm delivery, small for gestational age, placental abruption, and uterine rupture if attempting a trial of labor after cesarean section.57,59,60 Postpartum contraception is important to initiate before resuming sexual activity. Approximately 50% of women have resumed intercourse by 6 weeks after delivery.61,62 Adolescents in the postpartum period are particularly at high risk for short interval pregnancy, with more than 50% reporting having intercourse prior to starting any form of birth control.63 For fully breastfeeding people, ovulation occurs in 20% by 12 weeks; for not fully breastfeeding people, ovulation resumes in about 50% by 6 weeks; and for those who are not breastfeeding, ovulation resumes as early as 4 weeks.61
Prompt initiation of contraception after childbirth is critical to prevent unplanned and short interval pregnancies. Contraceptive plans should ideally be discussed at prenatal care visits. Counseling and a full range of contraceptive options should be available at the time of delivery and/or prior to discharge from the hospital. When counseling people about postpartum contraceptive options, a shared decision-making approach should be practiced, meeting the needs of the patient’s contraceptive and non-contraceptive goals. Examples include consideration of ease of use, barriers to access (monthly pharmacy trips), long-term contraception, future pregnancy goals, bleeding patterns, and partner knowledge.
Immediate postpartum contraception, which involves providing a method of contraception prior to discharge from the hospital, has been shown to be safe and effective. The Society of Family Planning has defined the different intervals at when contraception is initiated in the postpartum period (Figure 3).64 Placement of immediate postpartum long-acting reversible contraceptives (LARC), which includes IUDs and implants, has been gaining traction worldwide. Immediate postpartum LARC placement is associated with a higher continued contraception rate at 6 months postpartum in comparison to interval placement.65 Despite its higher expulsion rate in comparison to interval placement, immediate IUD placement has been found to be cost effective given the higher continued use rate and prevention of unplanned pregnancy.66 The current practice is placing an IUD within 10 minutes of placental delivery per ACOG recommendations,12 however, studies have demonstrated that placement up to 48 hours after delivery has similar or even higher retention rates.67 Waiting until the patient’s 6 week postpartum follow up to address contraceptive needs is not advantageous with a postpartum return rate as low as 60% in the United States.68

3
Definitions of contraceptive initiation timing. Reproduced with permission from Whitaker et al. (2017).64
Post abortion LARC is similarly effective and safe. In a study comparing patients who received immediate versus interval post abortion LARC, the interval group was associated with a higher rate of repeat pregnancy and additional abortions.69 Post abortion LARC should be an option offered to all patients receiving an abortion to prevent unwanted pregnancy.
Myths and barriers
The misconceptions about contraception from both adolescents and health care providers create major obstacles to achieving effective contraception for adolescents. Teenagers hold a number of misperceptions about birth control (Figure 4). They may mistakenly believe that they need to undergo a pelvic examination and cervical inspection before starting any method, which is not the case. The fear of weight gain is a common fear of initiating contraception, however, a causal relationship has not been established.70 A fear of compromised growth due to early closure of the growth plate is not true, because by the time menarche is achieved, adolescents have already acquired 95% of their final height. Birth control’s effect on fertility is another common fear among teenagers, although the pregnancy rate between groups who have and have not been on hormonal contraception are similar.71 Although estrogen-based contraception does put people at a higher risk of developing thrombosis, people fail to recognize that the risk of thrombosis in pregnancy is higher than with estrogen-based contraception use.72 They fear the possibility of an IUD or implant negatively affecting their health status in the present or the future (predisposition to infections, cancer, for example). Some adolescents express the fear of associated cancer with hormonal contraception use, however, hormonal contraception has been shown to decrease risk of endometrial, ovarian, and colon cancer, with no effect on breast cancer.47

4
Adolescent’s misconceptions and fears about contraception.
