This chapter should be cited as follows:
Millischer AE, Grevent D, et al., Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.409543
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 4
Fetal development and maternal adaptation
Volume Editor: Professor Asma Khalil, The Royal College of Obstetricians and Gynaecologists, London, UK; Fetal Medicine Unit, Department of Obstetrics and Gynaecology, St George’s University Hospitals NHS Foundation Trust, London, UK

Chapter
Fetal Magnetic Resonance Imaging
First published: February 2021
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
Ultrasound imaging (US) is the first-line modality to assess normal fetal development and to screen for congenital anomalies. These are essential elements of prenatal care. Although US is convenient, low cost and widely available, it can be limited by several factors, making US incompatible with women’s or doctors' expectations, or subject to misdiagnosis-related legal issues.1,2,3,4,5 Magnetic resonance imaging (MRI) is less limited than US by fetal lie, oligohydramnios, multiple pregnancy, or obesity. It offers low resolution but high-contrast images that can be acquired in true anatomical planes. Therefore, over the past decade, MRI has evolved from a highly targeted tool in selected fetuses (primarily those who required brain evaluation)6,7 to a feasible and reproducible modality for a number of fetal applications.8
However, MRI is not yet a standard screening tool, and should mainly be used to answer specific questions raised by targeted US, or to help refine prognosis in high-risk situations.9,10 MRI can also provide functional information, which may become a useful tool for the assessment of fetal cerebral or placental physiology.11,12 Importantly, the clinician performing and interpreting the MRI should have a clear understanding of previous US findings, and of the relevant fetal anatomy and pathophysiology.13,14
TIMING OF EXAMINATION
There are no contradictions to MRI as early as the first trimester of pregnancy,15,16 although it is typically performed from 20 to 22 weeks' gestation onwards because small fetal size and motion artifacts tend to compromise the exam before 18 weeks. Its intended role in guiding pregnancy management is key; if the purpose is to confirm ominous fetal findings, local laws regarding pregnancy termination may dictate timing. However, questions such as adequate cortical gyration or information about airway impingement at delivery can only be addressed late in pregnancy.
TECHNIQUE
Techniques have evolved dramatically over the past decades. Most fetal MRIs are performed at 1.5 T. Some recent studies have pointed out the potential benefits of stronger magnetic fields (3 T), but the advantages of additional signal intensity and resolution17,18 may be offset by increased artifacts due to fetal movement and by greater radiofrequency power deposition.
Developments in ultrafast acquisition have reduced fetal-motion based artifacts and, thus, the need for fetal sedation. Fast (typically 20–30 s) T2-weighted acquisitions, as in single-shot fast spin echo (SSFSE – GE) or half-Fourier acquisition HASTE (Siemens) and RARE (Brucker), are the mainstay of fetal MRI. These sequences provide good resolution and perform well for surface delineation (due to the hypersignal of water in amniotic and cerebrospinal fluid) and gyration analysis. Echo planar imaging with gradient-echo T2-weighted images (T2*) allows detection of chronic hemorrhagic lesions and calcifications, as hemosiderin deposits and calcifications lead to a loss of signal on sequences with a long echo time (TE).19
T1-weighted sequences have lower spatial resolution and little inherent contrast, owing to the large water content. However, they may complement T2-weighed sequences by detecting lesions that the latter may overlook, such as laminar necrosis and calcified leukomalacia.20 Generally, T1-weighted sequences are performed using gradient echo sequences and are useful for evaluating hemorrhage, the pituitary axis and the thyroid, and the presence of meconium in the large bowel. They can also help to locate the liver in some cases of diaphragmatic hernia.
Diffusion-weighted imaging (DWI) sequences, which can detect ischemic lesions at an early stage, may prove useful in high-risk settings.21 DWI is also useful for locating the kidneys, as functioning renal parenchyma gives a bright DWI signal.22 More generally, functional imaging sequences, especially for CNS indications, include diffusion tensor imaging (DTI) with tractography, single-voxel spectroscopy for brain metabolism, and blood-oxygen-level dependent (BOLD) imaging.12,15,23,24,25,26,27 Functional imaging is also emerging for in vivo assessment of the placenta.11
FETAL ANATOMICAL EVALUATION
Brain
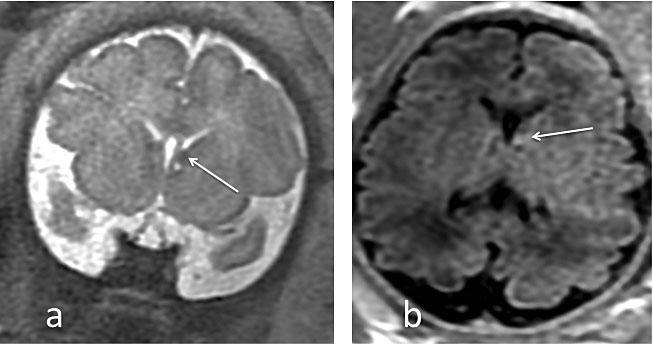
MRI has excellent tissue contrast and can easily distinguish fetal brain structures such as cortical sulcation/gyration, posterior fossa structures, and cerebrospinal fluid spaces. However, because brain development is a dynamic process, it is important to recognize the normal MRI aspect of the brain at different gestational ages in order to avoid false-positive or false-negative diagnoses. For instance, during the second trimester, cerebral malformations may result in morphologic features that differ from those seen in a more mature brain.28,29,30,31 Sulcation patterns should always be analyzed (Figure 1) and measurements should always be compared with published reference ranges.32 Importantly, MRI allows not only the skull, but also the developing brain to be measured, thus avoiding misinterpretations due to abnormal pericerebral spaces.
