This chapter should be cited as follows:
Vinturache A, Khalil A, Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.411323
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 4
Fetal development and maternal adaptation
Volume Editor: Professor Asma Khalil, The Royal College of Obstetricians and Gynaecologists, London, UK; Fetal Medicine Unit, Department of Obstetrics and Gynaecology, St George’s University Hospitals NHS Foundation Trust, London, UK

Chapter
Maternal Physiological Changes in Pregnancy
First published: February 2021
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
During pregnancy, a woman’s body undergoes significant changes that affect virtually every organ to adapt and support the pregnancy and the development of the growing fetus. The process of adaptation is continuous and dynamic. It begins after conception and continues throughout pregnancy. Most women experience uncomplicated pregnancy, with the anatomy and function of the organ system returning to their non-pregnant state after delivery, with minimal residual effects. In certain circumstances, however, these changes may unmask or worsen a pre-existing condition or disease. Physiological alterations that are part of the evolution in a normal gestation can be easily misinterpreted as disease or functional compromise. Thus, it is essential for the clinicians who provide antenatal care to understand the basic physiological adaptations of each body system related to pregnancy and to be able to differentiate from adaptations that are abnormal.
Overall, pregnancy is a hyperdynamic, hypermetabolic, hypervolemic, hypercoagulable, diabetogenic and low vascular resistance state. As such, the “normal” values of physiological variables in pregnant women may differ from those in the non-pregnancy state and may vary according to gestational age. The range of physiological variables in pregnancy that we use as reference today were defined in studies with small sample sizes performed 1970–1990. Recently, several studies have been undertaken to define the normal values of physiological variables in pregnancy on larger cohorts including pregnant women from different ethnicities.1,2,3 However, there are many aspects of physiological adaptation to pregnancy that require further investigation as our understanding of this complex phenomenon is still incomplete.
PHYSIOLOGICAL ADAPTATION OF THE HEMATOLOGICAL SYSTEM TO PREGNANCY
The physiological adaptation of the hematological system during pregnancy aims to ensure adequate blood supply to the new vascular bed, gravid uterus and its content, safeguard the mother and fetus against blood pressure changes and impaired venous return, compensate for blood loss at delivery, and support fetal hematopoiesis. Hematological adaptation to pregnancy begins as early as 4 weeks' gestation and includes progressive changes in plasma volume and red blood cells (physiological anemia), leukocyte and immunological function, thrombocytes levels, coagulation and fibrinolysis. Physiological changes of the hematological system are mediated by the changes in the hormonal milieu of pregnancy and puerperium.4
Plasma volume
The maternal blood volume increases by 1.5 l during pregnancy, at term being approximately 50% above the non-pregnant level. Of this, 1.0 l is contained within the uterus and maternal blood spaces of the placenta.4 Plasma volume increases by 10–15% at 6–12 weeks of gestation, expands rapidly until 30–34 weeks, after which there is only a modest rise, to an average of 100 ml/kg.5 This increase is mediated by the direct action of progesterone and estrogen on the kidney with release of renin and activation of the renin–angiotensin–aldosterone mechanism, which leads to renal sodium retention and increase in total body water. The activation of the renin–angiotensin system during pregnancy is accompanied by a reduced sensitivity of the vasculature to angiotensin. The competitive relationship between atrial natriuretic peptide (ANP) and the renin–aldosterone system in regulating sodium balance and fluid volume is preserved during pregnancy. ANP induces relaxation of the vascular smooth muscle pre-contracted by angiotensin.6 Although the circulating levels of ANP are decreased during pregnancy, the regulation of ANP secretion takes place around a new volume set-point, whereby blood volume is increased and maintained during pregnancy.7 Taken together, this suggests that the elevation in plasma volume in pregnancy might be in response to an underfilled vascular system resulting from systemic vasodilatation and increase in vascular capacitance, rather than actual blood volume expansion.8 Presence of a fetus is not essential for the hematological changes as an increase in blood volume has been seen in women with hydatidiform mole.9
The plasma volume regresses to about 10–15% above that of the non-pregnant state by the third week postpartum and returns to normal non-pregnant level by the 6th week postpartum.10
Red blood cells
Red blood cell (RBC) mass begins to increase at 8–10 weeks of gestation and steadily rises to 15–20% above non-pregnant levels by the end of pregnancy (average of 250–450 ml) (Table 1). In women who receive iron supplements, the increase in red blood cell mass is higher at term, by about 20–30% compared with the non-pregnant state. Increments in red cell and hemoglobin mass are maximal at 12–28 weeks of pregnancy. Due to a greater expansion of plasma volume relative to the increase in hemoglobin mass and erythrocyte volume there is a modest decrease in hemoglobin concentration, hematocrit and RBC count in healthy pregnant women that is termed physiological or dilutional anemia of pregnancy. The fall in hemoglobin levels is typically by 1–2 g/dl by the late second trimester and stabilizes thereafter in the third trimester, when a reduction in the maternal plasma volume occurs. Despite this hemodilution, there is no significant change in RBC indices, mean corpuscular volume (MCV) or mean corpuscular hemoglobin concentration (MCHC) in a normal pregnancy. The average life span of the RBCs is also slightly reduced.11 Women who take iron supplements have less pronounced changes in hemoglobin compared with those not taking hematinic supplements. The MCV is on average 4 fl higher in iron-replete women, without suggesting folate or vitamin B12 deficiency but decreases to an average of 80–84 fl in those not on iron supplements.12 Increased production of RBCs to meet the demands of pregnancy, with a higher proportion of young larger in size RBCs reasonably explains why there is an increased MCV (Table 1). Anemia in pregnancy is defined as hemoglobin levels of less than 11 g/dl in the first and third trimester and less than 10.5 g/dl in the second trimester.13 A hemoglobin concentration <9.5 g/dl in association with a mean corpuscular volume <84 fl probably indicates iron deficiency or other pathology.14
1
Normal reference range of hematological variables in pregnancy. (Adapted from Cunningham15)
Variable | Non-pregnant adult | First trimester | Second trimester | Third trimester |
Hemoglobin (g/dl) | 12.0–15.8 | 11.6–13.9 | 9.7–14.8 | 9.5–15.0 |
Hematocrit (%) | 35.4–44.4 | 31.0–41.0 | 30.0–39.0 | 28.0–40.0 |
Mean corpuscular hemoglobin (MCH) (pg/cell) | 27–32 | 30–32 | 30–33 | 29–32 |
Mean corpuscular volume (MCV) (μm3) | 79–93 | 81–96 | 82–97 | 81–99 |
Red blood cell count (RBC) (×106/mm3) | 4.00–5.20 | 3.42–4.55 | 2.81–4.49 | 2.71–4.43 |
Red cell distribution (RDW) (%) | <14.5 | 12.5–14.1 | 13.4–13.6 | 12.7–15.3 |
Platelets (×109/L) | 165–415 | 174–391 | 155–409 | 146–429 |
Mean platelet volume (MPV) (μm3) | 6.4–11.0 | 7.7–10.3 | 7.8–10.2 | 8.2–10.4 |
White blood cells (WBC) (×103/mm3) | 3.5–9.1 | 5.7–13.6 | 5.6–14.8 | 5.9–16.9 |
Neutrophils (×103/mm3) | 1.4–4.6 | 3.6–10.1 | 3.8–12.3 | 3.9–13.1 |
Monocytes (×103/mm3) | 0.1–0.7 | 0.1–1.1 | 0.1–1.1 | 0.1–1.4 |
Lymphocytes (×103/mm3) | 0.7–4.6 | 1.1–3.6 | 0.9–3.9 | 1.0–3.6 |
Eosinophils (×103/mm3) | 0–0.6 | 0–0.6 | 0–0.6 | 0–0.6 |
Basophils (×103/mm3) | 0–0.2 | 0–0.1 | 0–0.1 | 0–0.1 |
Ferritin (ng/ml) | 10–150 | 6–130 | 2–230 | 0–116 |
Iron, serum (μg/dl) | 41–141 | 72–143 | 44–178 | 30–193 |
Iron, total binding capacity (TIBC) (μg/dl) | 251–406 | 278–403 | Not reported | 359–609 |
Transferrin (mg/dl) | 200–400 | 254–344 | 220–441 | 288–530 |
Erythropoietin (U/L) | 4–27 | 12–25 | 8–67 | 14–222 |
Folate, serum (ng/ml) | 5.4–18.0 | 2.6–15.0 | 0.8–24.0 | 1.4–20.7 |
Erythropoietin levels increase by 50% in pregnancy due to the higher metabolic oxygen requirement, accounting for a moderate bone marrow erythroid hyperplasia and elevated reticulocyte count (Table 1). There is also an increased transportation of oxygen across the placenta due to the combination of a reduced maternal RBCs oxygen affinity from an elevated 2,3 diphosphoglycerate and a low maternal pCO2.16 Hemoglobin levels are restored to the non-pregnant levels by 4–6 months postpartum.