Some providers still hold misconceptions about IUD placement in nulliparous and adolescent patients (Table 4). A survey among providers demonstrated a lack of appropriate training to place an IUD.73 Additionally, the belief that IUDs cause pelvic inflammatory disease and the fear of litigation were associated with lower IUD insertions among providers.73 Barriers found in low to middle-income countries include unavailability of contraceptive supplies and gender-based barriers.74 Religious beliefs can create significant barriers since extramarital sex is considered a sin in some settings and IUDs are considered as "abortifacient" devices.
The cost and access to contraception creates a significant barrier for adolescents. If contraceptive methods are not included in public health systems or if access can only be achieved through parents' health insurance, the fear of disclosure may prevent adolescents from using contraception. Parental consent, depending on the regulations of each country or site of care, may also prevent adolescents from accessing contraception. Making contraception available and affordable is necessary to avoid unwanted and unplanned pregnancies in adolescents. The following video traces the history of the successful efforts of pioneers in Argentina to reduce unplanned pregnancy in adolescents through access to education, contraception and abortion services (Video 1).
1
A History of The Successful Reduction in the Rates of Adolescent Pregnancy in Argentina through Initiation of Comprehensive Reproductive Healthcare.
PRACTICE RECOMMENDATIONS
- Contraceptive counseling is an opportunity to promote a healthy and safe sexual life.
- Non-coercive contraception counseling is paramount to supporting adolescent decisions about family planning.
- Professionals should incorporate the reproductive justice framework when counseling by recognizing mistreatment experienced by marginalized groups and subsequent effects on their reproductive choices; potential unconscious bias that may influence patient counseling; and prioritizing patient values, preferences, and experiences when choosing a contraceptive method.
- Detailed counseling and anticipatory guidance about bleeding expectations is important at the time of contraceptive method initiation to improve continuation.
- Dual method use should always be recommended, which entails using a condom in conjunction with a more effective method of contraception to prevent sexually transmitted infections.
- After a contraceptive method is selected, attention should then be directed to ensure correct method use, continued use, and reliable follow-up care.
CONFLICTS OF INTEREST
Tyson HRA pharmaceuticals (consulted on Adolescent use of over the counter pill)
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
PROFESSIONAL ASSOCIATIONS
- American College of Obstetricians and Gynecologists.
- American Academy of Pediatrics.
- North American Society of Pediatric and Adolescent Gynecology.
REFERENCES
World Health Organization. Adolescent pregnancy. Accessed January 26, 2022. https://www.who.int/news-room/fact-sheets/detail/adolescent-pregnancy. | |
Darroch JE, Woog V, Bankole A, et al. Adding It Up: Costs and Benefits of Meeting the Contraceptive Needs of Adolescents. Published online May 17, 2016. Accessed January 26, 2022. https://www.guttmacher.org/report/adding-it-meeting-contraceptive-needs-of-adolescents. | |
Neal S, Matthews Z, Frost M, et al. Childbearing in adolescents aged 12–15 years in low resource countries: a neglected issue. New estimates from demographic and household surveys in 42 countries. Acta Obstet Gynecol Scand 2012;91(9):1114–8. doi:10.1111/j.1600-0412.2012.01467.x. | |