The high neuron density in the germinal matrix (a cell-dense layer immediately adjacent to the ventricular walls) produces a low signal on T2 and a higher signal on T1-weighted images. Fetal white matter, has the opposite signal characteristics (high T2 and lower T1) because of its higher water content and lack of myelination. Neurons from the germinal matrix migrate from the ventricular wall to the surface of the brain during the second trimester, producing a 5-layered appearance of migrating cells between 20 and 28 weeks of gestation (Figure 1). Myelination mainly occurs after birth, but can be detected in the fetus by high signal intensity on T1-weighted images in certain structures.33
1
Single-shot fast spin echo (SSFSE) T2-weighted images of a normal fetal brain, illustrating the dynamic brain maturation process on coronal planes. (a) 26 weeks of gestation: the germinal matrix (white arrows) is present as hyposignal T2 bands, underlying the wall of the ventricles; (b) 31 weeks of gestation; (c) 36 weeks of gestation: note the development of sulcation. The Sylvian fissure is a major landmark for the assessment of sulcation (white star on the left Sylvian fissure)
Common indications for fetal brain MRI include ventriculomegaly, midline defects, such as agenesis of the corpus callosum, posterior fossa anomalies, cerebral cortical malformations, and screening in a family with history of brain abnormalities such as tuberous sclerosis, corpus callosal dysgenesis or lissencephaly.13,14,34,35 Although most of these pathologies are amenable to US diagnosis, MRI can often improve US diagnosis and refine the prognosis.36
Cortex
MRI of the normal fetal cerebrum is characterized initially by a smooth surface that matures into cortical sulcations. The degree of sulcation on fetal MR images is an indicator of gestational age-related cortical development. The main landmark is the Sylvian sulcus, which is typically developed by 28–30 weeks, and therefore exclusion of cortical malformations cannot be excluded until after this point in gestation.20,37,38 Disorders of cortical development should be suspected in the case of abnormal gyration mimicking the preterm appearance of the gyri. Cortical lesions can be defined by pathophysiological processes, broadly subdivided into under-migration, over-migration, and organizational disorders.39,40,41 Under-migration leads to lissencephaly type 1 (pachygyri and shallow sulci) and gray matter heterotopia (T2 hyposignal foci, underlying the ventricle wall). Heterotopias must be distinguished from subependymal nodules of tuberous sclerosis. Tubers appear hypointense on T2-weighted images and hyperintense on T1-weighted images. Subependymal giant astrocytoma is rare, typically occurring in the wall of the lateral ventricle near the foramen of Monro (Figure 2). Over-migration leads to lissencephaly type 2 (Walker-Warburg Syndrome) with a typical Z-shaped brainstem.42,43,44 Polymicrogyria is due to abnormal organization of neuronal cells, with excessive folding of the cerebral cortical surface, and numerous small and shallow gyri. Associated causes such as infection (commonly cytomegalovirus), genetic abnormalities and ischemia should be suspected.9,10 The most commonly affected area is around the Sylvian fissure.
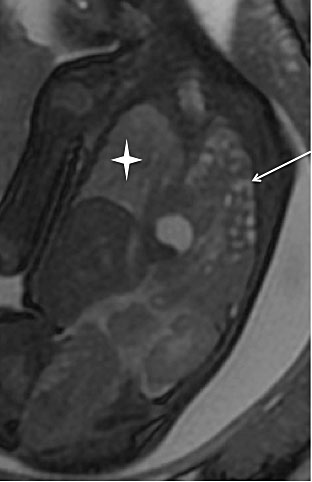
2
Tuberous sclerosis in a 32-week fetus. (a) Coronal single-shot fast spin echo (SSFSE) T2-weighted image: subependymal nodules located on the wall of the ventricles in hypo T2 signal, near the foramen of Monro (thin white arrow); (b) Axial FSE T1-weighted image: Subependymal nodule located typically in the wall of the lateral ventricle, in hyper T1 signal, near the foramen of Monro (white arrowhead)
Ventricles
Like the cortex, the fetal ventricles change during gestation.45,46 Early in gestation, they appear prominent because of the relative paucity of brain parenchyma. Physiologically disproportionate enlargement of the occipital horns in relation to the frontal horns is apparent on fetal MRI until 23 weeks, after which the occipital horns gradually diminish. In the absence of ventriculomegaly, the atrial diameter of normal lateral ventricles remains constant (<10 mm) from 15 to 35 weeks and the lateral borders are concave.47,48
Ventriculomegaly on US is one of the main indications for fetal MRI, which is used to look for associated cerebral lesions.7,14,48,49,50,51,52 The shape of the ventricles can provide important clues: colpocephaly is associated with corpus callosum agenesis and pointed posterior horns with Chiari II malformation.53 Aqueductal stenosis is associated with widened posterior horns and third ventricle, and reduced extracerebral fluid. A potential cause of aqueduct obliteration is hemorrhagic intraventricular material, which can be detected with MRI (T1 hypersignal). Ventriculomegaly due to ischemic or infectious events that cause cerebral atrophy can be associated with porencephaly, which may be seen with MRI.
Midline structures
The septum pellucidum is a thin vertical membrane walled off by the corpus callosum above and the fornix below, separating the frontal horns of the lateral ventricles (Figure 3). The septum pellucidum is in close developmental association with the corpus callosum. During fetal development, the septum pellucidum is filled with cerebrospinal fluid and is referred to as the cavum septi pellucidi.28,54 The corpus callosum appears on midline sagittal T2-weighted images as a C-shaped hypointense structure at the upper margin of the cavum septi pellucidi. It increases in length, width and thickness with gestational age.55,56
3
Mid-sagittal-plane single-shot fast spin echo (SSFSE) T2-weighted images of a normal fetal brain at 31 weeks. The corpus callosum is complete in hyposignal T2 (white arrowhead). Note the posterior fossa, with a normal vermis and the primary fissure (white arrow)
Agenesis/hypogenesis of the corpus callosum (Figure 4) is one of the most common congenital brain malformations suspected upon prenatal US by the presence of a colpocephalic configuration of the occipital horns with parallel orientation of the lateral ventricles, and absence of the septum pellucidum. MRI should be used to rule out associated intra- or extracranial anomalies, such as cortical malformations, parenchymal lesions, and brainstem or cerebellar abnormalities. Lesions such as interhemispheric lipoma and interhemispheric cyst can coexist.55,57,58