The normal total body iron content in a non-pregnant woman is about 2 g, with approximately 300 mg more in iron stores.14 Pregnancy is associated with a 2–3 fold increase in the iron requirements for hemoglobin synthesis, fetus, and production of certain enzymes. Approximately 1000 mg of iron supplementation is needed during pregnancy: 500 mg for the development of the maternal red blood cell mass, 300 mg for the fetus and placenta, whereas 200 mg of iron is excreted via the skin, urine and feces. Recommendation is for 30–60 mg of daily supplementation of elemental iron for anemia prevention; more is required for treatment, depending on the degree of maternal anemia. During pregnancy, there is a 10–20-fold increase in folate requirements and a 2-fold increase in the requirement for vitamin B12.
White blood cells
Pregnancy is associated with an increase in white blood cell (WBC) count. The onset of leucocytosis with neutrophilia is as early as the second month of pregnancy and follows an upward trend afterwards. Neutrophil count ranges between 5 and 12,000/μL, levels that may increase to 15,000/μL in the third trimester, and up to 25,000/μL or more in response to the stress of labor and delivery. By 4 weeks postdelivery, the WBC count returns to values similar to healthy non-pregnant women (Table 1).17 Lymphocyte count decreases during the first and second trimesters of pregnancy and increases during the third trimester. Monocyte count increases during pregnancy, especially in the first trimester, but decreases as gestation advances. Thus, the monocyte to lymphocyte ratio is markedly increased in pregnancy.18 There is evidence that monocytes help in preventing fetal allograft rejection by infiltrating the decidual tissue between 7th and 20th week of gestation, through PGE2-mediated immunosuppression.16 Eosinophil and basophil counts do not change significantly during pregnancy.17
Immunological function
Various changes in the immunological function during pregnancy prepare the maternal body to accept the fetal graft. As such, there is a decrease in cell-mediated immunity and an increase in the humoral or antibody-mediated immunity.19,20 Complement factors C3 and C4 are increased in the second and third trimester. C-reactive protein (CRP) level is elevated during pregnancy with further increase during labor and delivery.20,21
The suppression of T-helper 1 and T-cytotoxic 1 cells leads to decreased interleukin-2, tumor necrosis factor, interferon gamma and alpha levels. Up-regulation of T-helper 2 and T-cytotoxic 2 cell function leads to an increase in the levels of Interleukin 4, 6 and 13.22 An increase in the ratio of CD8 to CD4 T lymphocytes and in the levels of immunoglobulin A and G has also been reported.17
Platelets
Pregnancy is a relatively hypercoagulable state with increased platelet activity and consumption. The platelet count tends to fall progressively during normal pregnancy, and it usually remains within normal limits, although it may be slightly lower than in healthy non-pregnant women (Table 1). In 5–10% of women, the count could reach levels of 100–150 × 109 cells/l by term, in the absence of any pathological process.23 The decrease in platelet count during pregnancy occurs particularly in the third trimester. The accepted lower limit of platelet count in late pregnancy is considered 100 × 109 cells.24 Gestational thrombocytopenia is partly due to hemodilution and partly due to increased platelet activation and accelerated clearance.25 It does not typically have complications related to thrombocytopenia and babies do not have severe thrombocytopenia (platelet count <20,000/mm3).24
As with the other blood cells lineages, the platelet volume distribution width increases significantly and continuously as gestation advances. The mean platelet volume becomes a less sensitive measure of the platelet size as pregnancy progresses. Postdelivery the platelet count increases in response to increased platelet consumption during the process of delivery.25
Coagulation and fibrinolysis
Pregnancy is regarded as a procoagulant state and is associated with significant changes in the hemostatic profile. There is a 20–200% increase in the levels of fibrinogen and factors II, VII, VIII, X, XII and vWF, with activation of the coagulation cascade.26 Fibrinogen level increases from about 300 mg/dl pre-pregnancy to approximately 600 mg/dl at term (average 450 mg/dl). The level of coagulation factors XI and XIII decreases.27 Increased levels of coagulation factors are due to increased protein synthesis mediated by the rising estrogen levels.4
Pregnancy is a prothrombotic state. Plasma of pregnant women progressively increases thrombin generation throughout pregnancy26 as well as the levels of fibrinolytic inhibitors such as plasminogen activator inhibitor type 1 and 2 (PAI-1 and 2), and thrombin cleavage products.28 While the anticoagulant protein S and fibrinolysis are reduced, factors V, IX, antithrombin and protein C are relatively unchanged.28,29 The whole blood clotting and bleeding time are unchanged. Activated partial thromboplastin time (aPTT) is usually shorter during pregnancy, by up to 4 s in the third trimester, largely due to the hormonally influenced increase in factor VIII. There are no marked changes in prothrombin time (PT) or thrombin time (TT).30
Overall, the coagulation profile changes aim to prevent postpartum hemorrhage due to thrombus formation in addition to the myometrial contraction. The downside of activation of the coagulation process is the increased risk of venous thromboembolism (VTE). Hypercoagulation persists for the first 3 weeks after delivery and resolves by 6–8 weeks postpartum.30
D-dimer and the erythrocyte sedimentation rate are markedly elevated in pregnancy with typical reference range 10-fold higher in late pregnancy than in early pregnancy or in the non-pregnant state.31 Thus, their diagnostic usefulness during pregnancy is limited. The increase in D-dimers reflects the increase in total fibrin during pregnancy consequent to increased thrombin generation, increased fibrinolysis or a combination of both.32
PHYSIOLOGICAL ADAPTATION OF CARDIOVASCULAR SYSTEM TO PREGNANCY
Pregnancy is associated with significant anatomic and physiological remodeling of the cardiovascular system. Increased circulating maternal blood volume, fetal nutritional requirement, addition of the new placental circulatory system increase the cardiovascular load. The adaptation is mediated by circulating estrogens, progesterone, prostaglandins (PGE1 and PGE2), renin–angiotensin–aldosterone system, nitric oxide, and other vasoactive substances. Whereas in most women the increased demands are met without compromise, pre-existing disease states may impair homeostasis with marked clinical deterioration during pregnancy. The maternal cardiovascular system is further altered during the intrapartum and postpartum period. Furthermore, inadequate maternal hemodynamic adaptation may lead to defective uteroplacental circulation and fetal compromise. Therefore, the maternal cardiovascular system needs to achieve a balance between maternal tolerance and fetal needs.