Global Health Estimates: Life expectancy and leading causes of death and disability. Accessed January 26, 2022. https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates. | |
Were M. Determinants of teenage pregnancies: the case of Busia District in Kenya. Econ Hum Biol 2007;5(2):322–39. doi:10.1016/j.ehb.2007.03.005. | |
Abebe AM, Fitie GW, Jember DA, et al. Teenage Pregnancy and Its Adverse Obstetric and Perinatal Outcomes at Lemlem Karl Hospital, Tigray, Ethiopia, 2018. Biomed Res Int 2020;2020:3124847. doi:10.1155/2020/3124847. | |
Global Accelerated Action for the Health of Adolescents (AA-HA!). Accessed January 29, 2022. https://www.who.int/publications-detail-redirect/9789241512343. | |
Bitzer J, Abalos V, Apter D, Martin R, Black A, Global CARE (Contraception: Access, Resources, Education) Group. Targeting factors for change: contraceptive counselling and care of female adolescents. Eur J Contracept Reprod Health Care 2016;21(6):417–30. doi:10.1080/13625187.2016.1237629. | |
American College of Obstetricians and Gynecologists’ Committee on Health Care for Underserved Women, Contraceptive Equity Expert Work Group, and Committee on Ethics. Patient-Centered Contraceptive Counseling: ACOG Committee Statement Number 1. Obstet Gynecol 2022;139(2):350–3. doi:10.1097/AOG.0000000000004659. | |
Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep 2016;65(3):1–103. doi:10.15585/mmwr.rr6503a1. | |
Ott MA, Sucato GS, Committee on Adolescence. Contraception for adolescents. Pediatrics 2014;134(4):e1257–81. doi:10.1542/peds.2014-2300. | |
ACOG Practice Bulletin No. 121: Long-acting reversible contraception: Implants and intrauterine devices. Obstet Gynecol 2011;118(1):184–96. doi:10.1097/AOG.0b013e318227f05e. | |
Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med 2012;366(21):1998–2007. doi:10.1056/NEJMoa1110855. | |
Peipert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol 2011;117(5):1105–13. doi:10.1097/AOG.0b013e31821188ad. | |
McNicholas C, Madden T, Secura G, et al. The contraceptive CHOICE project round up: what we did and what we learned. Clin Obstet Gynecol 2014;57(4):635–43. doi:10.1097/GRF.0000000000000070. | |
Mestad R, Secura G, Allsworth JE, et al. Acceptance of long-acting reversible contraceptive methods by adolescent participants in the Contraceptive CHOICE Project. Contraception 2011;84(5):493–8. doi:10.1016/j.contraception.2011.03.001. | |
O’Neil-Callahan M, Peipert JF, Zhao Q, et al. Twenty-four-month continuation of reversible contraception. Obstet Gynecol 2013;122(5):1083–91. doi:10.1097/AOG.0b013e3182a91f45. | |
Jensen J, Creinin MD. Speroff & Darney’s Clinical Guide to Contraception, 6th edn. Wolters Kluwer, 2020. | |
Ali M, Akin A, Bahamondes L, et al. Extended use up to 5 years of the etonogestrel-releasing subdermal contraceptive implant: comparison to levonorgestrel-releasing subdermal implant. Hum Reprod 2016;31(11):2491–8. doi:10.1093/humrep/dew222. | |
Learn About Mirena® IUD. Homepage. Accessed January 29, 2022. https://www.mirena-us.com/. | |
Liletta HCP. Accessed January 29, 2022. https://www.liletta.com/. | |
https://www.prnewswire.com/news-releases/fda-approves-medicines360s-liletta-levonorgestrel-releasing-intrauterine-system-52-mg-to-prevent-pregnancy-for-up-to-eight-years-301676741.html. Accessed 8 February 2023. | |
McNicholas C, Swor E, Wan L, et al. Prolonged use of the etonogestrel implant and levonorgestrel intrauterine device: 2 years beyond Food and Drug Administration-approved duration. Am J Obstet Gynecol 2017;216(6):586.e1–6. doi:10.1016/j.ajog.2017.01.036. | |