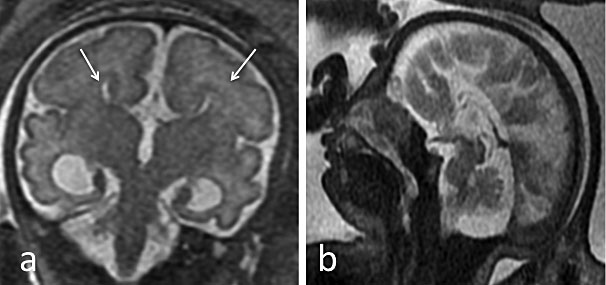
4
Isolated complete agenesis of the corpus callosum in a 31-week fetus. (a) Coronal plane single-shot fast spin echo (SSFSE) T2-weighted image: bilateral bi-convex dimorphic frontal horns (white arrow); (b) Mid-sagittal-plane SSFSE T2-weighted image: no corpus callosum, with typical convergence of the sulci
Posterior fossa
The pontine flexure develops at the same time as the cerebellar hemispheres, and the midsagittal plane is essential to assess the primary flexure after 25 weeks. The transverse cerebellar diameter increases throughout gestation and can be measured on axial and coronal MRI. The vermis can be detected on midsagittal and axial plane MRI after 20 weeks of gestation. The dorsal part of the midbrain and pons has low signal intensity on T2-weighted images and high signal intensity on T1-weighted images after 24 weeks of gestation, due to myelination.28,59,60,61
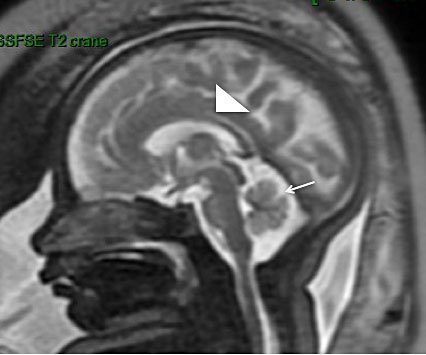
A common reason for MRI referral is an enlarged retrocerebellar fluid space (>10 mm). MRI is better than US for the evaluation and multidimensional analysis of the cerebellum, cerebellar parenchyma, vermis and brainstem.62 For instance, a significant reduction in the transverse cerebellar diameter is seen with cerebellar agenesis or hypoplasia, the cerebellum can be compressed in the case of an arachnoid cyst and the position of the tentorium cerebelli and the shape of the fourth ventricle determine diagnosis of Dandy Walker malformation (Figure 5). Other causes of enlarged CSF-filled spaces include Blake’s pouch cyst, defined as failure of regression of the rudimentary fourth ventricle. The retrocerebellar fluid space may be reduced in the case of Arnold–Chiari malformation, which should raise suspicions of myelomeningocele and prompt further MRI evaluation.63
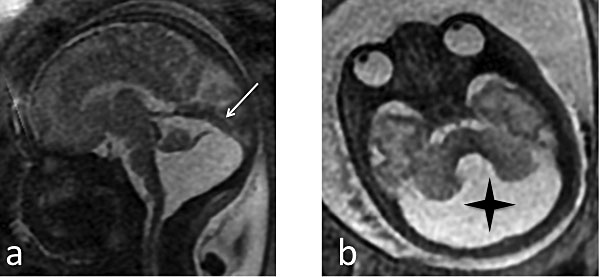
5
Dandy Walker malformation in a 33-week fetus. (a) Sagittal single-shot fast spin echo (SSFSE) T2-weighted image: elevation of the tent of the cerebellum, with the vermis located high and compressed (white arrow). The size and aspect of the vermis will mainly determine the prognosis; (b) Axial SSFSE T2-weighted image: enlargement of 4th ventricle and the posterior cisterna (black star) with absent vermis
Acquired brain lesions
Causes of acquired fetal brain lesions include ischemic infarction, hemorrhage, tumor or infection. The main intracranial signs of prenatal infection are ventriculomegaly, periventricular calcifications, microcephaly, polymicrogyria and cerebellar hypoplasia. MRI can detect cortical lesions and hypersignal in the white matter, specifically the temporal lobe64 and should be performed systematically in cases of proven fetal infection. Ischemic lesions can lead to a reduction in brain tissue, with porencephaly or schizencephaly.65 MRI can diagnose brain injury, including after laser therapy for twin-to-twin-syndrome.9
Hemorrhagic lesions can be detected by T1W and echoplanar sequences.66 Hemorrhagic and parenchymal atrophy may be seen with the most common vascular lesion, vein of Galen malformation.67,68
Thorax and lungs
Bronchopulmonary sequestration, congenital cystic adenomatoid malformation, bronchogenic cyst, congenital high airway obstruction syndrome, and bronchial plug
Because normal fetal lungs and airways are mainly filled with amniotic fluid, they have a relative hypersignal on T2-weighted sequences, allowing easy evaluation of lung volume and anatomy69 (Figure 6). MRI can distinguish cystic spaces from cysts and sequestration in the fetal lung, as well as identify vascular communication with the bronchial tree. The cystic spaces can be distinguished from cysts and sequestration as they communicate with the bronchial tree and are fed and drained by arteries and veins derived from the pulmonary circulation (in contrast with sequestration). Depending on the subtype, with variable sized cysts, their exact appearance can be better described with MRI. In the case of bronchial atresia or plug, segmental or subsegmental occlusion will lead to a dilated T2 hyperintense tubular structure that can be easily seen on MRI. MRI can evaluate pulmonary sequestration, which arises as an accessory pulmonary bud, composed of non-functioning lung tissue with no normal communication with the airways and an abnormal systemic arterial supply.
6
Fetal lung at 28 weeks. Coronal plane: Left isolated congenital diaphragmatic hernia in a 28-week fetus: note the herniation of bowel loops (white arrow), and the undetectable left lung. The right lung is slightly compressed (thick white star)
MRI has several advantages over US alone in the work-up of fetal lung lesions: it can help to confirm a diagnosis, evaluate tracheal anatomy and lung lesion size, detect subtle accompanying anomalies, and improve the detection of chest anomalies.31,70,71
Congenital diaphragmatic hernia
The biggest contribution of MRI is probably in congenital diaphragmatic hernia (Figure 6). Indeed, MRI can help to determine the position of the liver, assess the herniated content, detect commonly associated abnormalities and, evaluate the volume of the normal lung. Indeed, pulmonary volumes reflect the degree of lung hypoplasia and correlate directly with postnatal outcome. Pulmonary volume measurements must be standardized by gestational age, using the lung to head ratio (LHR) and/or volumes reported to expected measurements.72,73,74 MRI may also reveal the presence of a sac around herniated organs, a finding associated with better outcome.75 Finally, MRI may prove useful in the evaluation of lung maturity, based on diffusion-weighted imaging and spectroscopy.76,77
Neck and face
Neck masses are rare, and MRI is particularly helpful for detecting associated brain abnormalities, as well as assessing the extent of lesion to assist in delivery planning (e.g. need for ex-utero intrapartum treatment (EXIT)).78
Gastrointestinal and genitourinary tracts
MRI can improve prenatal diagnosis of esophageal atresia by demonstrating the pouch sign on dynamic sequences. It is also useful for detecting associated malformations, including tracheo-esophageal fistula.79,80,81 Because of its large field of view and better tissue contrast than US, and also because of the T1 hypersignal of meconium, MRI can assist in the evaluation of dilated bowel loops, and differentiation of proximal from distal obstruction (Figures 7 and 8).