The cardiovascular parameters that are altered during the pregnancy include plasma and total blood volume, cardiac output and stroke volume, blood pressure and heart rate, systemic vascular resistance and regional blood flow. The hemodynamic alterations along with physical and anatomical changes lead to different findings on cardiac examination and investigations in pregnant as compared to non-pregnant women.
Anatomical and physical changes
The extent of anatomical changes of the cardiovascular system in pregnancy is determined by the configuration of abdomen and thorax, strength of the anterior abdominal wall muscles, and the position and size of the uterus.33 The changes in cardiac and vascular compliance are mediated by the remodeling of the heart and blood vessels that takes place during pregnancy. With the increase in the size of the gravid uterus, the diaphragm is displaced upward, shifting the heart upward and laterally, closer to the chest wall. The ventricular wall mass, the size of the four chambers, myocardial contractility, and cardiac compliance increase.34 Through mechanisms not entirely elucidated, that might involve vascular smooth muscle hypertrophy and softening of the collagen, there is an increase in the compliance of the entire vasculature, with increase in aortic size and compliance as well as venous blood volume and compliance. There is also an increase in capillary permeability, which in conjunction with the decrease in colloid osmotic pressure and the increase in the femoral venous pressure, leads to edema formation which is common during pregnancy.35
Changes in the heart sounds auscultation may be found in up to 90% of pregnant women. A louder and more widely split of first sound is noted from 12 weeks’ gestation and until 2–4 weeks postpartum. This change results from an increased flow across and earlier closure of the mitral valve. Most women have pulmonary and tricuspid valve regurgitation, and approximately a third minor mitral regurgitation.36 During the last 10 weeks of gestation a persistent splitting of the aortic and pulmonary elements of the second heart sound is audible. A third heart sound is present in up to 90% of pregnant women.36,37 During the last two trimesters of pregnancy, more than 90% of women demonstrate a mid-systolic murmur along the left sternal border indicative of increased ejection load of the blood from right ventricle into the pulmonary trunk or from left ventricle into the brachiocephalic arteries.35
Alterations in cardiovascular parameters suggest alterations of the ECG during pregnancy. Most of the ECG changes that occur during pregnancy are due to the physiological adaptations in response to pregnancy, which include shift in the heart position, changes in the spatial arrangement of the chest organs, alterations in the electrical properties of the myocardium under the influence of the sympathetic and hormonal modulation of epinephrine and progesterone.38 The incidence of arrythmias, such as atrial and ventricular ectopics, supraventricular tachycardia, increase during pregnancy.39,40,41,42
Echocardiography during the second and third trimesters shows increased left ventricle systolic and diastolic dimensions and wall thickness, result of increased blood volume and diastolic filling.43 The changes in left ventricle might be accompanied by moderate increase in size of right atrium, left atrium, and right ventricle, as well as increase in left and right ventricles outflow obstruction velocities41,44,45
The findings of cardiac investigations during pregnancy are shown in Table 2.
2
Effects of pregnancy on cardiovascular investigations. (Adapted from Gei and Hankins41 and Kametas NA, et al.43)
Investigation | Changes |
Electrocardiography | Heart rate ↑ 10–15% Right axis deviation of QRS Inverted T-wave in lead III Small Q-wave in lead III and AVF Non-specific ST changes: ST-segment depression and T-wave inversion in the inferior and lateral leads |
Echocardiogram | Trivial tricuspid regurgitation Pulmonary regurgitation Mitral valve regurgitation at term (28%) Increase in left ventricular mass (52%) Increase in left ventricular end-diastolic diameter (12%) Increase in left ventricular end-systolic diameter (20%) Increase in left ventricular posterior wall diameter during diastole (22%) Increase in left ventricular posterior wall diameter during systole (13%) Increase in left intraventricular septum diameter during diastole (15%) Increase in left intraventricular septum diameter during systole (19%) |
Hemodynamic changes
The hemodynamic changes as a result of cardiovascular adaptation in pregnancy are summarized in Table 3. Stroke volume, heart rate, and cardiac output increase, whereas systemic vascular resistance, pulmonary vascular resistance, and colloid osmotic pressure decrease during pregnancy. Among the hemodynamic changes of pregnancy, the most dramatic is the one in the cardiac output, which increases by third trimester to 30–50% above the baseline in the non-pregnant state. These changes occur primarily early in pregnancy, with 50% increase occurring by 8 weeks’ gestation and 75% by the end of the first trimester.46,47 These substantial changes in cardiac output are mediated primarily by the decrease in systemic vascular resistance,45,48 decrease in preload and increase in the stroke volume. cardiac output plateaus between 28 and 32 weeks’ gestation, with not significant changes thereafter until delivery.49 During the third trimester, the increase in heart rate is primarily responsible for maintaining the increase in cardiac output.46 The changes in heart rate and stroke volume occur by 5–8 weeks’ gestation.50 Stroke volume increases by 25–30%, reaching a nadir at 16–24 weeks, to decline towards the non-pregnant levels by term.42,45,48,51 The increase in the stroke volume is mediated by the increase in ventricular mass and end-diastolic volume.50 Cardiac output falls to non-pregnant values in few weeks after delivery. The increase in cardiac output in multiple pregnancies is slightly greater than in singletons and the peak increase is greater (Figure 1).52
3
Physiological changes of the cardiovascular system in pregnancy. (Adapted from Gei and Hankins41 and Costantine53)
Parameter | Direction of change | Magnitude of change | Peak of change |
Myocardial contractility: | |||
Heart rate (HR) | ↑ | ↑ 10–20% from 80 bpm to 90–100 bpm | 28–32 weeks |
Stroke volume (SV) | ↑ | ↑ 30% to a maximum 85 mL | 20–24 weeks |
Cardiac output (HR × SV) | ↑ | ↑ 30–50% from 4.5 l/min to ~6 l/min Plateaus at 24–30 weeks ↓ to pre-pregnancy level after delivery (variable time) | 28–32 weeks |