Goldthwaite LM, Creinin MD. Comparing bleeding patterns for the levonorgestrel 52 mg, 19.5 mg and 13.5 mg intrauterine systems. Contraception 2019;100(2):128–31. doi:10.1016/j.contraception.2019.03.044. | |
Curtis KM. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep 2016;65. doi:10.15585/mmwr.rr6504a1. | |
Castaño PM, Westhoff CL. Experience with same-day placement of the 52 mg levonorgestrel-releasing intrauterine system. Am J Obstet Gynecol 2020;222(4S):S883.e1–6. doi:10.1016/j.ajog.2019.12.268. | |
Turok DK, Gero A, Simmons RG, et al. Levonorgestrel vs. Copper Intrauterine Devices for Emergency Contraception. N Engl J Med 2021;384(4):335–44. doi:10.1056/NEJMoa2022141. | |
Madden T, Proehl S, Allsworth JE, et al. Naproxen or estradiol for bleeding and spotting with the levonorgestrel intrauterine system: a randomized controlled trial. Am J Obstet Gynecol 2012;206(2):129.e1–8. doi:10.1016/j.ajog.2011.09.021. | |
Hou MY, McNicholas C, Creinin MD. Combined oral contraceptive treatment for bleeding complaints with the etonogestrel contraceptive implant: a randomised controlled trial. Eur J Contracept Reprod Health Care 2016;21(5):361–6. doi:10.1080/13625187.2016.1210122. | |
Mansour D, Bahamondes L, Critchley H, et al. The management of unacceptable bleeding patterns in etonogestrel-releasing contraceptive implant users. Contraception 2011;83(3):202–10. doi:10.1016/j.contraception.2010.08.001. | |
Guiahi M, McBride M, Sheeder J, et al. Short-Term Treatment of Bothersome Bleeding for Etonogestrel Implant Users Using a 14-Day Oral Contraceptive Pill Regimen: A Randomized Controlled Trial. Obstet Gynecol 2015;126(3):508–13. doi:10.1097/AOG.0000000000000974. | |
Weisberg E, Hickey M, Palmer D, et al. A randomized controlled trial of treatment options for troublesome uterine bleeding in Implanon users. Hum Reprod 2009;24(8):1852–61. doi:10.1093/humrep/dep081. | |
Bonny AE, Ziegler J, Harvey R, et al. Weight gain in obese and nonobese adolescent girls initiating depot medroxyprogesterone, oral contraceptive pills, or no hormonal contraceptive method. Arch Pediatr Adolesc Med 2006;160(1):40–5. doi:10.1001/archpedi.160.1.40. | |
Mangan SA, Larsen PG, Hudson S. Overweight teens at increased risk for weight gain while using depot medroxyprogesterone acetate. J Pediatr Adolesc Gynecol 2002;15(2):79–82. doi:10.1016/s1083-3188(01)00147-4. | |
Bahamondes L, Del Castillo S, Tabares G, et al. Comparison of weight increase in users of depot medroxyprogesterone acetate and copper IUD up to 5 years. Contraception 2001;64(4):223–5. doi:10.1016/s0010-7824(01)00255-4. | |
Hassan DF, Petta CA, Aldrighi JM, et al. Weight variation in a cohort of women using copper IUD for contraception. Contraception 2003;68(1):27–30. doi:10.1016/s0010-7824(03)00079-9. | |
Harel Z, Johnson CC, Gold MA, et al. Recovery of bone mineral density in adolescents following the use of depot medroxyprogesterone acetate contraceptive injections. Contraception 2010;81(4):281–91. doi:10.1016/j.contraception.2009.11.003. | |
Scholes D, LaCroix AZ, Ichikawa LE, et al. Change in bone mineral density among adolescent women using and discontinuing depot medroxyprogesterone acetate contraception. Arch Pediatr Adolesc Med 2005;159(2):139–44. doi:10.1001/archpedi.159.2.139. | |
Cromer BA, Scholes D, Berenson A, et al. Depot medroxyprogesterone acetate and bone mineral density in adolescents–the Black Box Warning: a Position Paper of the Society for Adolescent Medicine. J Adolesc Health 2006;39(2):296–301. doi:10.1016/j.jadohealth.2006.03.011. | |