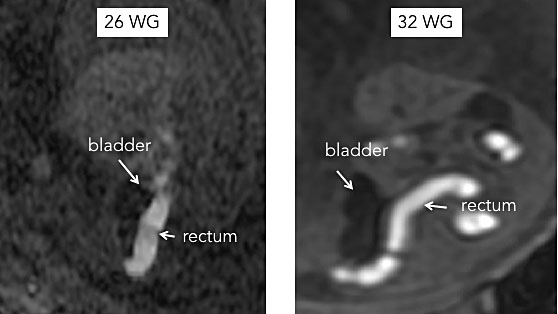
7
Normal bowel: sagittal T1-weighted gradient-echo image. (a) 26-week fetus; (b) 32-week fetus. The colon and rectum can be characterized early in gestation (MRI colonography), based on the high signal arising from meconium (a proteinaceous fluid) within the bowel on T1-weighted sequences after 20 weeks of gestation

8
Distal bowel atresia in a 29-week fetus. (a) Axial single-shot fast spin echo (SSFSE) T2 image: dilated bowel in hyper T2 signal (white star) adjacent to normal bowel (white arrow); (b) Coronal SSFSE T2 image: dilated bowel (white star); (c) Coronal T1 gradient-echo image: microcolon and microrectum in hyperT1 signal (white arrow)
In cases of abdominal wall defects such as gastroschisis or omphalocele the extracorporeal structures may be difficult to visualize by US alone. MRI can assess the size of the wall defect and calculate lung volume. Critically, MRI can be used to screen for omphalocele-associated anomalies that may be overlooked by US.82,83,84
Another application of fetal MRI is to characterize abdominal cystic masses85 such as ovarian cyst. In cystic lymphatic malformations. MRI can also help identify compression of adjacent structures in cystic lymphatic malformations, and identify location of choledocal cyst, intestinal duplication cyst, or renal/suprarenal cystic lesion.85
The most common fetal anomalies involve the kidney and bladder. US is the primary imaging modality, but may be compromised by oligohydramnios, which does not significantly affect MRI. In fact, thanks to multiplanar acquisitions, MRI can be used to analyze the renal parenchyma, the degree and extent of pyelectasis, ureteral dilatation, ureterocele, the posterior urethra in the male fetus, and the digestive tract (Figure 9).86,87
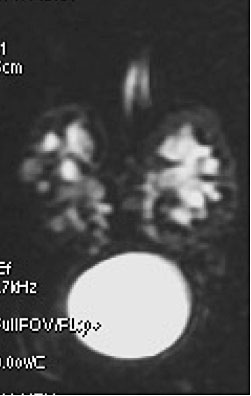
9
Uro MRI in a 33-week fetus: Coronal plane. A specific T2 sequence (HASTE sequence) allows the whole dilated tract to be visualized.
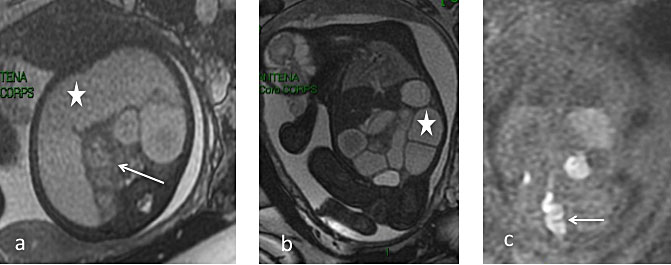
Finally, urogenital anomalies are complex because they often share similar clinical presentations despite markedly different prognoses. MRI can be helpful in evaluating these anomalies when US is unable to differentiate between persistent cloaca, urogenital sinus, and simple genital obstruction, and for abnormalities such as teratoma or anterior myelomeningocele.88,89 Specifically, MRI can differentiate a cloaca from urogenital sinus by validating the presence of the rectum (on hypersignal T1), hydrocolpos, uterus and bladder.102
Heart
Congenital heart defects (CHD) are among the most common congenital anomalies and are often associated with extra-cardiac anomalies.90 Fetal echocardiography remains the gold standard for evaluation, but MRI may detect extracardiac lesions and/or anomalies of brain development.91,92 MRI of the fetal heart requires further technical developments to overcome problems such as the need for gating, fast fetal heart movements, and fetal movements.90
Skeleton
Bones are usually better evaluated by US, but MRI may be useful in the evaluation of skeletal dysplasia when evaluation of cartilage and muscle is desired.6
OTHER APPLICATIONS
Placenta
Because of its wide field of view and high contrast resolution, MRI is mainly useful for analyzing an abnormally invasive placenta, placental tumors, or hematomas. The normal placenta has a low signal on T1-weighted images and a relatively high signal on T2-weighted images. Heterogeneous areas that develop in late pregnancy may correspond to regions of infarction, necrosis or fibrosis.93 Subchorionic hemorrhage can be seen as an increased signal on T1-weighted images. The last few years have seen increasing interest in the use of MRI for the diagnosis of abnormal invasive placenta. Functional MRI has also been used to study vascular particularities of the placenta.
The morbidly adherent placenta (MAP), including previa, accreta and percreta (Figure 10) is defined by a spectrum of abnormal presence of placental tissue throughout the myometrium. Prenatal detection can reduce maternal morbidity by allowing delivery planning.94 However, the exact role of MRI in the management of suspected MAP remains controverisal.95,96,97,98,99 The following features should be sought to document accreta: heterogeneous intraplacental signal intensity on T2-weighted images, focal interruption of the myometrial borders with exophytic placental tissue throughout the myometrium, dark placental bands on T2-weighted sequences, abnormal uterine bulging, visibility of the entire hypointense T2-weighted interface between the myometrium and the placenta, and placental invasion of adjacent organs (suspected in cases of bladder wall irregularity or tenting).99,100 Recently, contrast injection was shown to improve the accuracy of diagnosis, especially by inexperienced radiologists, but should be reserved for cases beyond 30 weeks in which the diagnosis cannot be confirmed or ruled out by anatomical sequences alone.101,102
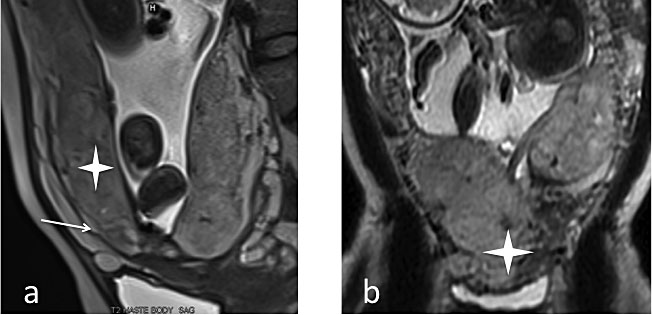
10
MRI of placenta previa. (a) Sagittal plane single-shot fast spin echo (SSFSE) T2: placenta previa without accreta. Myometrium is present (white arrow), with a distinct placenta (white star); (b) Sagittal plane SSFSET2: placenta accreta, with a heterogeneous and bulging placenta; note the lack of a clear demarcation between the myometrium and adjacent placenta (white star).