Systolic blood pressure | ↓ | ↓ 5 mmHg | from 8 to 36 week |
Diastolic blood pressure | ↓ | ↓ 10 mmHg | 24–32 weeks |
Systemic vascular resistance | ↓ | ↓ 20–30% | 16–24 weeks |
Pulmonary vascular resistance | ↓ | ↓ ~30% | 34 weeks |
Pulmonary capillary wedge pressure | ~ | No significant change | |
Colloid osmotic pressure | ↓ | ↓ 14% | |
Uteroplacental circulation | ↑ | Greater than 1000% | Term |
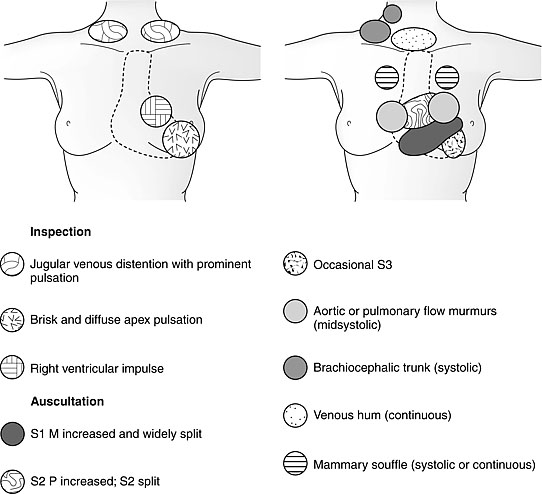
1
Findings of cardiovascular examination in the pregnant women. M, mitral; P, pulmonary; S1, first sound; S2, second sound; S3, third sound. (From Gei and Hankins41)
The heart rate increases by 10–20% (averaging 10–20 beats per minute higher) by 32 weeks’ gestation, probably mediated by an increase in myocardial alpha receptors due to estrogen.36,54 The increase in heart rate in multiple pregnancies occurs earlier and the peak reaches 40% above the non-pregnant level (Figure 2).55
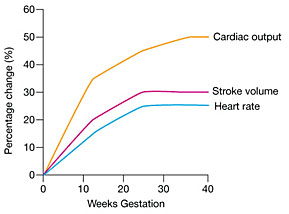
2
Maternal cardiovascular changes in cardiac output, stroke volume, and heart rate during pregnancy. (Adapted from Anesthesia UK, http://www.frca.co.uk/)
Consequential to the vasodilatory effects of progesterone, nitric oxide, and prostaglandins the blood pressure decreases early in pregnancy leading to physiological hypotension. Both, the systolic and diastolic blood pressures are reported to drop by 10–15 mmHg in the first trimester, and return to baseline in the second half of pregnancy.40,45 The decrease in systolic and diastolic blood pressure reaches a nadir by 24–32 weeks’ gestation, returning to non-pregnant state by term. Since the diastolic blood pressure declines more than systolic, the pulse pressure widens (Figure 3).42
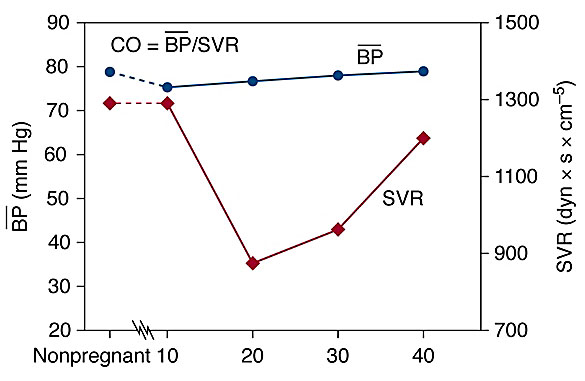
3
Maternal physiological changes in blood pressure and systemic vascular resistance during pregnancy. CO, cardiac output; BP, mean blood pressure; SVR, systemic vascular resistance.
As a result of the increase in blood volume during pregnancy balanced by the increased compliance and capacitance, under the effects of progesterone and nitric oxide acting on vascular smooth muscle and collagen, the venous pressure does not change significantly during pregnancy.56
The total peripheral resistance declines by 20–30% during pregnancy and parallels the decrease in blood pressure. Systemic vascular resistance decreases by 5 weeks, reaches a nadir between 26 and 34 weeks, then progressively increases towards term.45,48 The vascular remodeling during pregnancy is determined by the hypertrophy of vascular smooth muscle, softening of collagen fibers, fluid retention, and addition of the low-resistance uteroplacental circulation.45,57 These changes are potentiated by vasodilatory effects of progesterone, prostaglandins, prostacyclin and nitric oxide (Figure 3).45,57,58,59
Uterine blood flow increases 10 fold from early in pregnancy. The uterus receiving 50 ml/min by 10 weeks and 500 ml/min at term.45 The renal blood flow increases by the end of the first trimester to decrease again towards term. Whereas the renal blood flow increases by 50%, there are minimal alterations to the liver and brain blood flow.60 Nitric oxide, prostacyclin, and atrial natriuretic factor mediate a decrease in the renal vascular resistance. Overall, the proportion of cardiac output perfusing the kidneys remains similar to that in non-pregnant women, around 18%. The increase in renal blood flow leads to a 40–50% increase in the glomerular filtration rate.61 The increase in the pulmonary blood flow is compensated by the decrease in the pulmonary vascular resistance.62 The blood flow to breast tissue, skeletal muscle and splanchnic bed decreases.36 In contrast, the cerebral blood flow remains at baseline proportions, not altered during pregnancy.45,63
Circulating maternal blood volume increases by 30–40% above non-pregnant volumes starting from 6–8 weeks of gestation and peaking at 28–34 weeks.64 This, coupled with the drop in the serum albumin concentration, leads to decreased serum colloid osmotic pressure and hemodilutional anemia. The mechanism of the increase in blood volume is not fully understood. Nitric oxide-mediated vasodilatation and increased arginine vasopressin production and mineralocorticoid activity, may lead to water and sodium retention and hypervolemia.47,65,66 The pregnancy-induced hypervolemia is thought to provide survival advantage to the pregnant women, protecting her from the hemodynamic instability caused by the blood loss at the time of delivery.46,58
Up to 75% of the increase in total blood volume is through the plasma volume. Plasma volume increases by 45–50% compared to non-pregnant values. The increase is rapid from 6 to 8 weeks until the second trimester and progressively slower afterwards. Plasma volume reaches 4700–5200 ml by 32 weeks’ gestation, an increase that is mediated by the renin–angiotensin–aldosterone system via nitric oxide, prostaglandins, estrogen, and progesterone.42,45,48
PHYSIOLOGICAL ADAPTATION OF RESPIRATORY SYSTEM TO PREGNANCY
The physiological changes of the respiratory system in pregnancy aim to increase the volume of air and gas exchange in order to increase oxygen availability and remove carbon dioxide. The alterations in the respiratory system mediated by mechanical, hormonal, and biochemical factors lead to alterations in the acid–base homeostasis, with implications for the mother, fetus and neonate.
Anatomical and mechanical factors
With gradual enlargement of the uterus, the resting position of the diaphragm shifts approximately 4–5 cm upwards. The movement of the diaphragm increases about 2 cm during pregnancy, with a conversion from thoracic to abdominal breathing (Figure 4).55,67 In conjunction with the increase in the intra-abdominal pressure, the relaxation of the muscles and cartilage of the thorax under the influence of relaxin allows broadening of the thoracic circumference by 5–7 cm and the transverse diameter by 2 cm.55,68,69,70 As the pregnancy progresses, the subcostal angle increases from 69° to 103° by 37 weeks. The anatomical changes allow for the shifting of abdominal contents to accommodate the growing uterus.71
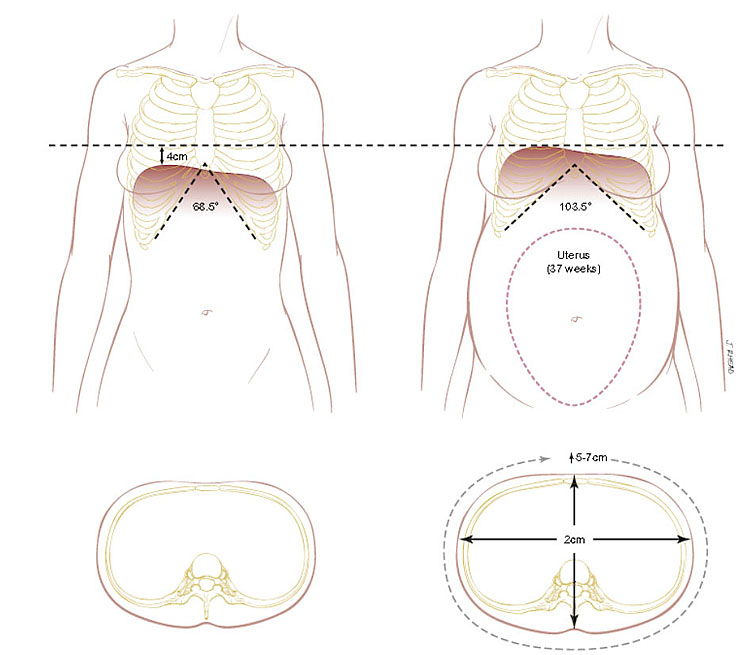
4
Chest wall changes that occur during pregnancy. (Adapted from Hegewaldand Crapo69)
The increase in intra-abdominal pressure and the changes in the thoracic configuration have a major impact on the lung volumes. The upward shift of the diaphragm leads to basilar alveolar collapse, basilar atelectasis, and a decrease in both functional residual capacity and total lung capacity by 10–20%.72,73 The decrease in chest wall compliance results in a decrease in residual volume.