American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Committee Opinion No. 415: Depot medroxyprogesterone acetate and bone effects. Obstet Gynecol 2008;112(3):727–30. doi:10.1097/AOG.0b013e318188d1ec. | |
Dragoman M, Petrie K, Torgal A, et al. Contraceptive vaginal ring effectiveness is maintained during 6 weeks of use: a prospective study of normal BMI and obese women. Contraception 2013;87(4):432–6. doi:10.1016/j.contraception.2012.12.001. | |
Zieman M, Guillebaud J, Weisberg E, et al. Contraceptive efficacy and cycle control with the Ortho Evra/Evra transdermal system: the analysis of pooled data. Fertil Steril 2002;77(2 Suppl 2):S13–8. doi:10.1016/s0015-0282(01)03275-7. | |
van den Heuvel MW, van Bragt AJM, Alnabawy AKM, et al. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception 2005;72(3):168–74. doi:10.1016/j.contraception.2005.03.005. | |
Jick SS, Hagberg KW, Hernandez RK, et al. Postmarketing study of ORTHO EVRA and levonorgestrel oral contraceptives containing hormonal contraceptives with 30 mcg of ethinyl estradiol in relation to nonfatal venous thromboembolism. Contraception 2010;81(1):16–21. doi:10.1016/j.contraception.2009.07.004. | |
Daniels K, Mosher WD. Contraceptive methods women have ever used: United States, 1982–2010. Natl Health Stat Report 2013;(62):1–15. | |
Bertrand JT, Ross J, Sullivan TM, et al. Contraceptive Method Mix: Updates and Implications. Glob Health Sci Pract 2020;8(4):666–79. doi:10.9745/GHSP-D-20-00229. | |
ACOG Practice Bulletin No. 110: noncontraceptive uses of hormonal contraceptives. Obstet Gynecol 2010;115(1):206–18. doi:10.1097/AOG.0b013e3181cb50b5. | |
Ruan X, Mueck AO. Oral contraception for women of middle age. Maturitas 2015;82(3):266–70. doi:10.1016/j.maturitas.2015.06.030. | |
Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet 2010;375(9714):555–62. doi:10.1016/S0140-6736(10)60101-8. | |
Piaggio G, Kapp N, von Hertzen H. Effect on pregnancy rates of the delay in the administration of levonorgestrel for emergency contraception: a combined analysis of four WHO trials. Contraception 2011;84(1):35–9. doi:10.1016/j.contraception.2010.11.010. | |
Practice Bulletin No. 152: Emergency Contraception. Obstet Gynecol 2015;126(3):e1–11. doi:10.1097/AOG.0000000000001047. | |
Kapp N, Abitbol JL, Mathé H, et al. Effect of body weight and BMI on the efficacy of levonorgestrel emergency contraception. Contraception 2015;91(2):97–104. doi:10.1016/j.contraception.2014.11.001. | |
Praditpan P, Hamouie A, Basaraba CN, et al. Pharmacokinetics of levonorgestrel and ulipristal acetate emergency contraception in women with normal and obese body mass index. Contraception 2017;95(5):464–9. doi:10.1016/j.contraception.2017.01.004. | |
Banh C, Rautenberg T, Duijkers I, et al. The effects on ovarian activity of delaying versus immediately restarting combined oral contraception after missing three pills and taking ulipristal acetate 30 mg. Contraception 2020;102(3):145–51. doi:10.1016/j.contraception.2020.05.013. | |
I T, Jn S, Eb S, et al. Copper intrauterine device placement 6–14 days after unprotected sex. Contraception 2019;100(3). doi:10.1016/j.contraception.2019.05.015. | |
Hatcher, Robert. Contraceptive Technology, 21st edn. Ayer Company Publishers Inc., 2018. | |
ACOG Committee Opinion No. 762: Prepregnancy Counseling. Obstet Gynecol 2019;133(1):e78–89. doi:10.1097/AOG.0000000000003013. | |
Zapata LB, Murtaza S, Whiteman MK, et al. Contraceptive counseling and postpartum contraceptive use. Am J Obstet Gynecol 2015;212(2):171.e1–8. doi:10.1016/j.ajog.2014.07.059. | |