Recent developments in MRI offer new perspectives for placental imaging, based on functional MRI (fMRI) tools to explore vascularization, oxygenation and metabolism.11 Diffusion-weighted imaging (DWI) and intravoxel incoherent motion (IVIM) MRI studies of the movement of water molecules within tissues provide information on tissue vascularization and structure. The apparent diffusion coefficient (ADC) and the perfusion fraction (f) are significantly decreased in intrauterine growth retardation (IUGR) when compared to normal pregnancy.103 Blood oxygen level dependent (BOLD) and oxygen-enhanced (OE) MRI uses hemoglobin (Hb) as an endogenous contrast agent. Changes in T2* signal intensity can be seen when the oxygenation status of the organ of interest is modified. BOLD MRI could be used as a non-invasive diagnostic tool to detect placental insufficiency and to distinguish healthy fetuses from those with IUGR. Arterial spin labeling (ASL) MRI relies on magnetically labeled water to quantify blood flow and was the first functional MRI technique used to study the human placenta, in 1998.104 It remains a very promising technique.105 Finally, dynamic contrast-enhanced MRI (DCE-MRI) can quantify placental perfusion, permeability and blood volume fractions but requires a gadolinium-based contrast agent, the use of which should be limited to situations where the benefits outweigh the risks. fMRI of the placenta is still evolving, but shows promise for detection and management of placental insufficiency.11,16,103
Virtuopsy
MRI may offer an acceptable alternative to conventional autopsy. Minimally invasive autopsy combines CT/MR imaging with targeted biopsy and has similar accuracy to conventional autopsy for determining the cause of death and detecting major abnormalities. MRI could therefore help to increase autopsy rates for stillbirths and terminations with anomalies.106
Labor and delivery
MRI has been used to image a normal delivery,107 and can be used to measure fetal volumes and weight108 and maternal pelvic dimensions,109 potentially making it useful for monitoring birth,110 although current cost, convenience and access issues make that impractical presently.
SAFETY ISSUES
MRI does not expose the fetus to ionizing radiation, but the magnetic fields and gradients used to build the images are potentially harmful. ACR guidelines state that “present data have not conclusively documented any deleterious effects of MR imaging exposure of the developing fetus" but the number of cases is insufficient for definitive conclusions, especially when higher-strength (greater than 1.5T) magnets are used.111 A large cohort study has shown no adverse effect of MRI.16 The same study as well as the Guidelines for Diagnostic Imaging During Pregnancy and Lactation advise against gadolinium contrast enhancement unless benefits clearly outweigh risks.16,101 Also, the specific absorption rate (the potential for heating of a tissue related to the radiofrequency energy necessary to produce the MR signal) should be closely monitored because system limits are not always programmed for pregnancy. The advantages and safety of fetal MRI, including its impact on long-term outcomes, should be evaluated in large studies.
WHAT THE REFERRING PHYSICIAN NEEDS TO KNOW
MRI has become an increasingly routine prenatal imaging procedure. It may be useful when US suggests, but cannot confirm, an anomaly, and as a tool to provide functional information. The development of open or semi-open magnets, as well as tables capable of handling patients weighing up to 250 kg, will likely broaden obstetric indications. A team approach to the development and interpretation of a fetal MRI program is essential, and, in addition to the obstetric care provider, should involve experts in US imaging, MR imaging, high-risk pregnancy, and pediatric imaging.
PRACTICE RECOMMENDATIONS
- Fetal MRI is not yet standard, and should be used to answer specific questions raised by targeted US, or in certain high-risk situations.
- Indications for fetal MRI have grown recently, and are likely to broaden further in the near future.
- Conducting and interpreting an MRI study requires a clear understanding of the US findings leading to the referral, as well as relevant fetal biometry, anatomy and pathophysiology.
- The advantages of fetal MRI for perinatal care and its impact on long-term outcomes should be evaluated in large prospective studies.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Maxwell C, Glanc P. Imaging and obesity: a perspective during pregnancy. AJR Am J Roentgenol 2011;196(2):311–9. | |
Frates MC, Kumar AJ, Benson CB, et al. Fetal anomalies: comparison of MR imaging and US for diagnosis. Radiology 2004;232(2):398–404. | |
Paladini D. Sonography in obese and overweight pregnant women: clinical, medicolegal and technical issues. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol 2009;33(6):720–9. | |
Racusin D, Stevens B, Campbell G, et al. Obesity and the risk and detection of fetal malformations. Semin Perinatol 2012;36(3):213–21. | |
Fuchs F, Houllier M, Voulgaropoulos A, et al. Factors affecting feasibility and quality of second-trimester ultrasound scans in obese pregnant women. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol 2013;41(1):40–6. | |
Lyons K, Cassady C, Mehollin-Ray A, et al. Current Role of Fetal Magnetic Resonance Imaging in Body Anomalies. Semin Ultrasound CT MRI 2015;36(4):310–23. | |
Lyons K, Cassady C, Jones J, et al. Current Role of Fetal Magnetic Resonance Imaging in Neurologic Anomalies. Semin Ultrasound CT MRI 2015;36(4):298–309. | |
Millischer A, Sonigo P, Ville Y, et al. Standardized anatomical examination of the fetus at MRI. A feasibility study. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol 2013. | |
Stirnemann J, Chalouhi G, Essaoui M, et al. Fetal brain imaging following laser surgery in twin-to-twin surgery. BJOG Int J Obstet Gynaecol 2016. | |
Benoist G, Salomon LJ, Mohlo M, et al. Cytomegalovirus-related fetal brain lesions: comparison between targeted ultrasound examination and magnetic resonance imaging. Ultrasound Obstet Gynecol 2008;32(7):900–5. | |
Siauve N, Chalouhi GE, Deloison B, et al. Functional imaging of the human placenta with magnetic resonance. Am J Obstet Gynecol 2015;213(4):S103–14. | |