The changes in the upper respiratory tract are a result of capillary engorgement and changes in the airways caliber that affect the nasopharynx, larynx, trachea, and bronchi.69,74 These effects are mediated by hormonal changes, with an increase in estrogen, increase in blood volume, edema, hyperglandular secretion, and increase in phagocytes activity.75 Progesterone and increased blood volume contribute to vascular pooling. Smooth muscle relaxation under the action of progesterone contributes further to the vascular pooling. The mucosa is erythematous and swollen throughout the upper airways.76 This contributes to the difficulty in nasal breathing, spontaneous epistaxis, hoarsened voice, and symptoms of nasal congestion and rhinitis, increase in snoring and exacerbation of obstructive sleep apnea.77 Minor upper respiratory tract infections can markedly aggravate the above symptoms, increasing the discomfort women may experience.
Lung volume and function
Changes in lung volume start early in the first trimester and progress through the second trimester towards term.78 Pregnancy is associated with increase in tidal volume by 30–50%, (from 500 to 700 ml) and progressive decrease in expiratory reserve volume by 15–20%, residual volume by 20–25%, and functional residual capacity by 20–30%.33,78 The decrease in residual volume by 200–300 ml and in expiratory reserve volume by 200 ml leads to a deficit in functional residual capacity of approximately 500 ml.67,68 While the respiratory rate is not different compared to non-pregnant state, minute ventilation is significantly increased (30–50%).79,80 The increase in tidal volume is accompanied by an increase in inspiratory capacity by 5–10%, which allows the total lung capacity to remain stable. Vital capacity (VC) and inspiratory reserve volume are unaltered, while the physiological dead space increases by 60 ml. Forced expiratory volume in 1 sec (FEV1) and the ratio FEV1/VC does not change. Peak expiratory flow rates and peak inspiratory flow rates tend to decrease in pregnancy (Figure 5).67,68,78,81
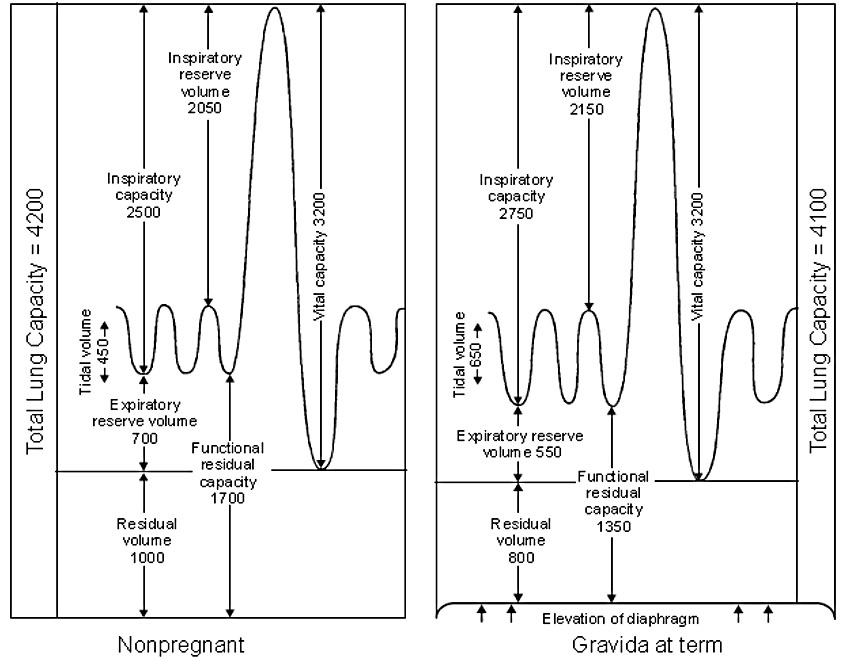
5
Lung volume changes in pregnancy (ml). (From Gardner and Doyle78)
The changes in lung function involve alterations in ventilation, airflow and diffusing capacity.68 The changes in the minute ventilation start in early pregnancy, from approximately 8 weeks’ gestation. The increase of 30–50% represents an increase from 6.5–7.5 l/min in early pregnancy to 10–10.5 l/min at term.71 The increase in minute ventilation is due to changes in tidal volume rather than changes in the respiratory rate which remains relatively stable (Figure 5).67,69,75 Airway resistance and lung compliance remain unchanged during pregnancy.
Pregnancy is associated with an increase in basal metabolism, oxygen consumption and carbon dioxide production. The increase in oxygen consumption in a singleton pregnancy is usually 15–20%, from 32 to 58 ml/min. The increase can be even higher in multiple pregnancies, in order to meet the increased demand placed on the mother by the growing fetus.71 Carbon dioxide production increases by 30% due to an increase in cholesterol and fat metabolism.82,83
Pregnant women are in a state of compensated respiratory alkalosis, due to an increase in paO2 and fall in paCO2. The arterial partial pressure of oxygen (paO2) is increased from 90–100 mmHg to 104–108 mmHg and arterial partial pressure of carbon dioxide (paCO2) decreased from 35–45 mmHg to 27–32 mmHg. The decrease in paCO2 creates a gradient between the paCO2 of the mother and fetus, which allows CO2 to diffuse freely from the fetus, through the placenta, and into the mother, where it can be eliminated through the maternal lungs.46 Maternal arterial blood pH is slightly increased from 7.35–7.45 to 7.40–7.45, consistent with mild respiratory alkalosis that facilitates the transfer of carbon dioxide from the fetus to the mother.53
This alkalosis is partially corrected by increased renal excretion of bicarbonate, leading to reduced serum bicarbonate level from 22–26 mEq/L to 18–21 mEq/L and reduced buffering capacity.46,79 This partially compensated respiratory alkalosis slightly shifts the oxygen-hemoglobin dissociation curve to the right, thereby favoring dissociation of oxygen to enhance oxygen release to the fetus and transfer across the placenta (Figure 6).72 The normal arterial blood values in pregnancy at sea level are summarized in Table 4.