Schummers L, Hutcheon JA, Hernandez-Diaz S, et al. Association of Short Interpregnancy Interval With Pregnancy Outcomes According to Maternal Age. JAMA Intern Med 2018;178(12):1661–70. doi:10.1001/jamainternmed.2018.4696. | |
Haight SC, Hogue CJ, Raskind-Hood CL, et al. Short interpregnancy intervals and adverse pregnancy outcomes by maternal age in the United States. Ann Epidemiol 2019;31:38–44. doi:10.1016/j.annepidem.2018.12.002. | |
Speroff L, Mishell DR. The postpartum visit: it’s time for a change in order to optimally initiate contraception. Contraception 2008;78(2):90–8. doi:10.1016/j.contraception.2008.04.005. | |
Lewis LN, Doherty DA, Hickey M, et al. Implanon as a contraceptive choice for teenage mothers: a comparison of contraceptive choices, acceptability and repeat pregnancy. Contraception 2010;81(5):421–6. doi:10.1016/j.contraception.2009.12.006. | |
Tocce K, Sheeder J, Python J, et al. Long acting reversible contraception in postpartum adolescents: early initiation of etonogestrel implant is superior to IUDs in the outpatient setting. J Pediatr Adolesc Gynecol 2012;25(1):59–63. doi:10.1016/j.jpag.2011.09.003. | |
Whitaker AK, Chen BA. Society of Family Planning Guidelines: Postplacental insertion of intrauterine devices. Contraception 2018;97(1):2–13. doi:10.1016/j.contraception.2017.09.014. | |
Levi EE, Stuart GS, Zerden ML, et al. Intrauterine Device Placement During Cesarean Delivery and Continued Use 6 Months Postpartum: A Randomized Controlled Trial. Obstet Gynecol 2015;126(1):5–11. doi:10.1097/AOG.0000000000000882. | |
Rodriguez MI, Caughey AB, Edelman A, et al. Cost-benefit analysis of state- and hospital-funded postpartum intrauterine contraception at a university hospital for recent immigrants to the United States. Contraception 2010;81(4):304–8. doi:10.1016/j.contraception.2009.11.002. | |
Blumenthal PD, Chakraborty NM, Prager S, et al. Programmatic experience of post-partum IUD use in Zambia: an observational study on continuation and satisfaction. Eur J Contracept Reprod Health Care 2016;21(5):356–60. doi:10.1080/13625187.2016.1201655. | |
Goldthwaite LM, Cahill EP, Voedisch AJ, et al. Postpartum intrauterine devices: clinical and programmatic review. Am J Obstet Gynecol 2018;219(3):235–41. doi:10.1016/j.ajog.2018.07.013. | |
Langston AM, Joslin-Roher SL, Westhoff CL. Immediate postabortion access to IUDs, implants and DMPA reduces repeat pregnancy within 1 year in a New York City practice. Contraception 2014;89(2):103–8. doi:10.1016/j.contraception.2013.10.014. | |
Gallo MF, Grimes DA, Schulz KF, et al. Combination estrogen-progestin contraceptives and body weight: systematic review of randomized controlled trials. Obstet Gynecol 2004;103(2):359–73. doi:10.1097/01.AOG.0000107298.29343.6a. | |
Girum T, Wasie A. Return of fertility after discontinuation of contraception: a systematic review and meta-analysis. Contracept Reprod Med 2018;3:9. doi:10.1186/s40834-018-0064-y. | |
Gordon CM, Pitts SAB. Approach to the adolescent requesting contraception. J Clin Endocrinol Metab 2012;97(1):9–15. doi:10.1210/jc.2011-1780. | |
Stanwood NL, Garrett JM, Konrad TR. Obstetrician-gynecologists and the intrauterine device: a survey of attitudes and practice. Obstet Gynecol 2002;99(2):275–80. doi:10.1016/s0029-7844(01)01726-4. | |
Chandra-Mouli V, McCarraher DR, Phillips SJ, et al. Contraception for adolescents in low and middle income countries: needs, barriers, and access. Reprod Health 2014;11(1):1. doi:10.1186/1742-4755-11-1. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards programme CLICK HERE)