Jakab A, Pogledic I, Schwartz E, et al. Fetal Cerebral Magnetic Resonance Imaging Beyond Morphology. Semin Ultrasound CT MR 2015;36(6):465–75. | |
Reddy UM, Abuhamad AZ, Levine D, Saade GR, Fetal Imaging Workshop Invited Participants. Fetal imaging: Executive summary of a Joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, American Institute of Ultrasound in Medicine, American College of Obstetricians and Gynecologists, American College of Radiology, Society for Pediatric Radiology, and Society of Radiologists in Ultrasound Fetal Imaging Workshop. Am J Obstet Gynecol 2014;210(5):387–97. | |
Prayer D, Malinger G, Brugger PC, et al. ISUOG Practice Guidelines: performance of fetal magnetic resonance imaging. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol 2017;49(5):671–80. | |
Girard N, Fogliarini C, Viola A, et al. MRS of normal and impaired fetal brain development. Eur J Radiol 2006;57:217–25. | |
Ray JG, Vermeulen MJ, Bharatha A, et al. Association Between MRI Exposure During Pregnancy and Fetal and Childhood Outcomes. JAMA 2016;316(9):952–61. | |
Victoria T, Jaramillo D, Robertz HAA, et al. Fetal magnetic resonance imaging: jumping from 1.5 to 3 tesla (preliminary experience). Pediatr Radiol 2014;44(4):376–86. | |
Victoria T, Johnson AM, Edgar JC, et al. Comparison Between 1.5-T and 3-T MRI for Fetal Imaging: Is There an Advantage to Imaging With a Higher Field Strength? Am J Roentgenol 2016;206(1):195–201. | |
Salomon LJ, Garel C. Magnetic resonance imaging examination of the fetal brain. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol 2007;30(7):1019–32. | |
Salomon LJ, Garel C. How we do it? MRI of the fetal brain. Ultrasound Obstet Gynecol 2007;30(7):1019–32. | |
Weisz B, Hoffmann C, Ben-Baruch S, et al. Early Detection of Severe Brain lesions After Fetoscopic Laser Treatment in Twin-Twin Transfusion Syndrome by Diffusion weighted Sequence MRI. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol 2013. | |
Savelli S, Di Maurizio M, Perrone A, et al. MRI with diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) assessment in the evaluation of normal and abnormal fetal kidneys: preliminary experience. Prenat Diagn 2007;27(12):1104–11. | |
Wedegartner U, Tchirikov M, Schafer S, et al. Functional MR imaging: comparison of BOLD signal intensity changes in fetal organs with fetal and maternal oxyhemoglobin saturation during hypoxia in sheep. Radiology 2006;238:872–80. | |
Girard N, Chaumoitre K, Confort-Gouny S, et al. Magnetic resonance imaging and the detection of fetal brain anomalies, injury, and physiologic adaptations. Curr Opin Obstet Gynecol 2006;18(2):164–76. | |
Schöpf V, Kasprian G, Brugger PC, et al. Watching the fetal brain at “rest.” Int J Dev Neurosci Off J Int Soc Dev Neurosci [Internet] 2011 Oct 26 [cited 2011 Nov 20]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22044604 | |
Kasprian G, Brugger PC, Weber M, et al. In utero tractography of fetal white matter development. NeuroImage 2008;43(2):213–24. | |
Mitter C, Jakab A, Brugger PC, et al. Validation of In utero Tractography of Human Fetal Commissural and Internal Capsule Fibers with Histological Structure Tensor Analysis. Front Neuroanat 2015;9:164. | |
Saleem SN. Fetal Magnetic Resonance Imaging (MRI): A Tool for a Better Understanding of Normal and Abnormal Brain Development. J Child Neurol 2013;28(7):890–908. | |
Behairy NH, Talaat S, Saleem SN, et al. Magnetic resonance imaging in fetal anomalies: What does it add to 3D and 4D US? Eur J Radiol 2010;74(1):250–5. | |
Prayer D, Kasprian G, Krampl E, et al. MRI of normal fetal brain development. Eur J Radiol 2006;57(2):199–216. | |
Kul S, Korkmaz HAA, Cansu A, et al. Contribution of MRI to ultrasound in the diagnosis of fetal anomalies. J Magn Reson Imaging JMRI [Internet] 2011 Nov 29 [cited 2012 Jan 11]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22127893 | |
Tilea B, Alberti C, Adamsbaum C, et al. Cerebral biometry in fetal magnetic resonance imaging: new reference data. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol 2009;33(2):173–81. | |
Chung HW, Chen CY, Zimmerman RA, et al. T2-Weighted fast MR imaging with true FISP versus HASTE: comparative efficacy in the evaluation of normal fetal brain maturation. AJR Am J Roentgenol 2000;175(5):1375–80. | |
Levine D, Barnes PD, Madsen JR, et al. Fetal central nervous system anomalies: MR imaging augments sonographic diagnosis. Radiology 1997;204(3):635–42. | |
Whitby E, Paley MN, Davies N, et al. Ultrafast magnetic resonance imaging of central nervous system abnormalities in utero in the second and third trimester of pregnancy: comparison with ultrasound. BJOG Int J Obstet Gynaecol 2001;108(5):519–26. | |
Griffiths PD, Bradburn M, Campbell MJ, et al. Use of MRI in the diagnosis of fetal brain abnormalities in utero (MERIDIAN): a multicentre, prospective cohort study. Lancet Lond Engl 2017;389(10068):538–46. | |
Glenn OA, Barkovich AJ. Magnetic resonance imaging of the fetal brain and spine: an increasingly important tool in prenatal diagnosis, part 1. AJNR Am J Neuroradiol 2006;27(8):1604–11. | |
Brisse H, Fallet C, Sebag G, et al. Supratentorial parenchyma in the developing fetal brain: in vitro MR study with histologic comparison. AJNR Am J Neuroradiol 1997;18(8):1491–7. | |
Barkovich AJ, Guerrini R, Kuzniecky RI, et al. A developmental and genetic classification for malformations of cortical development: update 2012. Brain J Neurol 2012;135(Pt 5):1348–69. | |
Striedter GF, Srinivasan S, Monuki ES. Cortical Folding: When, Where, How, and Why? Annu Rev Neurosci 2015;38(1):291–307. | |
Cavallin M, Bery A, Maillard C, et al. Recurrent RTTN mutation leading to severe microcephaly, polymicrogyria and growth restriction. Eur J Med Genet 2018. | |
Ghai S, Fong KW, Toi A, et al. Prenatal US and MR imaging findings of lissencephaly: review of fetal cerebral sulcal development. Radiographics 2006;26(2):389–405. | |
Fogliarini C, Chaumoitre K, Chapon F, et al. Assessment of cortical maturation with prenatal MRI: part II: abnormalities of cortical maturation. Eur Radiol 2005;15(9):1781–9. | |
Righini A, Parazzini C, Doneda C, et al. Early formative stage of human focal cortical gyration anomalies: fetal MRI. AJR Am J Roentgenol 2012;198(2):439–47. | |
Salomon LJ, Chalouhi GE, Stirnemann JJ, et al. Cerebral ventricle width measurements vary in relation to gestational age, fetal gender and cephalometry. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol 2011;37(3):370–1. | |