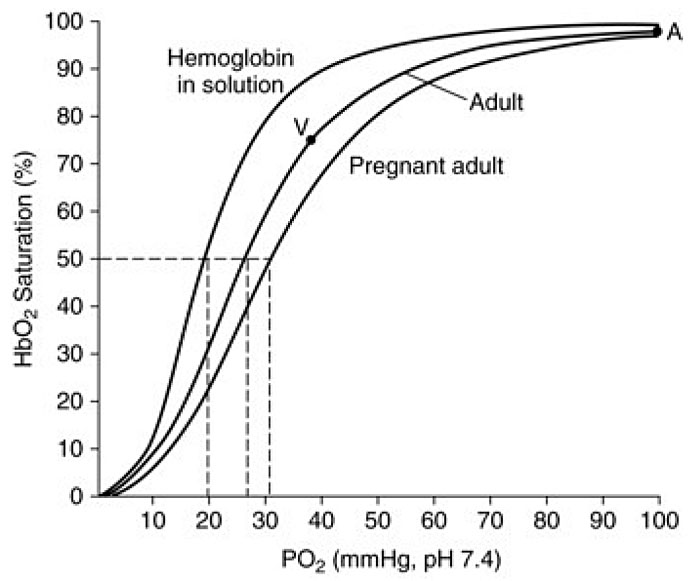
6
Oxygen–hemoglobin dissociation curve. The graph shows the curve for pregnant and non-pregnant adult in normal conditions and the dissociation curve of hemoglobin in solution. (Adapted from Delivoria-Papadopoulos et al.84)
Arterial blood gas measurement | Non-pregnant state | Pregnancy |
pH | 7.35–7.45 | 7.40–7.45 |
paO2 (mmHg) | 90–100 | 104–108 |
paCO2 (mmHg) | 35–45 | 27–32 |
Serum HCO3 (mEq/l) | 22–26 | 18–21 |
All the adaptive respiratory changes that occur make breathing easier for the mother, increase excretion of HCO3 through kidneys, and increase oxygen availability, for the benefit of the developing fetus.
Hormonal and biochemical changes
Progesterone is a respiratory stimulant and appears to mediate the changes in ventilation throughout pregnancy by increasing minute ventilation and enhancing the response to hypercapnia through lowering the carbon dioxide threshold of the respiratory centers. These mechanisms may explain the sensation of dyspnea and hyperventilation experienced by many women.68 Progesterone causes water retention in the lung which leads to a decrease in the diffusion capacity, which in turn leads to hyperventilation to maintain paO2 levels.67 By relaxing the bronchiolar smooth muscle, progesterone decreases the airway resistance which facilitates the airflow and work of breathing.33 The bronchiolar smooth muscles are regulated by prostaglandins, with PGF2α being a constrictor whereas PGE1 and PGE2 are bronchodilators. Prostaglandins contribute to respiratory adaptations by increasing tidal volume and lowering paCO2.
PHYSIOLOGICAL ADAPTATION OF THE RENAL SYSTEM TO PREGNANCY
Pregnancy affects all aspects of the urinary tract as early as 6 weeks after conception. Anatomical changes, with increase in size of the kidneys and dilatation of the urinary collecting system involving calices, renal pelvis, and ureters have been long appreciated.87,88 Renal and systemic hemodynamics are characterized by vasodilation and volume expansion, following alteration of the renal vascular and tubular responsiveness to the circulating hormones.89 The functional impact of pregnancy on kidney physiology is widespread, involving all aspects of kidney function: hemodynamics, glomerular filtration, and tubular handling of water, electrolytes, and other substances and alterations of the renin–angiotensin system (RAS).88 Alterations of the renal system during pregnancy are summarized in Table 5.
5
Renal hemodynamic and functional adaption of the renal system in normal human pregnancy. (Adapted from Blackburn33)
Parameters | Change | Significance |
Collecting system (calyces, pelvis, and ureters) | Dilatation (predominant on the right) Hypertonicity, decreased motility, elongation | Hydronephrosis Increased risk of urinary tract infections Altered accuracy of 24 hours urine collection |
Kidney | Size increase by 1 cm | |
Bladder | Decreased tone Increased capacity Hyperemic and edematous mucosa Displaced in late pregnancy Incompetence of vesicoureteral valve | Urinary frequency and incontinence Altered accuracy of 24 hours urine collection Increased risk of urinary tract infection Risk of trauma and reflux Risk of urinary retention and inadequate emptying |
Renal blood flow (RBF) | Vasodilation Increase by 50–80% by second trimester then decrease to term | Increase in renal plasma flow (RPF) Increase in glomerular filtration rate (GFR) Increase solute delivery to the kidney |
Glomerular filtration rate (GFR) | Increases by 40–60% | Inadequate assessment of creatinine clearance due to altered accuracy of urine collection Increased filtration and excretion of water and solutes Predominant natriuretic effect Increased urine volume and flow Decreased serum creatinine, blood urea nitrogen, uric acid Altered renal excretion of drugs |
Renal tubular function | Increased reabsorption of solutes Increased renal excretion of proteins, amino acids, glucose, urea, uric acid, calcium, phosphate, bicarbonate, hydrogen ions, water-soluble vitamins Retention of sodium and water | Maintain homeostasis with expanded extracellular volume Prevent solute and water loss Tendency to proteinuria, glucosuria Decreased serum bicarbonate levels Compensate for respiratory alkalosis Accumulation of sodium and water Increased nutritional needs, including calcium and water-soluble vitamins |
Renin–angiotensin–aldosterone system | Upregulation of all components of the system Reduced sensitivity to the pressor effects of angiotensin II (ANG II) | Decrease in systemic and renal vascular resistance Retention of water and sodium Reduced excretion of sodium Maintain the blood pressure |
Arginine-vasopressin and osmolarity regulation | Osmostat reset at lower baseline of osmolarity | Decrease in plasma osmolarity Expansion of plasma and extracellular volume Maintain volume homeostasis |
Anatomical changes
The kidneys are displaced in a cephalad direction by the enlarging uterus. The length of the kidneys also increases by 1–1.5 cm during pregnancy and decreases to pre-pregnancy measurements over 6 months postpartum.90 Renal volume increases by 30%.91,92 The increase in size of the kidney is probably due to increased vasculature, interstitial volume and dead space.93 Although this growth was initially attributed to hydronephrosis, some studies showed growth in kidney volume in women without evidence of renal pelvic dilatation.94
The renal collecting system dilates from the first trimester, as early as 6 weeks gestation, under the effects of progesterone and compression of the ureters at the pelvic brim.95,96 These changes affect renal pelvis and the upper portion of the ureters to the pelvic brim.96 Hydroureteronephrosis is more common on the right side due to a combination of mechanical compression from the enlarging, dextrorotated uterus, and progesterone-mediated smooth muscle relaxation.97 The sigmoid colon contributes to the dextroversion of the uterus and increases the ureteral compression on the left side in the last trimester.45,92 Compression of the ureter at the pelvic brim is maximal by around 30 weeks’ gestation. Dilatation of the ureteral lumen is associated with hypertonicity, hypomotility, and reduction of peristaltic movements mediated by PGE2.98 As pregnancy progresses, the elongated and tortuous ureters are laterally displaced by the growing uterus.98,99