Salomon LJ, Bernard JP, Ville Y. Reference ranges for fetal ventricular width: a non-normal approach. Ultrasound Obstet Gynecol [Internet] 2007. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17506037 | |
Guibaud L. Fetal cerebral ventricular measurement and ventriculomegaly: time for procedure standardization. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol 2009;34(2):127–30. | |
Guibaud L, Lacalm A. Etiological diagnostic tools to elucidate “isolated” ventriculomegaly. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol 2015;46(1):1–11. | |
Salomon L, Ouahba J, Delezoide AL, et al. Third-trimester fetal MRI in isolated 10- to 12-mm ventriculomegaly: is it worth it? Bjog [Internet] 2006. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16827833 | |
Parazzini C, Righini A, Doneda C, et al. Is fetal magnetic resonance imaging indicated when ultrasound isolated mild ventriculomegaly is present in pregnancies with no risk factors? Prenat Diagn 2012;1–6. | |
Blondiaux E, Garel C. Fetal cerebral imaging–ultrasound vs. MRI: an update. Acta Radiol [Internet] 2012 [cited 2013 Jan 23]. Available from: http://ar.rsmjournals.com/content/early/2012/09/25/ar.2012.120428.abstract | |
Manganaro L, Savelli S, Francioso A, et al. Role of fetal MRI in the diagnosis of cerebral ventriculomegaly assessed by ultrasonography. Radiol Med (Torino) 2009;114(7):1013–1023. | |
Mehta TS, Levine D. Imaging of fetal cerebral ventriculomegaly: A guide to management and outcome. Semin Fetal Neonatal Med [Internet] 2005. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15985390 | |
Cagneaux M, Guibaud L. From cavum septi pellucidi to anterior complex: how to improve detection of midline cerebral abnormalities. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol 2013;42(4):485–6. | |
Raybaud C. The corpus callosum, the other great forebrain commissures, and the septum pellucidum: anatomy, development, and malformation. Neuroradiology 2010;52(6):447–77. | |
Harreld JH, Bhore R, Chason DP, et al. Corpus callosum length by gestational age as evaluated by fetal MR imaging. AJNR Am J Neuroradiol 2011;32(3):490–4. | |
Rossi AC, Prefumo F. Additional value of fetal magnetic resonance imaging in the prenatal diagnosis of central nervous system anomalies: a systematic review of the literature. Ultrasound Obstet Gynecol 2014;44(4):388–93. | |
Glenn OA, Goldstein RB, Li KC, et al. Fetal magnetic resonance imaging in the evaluation of fetuses referred for sonographically suspected abnormalities of the corpus callosum. J Ultrasound Med Off J Am Inst Ultrasound Med 2005;24(6):791–804. | |
Chapman T, Mahalingam S, Ishak GE, et al. Diagnostic imaging of posterior fossa anomalies in the fetus and neonate: part 2, posterior fossa disorders. Clin Imaging 2015;39(2):167–75. | |
Chapman T, Mahalingam S, Ishak GE, et al. Diagnostic imaging of posterior fossa anomalies in the fetus and neonate: Part 1, normal anatomy and classification of anomalies. Clin Imaging 2015;39(1):1–8. | |
Ber R, Bar-Yosef O, Hoffmann C, et al. Normal fetal posterior fossa in MR imaging: new biometric data and possible clinical significance. AJNR Am J Neuroradiol 2015;36(4):795–802. | |
Guibaud L, des Portes V. Plea for an anatomical approach to abnormalities of the posterior fossa in prenatal diagnosis. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol 2006;27(5):477–81. | |
Sudhakaran N, Sothinathan U, Patel S. Best practice guidelines: Fetal surgery. Early Hum Dev 2012;88(1):15–9. | |
Picone O, Simon I, Benachi A, et al. Comparison between ultrasound and magnetic resonance imaging in assessment of fetal cytomegalovirus infection. Prenat Diagn 2008;28(8):753–8. | |
Garel C, Delezoide AL, Elmaleh-Berges M, et al. Contribution of fetal MR imaging in the evaluation of cerebral ischemic lesions. AJNR Am J Neuroradiol 2004;25(9):1563–8. | |
Zizka J, Elias P, Hodik K, et al. Liver, meconium, haemorrhage: the value of T1-weighted images in fetal MRI. Pediatr Radiol 2006;36(8):792–801. | |
Deloison B, Chalouhi GE, Sonigo P, et al. Hidden mortality of prenatally diagnosed vein of Galen aneurysmal malformation: retrospective study and review of the literature. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol 2012;40(6):652–8. | |
Paladini D, Deloison B, Rossi A, et al. Vein of Galen aneurysmal malformation (VGAM) in the fetus: retrospective analysis of perinatal prognostic indicators in a two-center series of 49 cases. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol 2017;50(2):192–9. | |
Recio Rodríguez M, Martínez de Vega V, Cano Alonso R, et al. MR imaging of thoracic abnormalities in the fetus. Radiogr Rev Publ Radiol Soc N Am Inc 2012;32(7):E305–321. | |
Levine D, Barnewolt CE, Mehta TS, et al. Fetal thoracic abnormalities: MR imaging. Radiology 2003;228(2):379–88. | |
Bulas D, Egloff AM. Fetal chest ultrasound and magnetic resonance imaging: recent advances and current clinical applications. Radiol Clin North Am 2011;49(5):805–23. | |
Gajewska-Knapik K, Impey L. Congenital lung lesions: Prenatal diagnosis and intervention. Semin Pediatr Surg 2015;24(4):156–9. | |
Victoria T, Danzer E, Scott Adzick N. Use of ultrasound and MRI for evaluation of lung volumes in fetuses with isolated left congenital diaphragmatic hernia. Semin Pediatr Surg 2013;22(1):30–6. | |
Jani J, Cannie M, Sonigo P, et al. Value of prenatal magnetic resonance imaging in the prediction of postnatal outcome in fetuses with diaphragmatic hernia. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol 2008;32(6):793–9. | |
Zamora IJ, Mehollin-Ray AR, Sheikh F, et al. Predictive Value of MRI Findings for the Identification of a Hernia Sac in Fetuses With Congenital Diaphragmatic Hernia. Am J Roentgenol 2015;205(5):1121–5. | |
Joe BN, Vahidi K, Zektzer A, et al. (1)H HR-MAS spectroscopy for quantitative measurement of choline concentration in amniotic fluid as a marker of fetal lung maturity: inter- and intraobserver reproducibility study. J Magn Reson Imaging JMRI 2008;28(6):1540–5. | |
Cannie M, Jani J, De Keyzer F, et al. Diffusion-weighted MRI in lungs of normal fetuses and those with congenital diaphragmatic hernia. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol 2009;34(6):678–86. | |
Mirsky DM, Shekdar KV, Bilaniuk LT. Fetal MRI: head and neck. Magn Reson Imaging Clin N Am 2012;20(3):605–18. | |
Spaggiari E, Faure G, Rousseau V, et al. Performance of prenatal diagnosis in esophageal atresia. Prenat Diagn 2015;35(9):888–93. | |
Salomon LJ, Sonigo P, Ou P, et al. Real-time fetal magnetic resonance imaging for the dynamic visualization of the pouch in esophageal atresia. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol 2009;34(4):471–4. | |
Hochart V, Verpillat P, Langlois C, et al. The contribution of fetal MR imaging to the assessment of oesophageal atresia. Eur Radiol 2015;25(2):306–14. | |
Brugger PC, Prayer D. Development of gastroschisis as seen by magnetic resonance imaging. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol 2011;37(4):463–70. | |
Danzer E, Victoria T, Bebbington MW, et al. Fetal MRI-calculated total lung volumes in the prediction of short-term outcome in giant omphalocele: preliminary findings. Fetal Diagn Ther 2012;31(4):248–53. | |
Nakagawa M, Hara M, Shibamoto Y. MRI findings in fetuses with an abdominal wall defect: gastroschisis, omphalocele, and cloacal exstrophy. Jpn J Radiol 2013;31(3):153–9. | |
Gupta P, Sharma R, Kumar S, et al. Role of MRI in fetal abdominal cystic masses detected on prenatal sonography. Arch Gynecol Obstet 2010;281(3):519–526. | |
Pico H, Dabadie A, Bourliere-Najean B, et al. Contribution of the foetal uro-MRI in the prenatal diagnosis of uronephropathies. Diagn Interv Imaging 2014;95(6):573–8. | |
Alamo L, Laswad T, Schnyder P, et al. Fetal MRI as complement to US in the diagnosis and characterization of anomalies of the genito-urinary tract. Eur J Radiol 2010;76(2):258–64. | |
Capito C, Belarbi N, Paye Jaouen A, et al. Prenatal pelvic MRI: additional clues for assessment of urogenital obstructive anomalies. J Pediatr Urol 2014;10(1):162–6. | |
Millischer AE, Grevent D, Rousseau V, et al. Fetal MRI compared with ultrasound for the diagnosis of obstructive genital malformations. Prenat Diagn 2017;37(11):1138–45. | |
Wielandner A, Mlczoch E, Prayer D, et al. Potential of magnetic resonance for imaging the fetal heart. Semin Fetal Neonatal Med 2013;18(5):286–97. | |
Khalil A, Bennet S, Thilaganathan B, et al. Prevalence of Prenatal Brain Abnormalities in Fetuses with Congenital Heart Disease: Systematic Review. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol 2016. | |
Jansen FAR, Everwijn SMP, Scheepjens R, et al. Fetal brain imaging in isolated congenital heart defects - a systematic review and meta-analysis. Prenat Diagn 2016;36(7):601–13. | |
Messerschmidt A, Baschat A, Linduska N, et al. Magnetic resonance imaging of the placenta identifies placental vascular abnormalities independently of Doppler ultrasound. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol 2011;37(6):717–22. | |
Sentilhes L, Goffinet F, Kayem G. Management of placenta accreta. Acta Obstet Gynecol Scand 2013;92(10):1125–34. | |
Jauniaux E, Chantraine F, Silver RM, Langhoff-Roos J, FIGO Placenta Accreta Diagnosis and Management Expert Consensus Panel. FIGO consensus guidelines on placenta accreta spectrum disorders: Epidemiology. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet 2018;140(3):265–73. | |
Jauniaux E, Ayres-de-Campos D, FIGO Placenta Accreta Diagnosis and Management Expert Consensus Panel. FIGO consensus guidelines on placenta accreta spectrum disorders: Introduction. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet 2018;140(3):261–4. | |
Jauniaux E, Bhide A, Kennedy A, et al. FIGO consensus guidelines on placenta accreta spectrum disorders: Prenatal diagnosis and screening. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet 2018;140(3):274–80. | |
D’Antonio F, Iacovella C, Bhide A. Prenatal identification of invasive placentation using ultrasound: systematic review and meta-analysis: Prenatal identification of invasive placentation. Ultrasound Obstet Gynecol 2013;42(5):509–17. | |
D’Antonio F, Iacovella C, Palacios-Jaraquemada J, et al. Prenatal Identification Of Invasive Placentation Using Magnetic Resonance Imaging (Mri): A Systematic Review And Meta-Analysis. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol 2014. | |
Lax A, Prince MR, Mennitt KW, et al. The value of specific MRI features in the evaluation of suspected placental invasion. Magn Reson Imaging 2007;25(1):87–93. | |
Millischer A-E, Salomon LJ, Porcher R, et al. Magnetic resonance imaging for abnormally invasive placenta: the added value of intravenous gadolinium injection. BJOG Int J Obstet Gynaecol 2016. | |
Millischer AE, Deloison B, Silvera S, et al. Dynamic contrast enhanced MRI of the placenta: A tool for prenatal diagnosis of placenta accreta? Placenta 2017;53:40–7. | |
Siauve N, Hayot PH, Deloison B, et al. Assessment of human placental perfusion by intravoxel incoherent motion MR imaging. J Matern-Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet 2017:1–8. | |
Gowland PA, Francis ST, Duncan KR, et al. In vivo perfusion measurements in the human placenta using echo planar imaging at 0.5 T. Magn Reson Med 1998;40(3):467–73. | |
Zun Z, Limperopoulos C. Placental perfusion imaging using velocity-selective arterial spin labeling. Magn Reson Med 2018;80(3):1036–47. | |
Thayyil S, Sebire NJ, Chitty L, et al. Post-mortem MRI versus conventional autopsy in fetuses and children: a prospective validation study. Lancet Lond Engl 2013;382(9888):223–33. | |
Bamberg C, Rademacher G, Güttler F, et al. Human birth observed in real-time open magnetic resonance imaging. Am J Obstet Gynecol 2012;206(6):505.e1–505.e6. | |
Kacem Y, Cannie MM, Kadji C, et al. Fetal Weight Estimation: Comparison of Two-dimensional US and MR Imaging Assessments. Radiology [Internet] 2013 [cited 2013 Jun 20]. Available from: http://radiology.rsna.org/content/early/2013/01/15/radiol.12121374.short | |
Zaretsky MV, Alexander JM, McIntire DD, et al. Magnetic resonance imaging pelvimetry and the prediction of labor dystocia. Obstet Gynecol 2005;106(5 Pt 1):919–26. | |
Bamberg C, Deprest J, Sindhwani N, et al. Evaluating fetal head dimension changes during labor using open magnetic resonance imaging. J Perinat Med 2016. | |
Strizek B, Jani JC, Mucyo E, et al. Safety of MR imaging at 1.5 T in fetuses: a retrospective case-control study of birth weights and the effects of acoustic noise. Radiology 2015;275(2):530–537. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards programme CLICK HERE)