Under the influence of progesterone, the bladder tone decreases and its capacity increases, almost doubling at term. Increased estrogen mediates hyperplasia and hypertrophy of bladder smooth muscle. The bladder mucosa becomes hyperemic and edematous, with increased vulnerability to infection and trauma.71 The bladder is displaced superiorly and anteriorly by the second trimester, which also alters the position of the intravesical portion of the ureters, increasing the risk of vesicoureteral reflux.99 Pregnant women have increased urinary frequency, urgency, and incontinence.71 These symptoms are potentiated in the third trimester by the fetal head engaged in the pelvis. The production of urine increases. Following alterations in sodium excretion, the mean urine output increases from 1475 to 1919 ml/24 hours. The number of voids per day also increases.99
Renal hemodynamics
Renal hemodynamic changes are related to the systemic vasodilatation and decrease in systemic vascular resistance and are carefully coordinated by several hormones. Renal blood flow (RBF) increases by 50–80% by the mid-second trimester and decreases slightly to term. The renal vascular dilatation and increase in the blood flow delivered to the kidney leads to an increase in glomerular filtration rate (GFR) and effective renal plasma flow (RPF).45,100,101 Effective renal plasma flow increases by 60–80% by mid-pregnancy.93,102 Although the increase in RPF parallels the increase in GFR, the increment in RPF surpasses those of the GFR in early pregnancy (60–80% versus 40–60%) until the RPF falls rapidly in the third trimester.101 As such, the ratio between GFR and RPF, the filtration fraction is altered throughout pregnancy.100,101 The filtration fraction is decreased in the first half of pregnancy and increases in late gestation (Table 5 and Figure 7).101,103
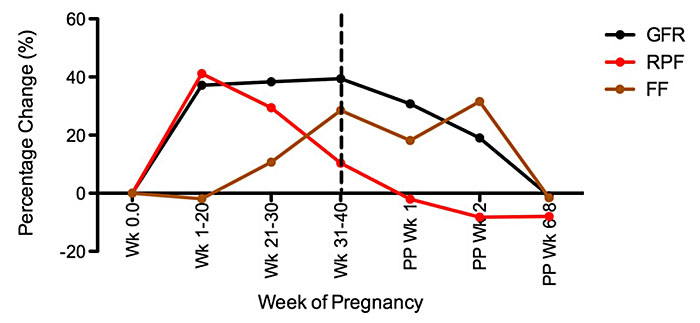
7
Percentage change from baseline of glomerular filtration rate (GFR), renal plasma flow (RPF), and filtration fraction (FF) throughout pregnancy and postpartum. Mean values are measured by inulin or isothalamate and p-aminohippurate clearance, respectively, at different points during gestation and postpartum (PP). (From Odutayo and Hladunewich101)
The changes in renal hemodynamics are under local and systemic hormonal influences. Progesterone can determine an increase in RPF and GFR during pregnancy but cannot account for the magnitude of changes in the two renal variables that occur during pregnancy. Maternal blood relaxin, secreted from the placenta and deciduas, shows a steady increase during pregnancy and decrease in the postpartum period.104 The relaxin contribution to renal vasodilation in humans remains unclear. Although no significant correlations have been found between relaxin levels and renal hemodynamic variables in gestation or postpartum, it was hypothesized that relaxin may mediate vasodilatation through an increase in nitric oxide production via upregulation of vascular gelatinase activity, which acts through the endothelial endothelin B receptor-nitric oxide pathway.104,105 In spite of upregulation of the renin-angiotensin system in pregnancy, the pregnancy is characterised by a state of vasodilation and decrease in systolic blood pressure.106 The insensitivity to increased levels of ANG II may be explained by the presence of hormones and other vasoactive factors, including progesterone, vascular endothelial growth factor, prostacyclins, nitric oxide as well as decreased responsiveness of the of angiotensin 1 (AT1) receptors.107 Other factors that may play a role include prostacyclin, atrial natriuretic factor (ANF), human placental lactogen and human chorionic gonadotropin.104
Changes in glomerular function
The GFR increases by 40–60% by the end of the first trimester, peaking at 180 ml/min.108 The increase in GFR begins at around 6 weeks’ gestation, preceding plasma volume expansion and the increase in the cardiac output. The GFR level peaks at 16 weeks and is maintained until 36 weeks’ gestation. GFR values, measured by creatinine clearance, average 110–150 ml/min during pregnancy.101,103 Creatinine clearance increases by 25% by 4 weeks’ gestation, and by 45% at 9 weeks’ gestation.45 The increase in GFR is probably secondary to vasodilatation of afferent and efferent arterioles and mediated by the increase in glomerular blood flow, glomerular capillary hydrostatic pressure, and the decrease in colloid osmotic pressure following the decrease in plasma proteins.53,108 Whereas the contribution of other GFR determinants is not clear in the first and second trimester, some studies have suggested that elevated GFR at 36 weeks may be due to a combination of increased RPF, decreased oncotic pressure and increased ultrafiltration coefficient.109
As result of the increase in GFR and changes in electrical charge selectivity of the glomerular membrane, there is increased urinary excretion of protein and albumin.88
The increase in GFR increases the concentration of solutes and volume of fluid delivered into the tubular lumen by 50–100%.110 As such, tubular reabsorption increases to avoid depletion of sodium, potassium, chloride, glucose, and water, with retention of these substances.33 Changes in glomerular function are summarized in Table 5 and Figure 7.
Changes in tubular function
During pregnancy there are alterations in tubular handling of waste and nutrients. The excretion of urea, amino acids, and proteins increases in pregnancy. Increased urea clearance in pregnancy leads to decreased plasma urea nitrogen levels by 8–10 weeks’ gestation, representing 63% of non-pregnant values by third trimester.111 Filtration of uric acid increases up to 30% by 16 weeks gestation, that is accompanied by decreased reabsorption and increased secretion due to increased GFR. As a result serum uric acid levels decrease by 25–35% (2–3 mg/dl) by 22–24 weeks,45,88 followed by gradual rise to normal by term. The increased clearance of uric acid is necessary to handle the increased production from placenta and fetus.
In normal pregnancy there is an increase in total urinary protein and albumin excretion, especially after 20 weeks. An average, from <100 mg up to 200–300 mg protein is excreted daily in a normal pregnancy.112 The proteins excreted in urine during pregnancy include mostly Tamm-Horsfall glycoprotein, with a small amount of albumin and other circulating proteins.88 There is marked day-to-day variation, with some evidence that the amount of albuminuria increases in late pregnancy, albeit the levels that do not exceed the upper limit of normal.113 Values of proteinuria higher than 300 mg/24 hours are considered abnormal, although protein excretion does not correlate with the severity of the renal disease.88,110,114 The mechanism of proteinuria in pregnancy is not fully understood, being only partially attributable to the rise in GFR and selective alterations in glomerular charge in the third trimester.112 Circulating soluble antiangiogenic factors (i.e. soluble fms-like tyrosine kinase 1 (sFlt-1) and vascular endothelial growth factor (VEGF) levels increase late in normal pregnancy and may explain late term elevations in proteinuria by disruptions in the podocyte slit diaphragm.115
Glucosuria is common in pregnancy and is thought to reflect the increased filtered load of glucose that exceeded the maximal tubular reabsorptive capacity. Increased GFR combined with less effective reabsorption of glucose overwhelm the capacity of the proximal tubule, loop of Henle, or collecting ducts, thus leading to glucosuria with normoglycemia or a physiological glucosuria.45,88,116 There is variability in glucose excretion, urinary glucose values reaching anywhere between 10 and 100-fold higher than the non-pregnant values (Table 6).45,88,116
6
Laboratory values of renal function in pregnancy. (Adapted from Blackburn33)
Variable/test | Non-pregnant values | Pregnant values | Values in pregnancy that require further investigation | Average values in pregnancy |
Plasma creatinine | 0.65 ± 0.14 mg/dl | 0.46 ± 0.13 mg/dl | >0.80 mg/dl | 0.5 mg/dl |
Creatinine clearance | 85–120 ml/min | 10–150 ml/min | ||
Blood urea nitrogen | 13 ± 3 mg/dl | 8.7 ± 1.5 mg/dl | >14 mg/dl | 9.0 mg/dl |
Plasma urate | 4–6 mg/dl | 2.5–4 mg/dl | >5.8 mg/dl | 2–3 mg/dl |
Serum sodium | 136–146 mEq/l | 133–148 mEq/l | <128 mEq/l | 135 mEq/l |
Serum potassium | 3.5–5.0 mEq/l | 3.6–5.0 mEq/l | >5.1 mEq/l | 3.8 mEq/l |
Serum chloride | 102–109 | 97–109 | >110 mEq/l | 103 mEq/l |
Serum bicarbonate | 27–28 mEq/l | 20–22 mEq/l | <20 mEq/l | 18–20 mEq/l |
Plasma osmolality | 275–295 mOsm/kg H2O | 276–289 mOsm/kg H2O | >290 mOsm/kg H2O | 270 mOsm/kg H2O |
Urinary glucose | 20–100 mg/24 h | >100 mg/24 h | – | – |
Urinary protein | <100–150 mg/24 h | <250–300 mg/24 h | >300 mg/24 h | – |
Urinary amino acids | – | Up to 2 g/24 h | >2 g/24 h | – |
The osmostat is reset in pregnancy to lower values of osmolarity as the threshold for stimulating the osmoreceptors for antidiuretic hormone (ADH) and thirst are lowered during pregnancy. Plasma osmolarity decreases from conception with 8–10 mOsm/kg by 10 weeks’ gestation and remain low until term. At the new set baseline of osmolarity the response to water loading or deprivation and concentration and dilution of urine occur similar to non-pregnant individuals. Maternal circulating levels of arginine and vasopressin are maintained constant, the 3–4-fold increase in plasma arginine-vasopressin (AVP) clearance by the placental vasopressinases being balanced by the increased release of the hormone from the posterior pituitary.117
Although the filtered load of sodium increases during pregnancy, reabsorption from renal tubules is also increased.111 There is a net retention of 900–950 mEq of sodium during pregnancy, which helps to sustain the plasma volume increase in the dilated systemic vasculature. The specific mechanisms of sodium retention are unclear, probably involving a balance between natriuretic factors such as increased GFR, decreased renal vascular resistance, decreased oncotic pressure, decreased serum albumin, vasodilatory prostaglandins, progesterone, increased atrial natriuretic peptide and antinatriuretic factors such as increased renin, aldosterone, human placental lactogen, estrogen and corticosterone.33,118 Relaxin also mediates sodium balance. However, pregnant women remain in homeostatic balance, as the retention of sodium being compensated by the water accumulation.
Potassium excretion is constant throughout pregnancy, with an increase in tubular reabsorption adapting to alterations in filtered load. This leads to retention and increase in total body potassium stores by approximately 300–350 mEq by the end of gestation. However, the maternal serum potassium level does not rise as the additional potassium is used by the maternal and fetal tissues. The retention of potassium occurs due to anti-kaliuretic effects of progesterone that antagonizes the kaliuretic effects of aldosterone.119
The increase in GFR leads to increase in urinary calcium excretion. Although there is a decrease in the serum levels of calcium and phosphate, serum ionic calcium levels remain stable through increased intestinal absorption of calcium. Pregnant women require a daily 1200 mg calcium intake to maintain homeostasis and meet fetal demands. Whereas parathyroid hormone plays a major role in calcium and bone metabolism in the non-pregnant state, vitamin D appears to be a prominent regulator during pregnancy.120,121 In pregnancy the 1,25(OH)2D levels rise 2–3 fold in order to increase intestinal calcium absorption and ensure mineralization of the fetal skeleton.121
The renal acid–base balance is altered to compensate for the respiratory alkalosis, resulting from increased ventilation in pregnancy. Increase in bicarbonate excretion with renal retention of hydrogen ions balances the loss of respiratory carbon dioxide. As a result, during the first trimester the serum bicarbonate levels decrease by 4–5 mEq/L, to reach values of 20–22 mEq/L. Thus, the buffering capacity of pregnant women is limited.122 The changes in the tubular function are summarized in Table 5 and Table 6.
PHYSIOLOGICAL ADAPTATION OF DIGESTIVE SYSTEM TO PREGNANCY
The anatomical and physiological adaptation of the gastrointestinal and hepatic systems is critical for supporting maternal and fetal nutrition. The changes are favored by mechanical forces of the expanding uterus with displacement of digestive organs and hormonal influences of progesterone on the smooth muscle and estrogen on liver function. Alterations in the gastrointestinal system during pregnancy are summarized in Table 7.
7
Alterations in the gastrointestinal system during pregnancy. (Adapted from Blackburn33)
Organ | Alteration | Significance |
Mouth | Gingivitis Epulis formation Increased saliva production | Bleeding form gums Interference with chewing Increase risk of periodontal diseases |
Esophagus | Decreased lower esophageal sphincter tone decrease tone of hiatus | Heartburn Increase risk of hiatus hernia |
Stomach | Decreased tone and motility Delayed gastric emptying Decreased gastric acidity and histamine output Incompetence of pyloric sphincter | Risk of gastroesophageal reflux and vomiting Reflux of biliary material in the stomach Increased risk of vomiting and aspiration with sedatives and anesthetics Improvement of peptic ulcer symptoms |
Small and large bowel | Decreased intestinal tone and motility Increased transit time Increased height of duodenal villi Increased activity of brush border enzymes Upward displacement of the cecum and appendix | Increased absorption of nutrients: iron, calcium, amino acids, vitamins and other substances Increased sodium and water absorption with predisposition to flatulence and constipation Difficult diagnosis of appendicitis |
Gallbladder | Decreased tone and motility | Alteration of gallbladder function Increased risk of gallstones |
Liver | Upward displacement Altered production of enzymes, bilirubin, proteins and lipids | Delayed recognition of hepatomegaly Altered early recognition of liver dysfunction Discomfort due to itching |
Anatomical changes
The displacement of the gastrointestinal tract by the growing uterus leads to increased in the intragastric pressures. Along with the reduced motility of the bowel, the lower tone of the lower oesophageal sphincter predisposes to reflux and heartburn. Reduced gastric and intestinal motility causes lower transit times, contributing to the sensation of bloating and constipation.123 Gastric volume is unchanged during pregnancy.124
Functional changes
Recent studies stress the relationship between oral health and risk of pregnancy complications such as preterm birth and low birth weight, although there is no definitive agreement regarding this relationship.125,126,127 Due to increased gum vascularization, gingival edema, and decrease thickness of the gingival epithelium bleeding from gum tissue is more frequent during pregnancy, especially in multiparous women, those with poor dentition or periodontal disease.128 The risk of gingivitis increases from early pregnancy affecting up to 30–80% of women by third trimester.128,129,130 Development of gingivitis may be related to alterations in the local inflammatory process stimulated by estrogen and progesterone.131 Advanced and severe gingivitis may favor development of epulis, which occurs in approximately 5% of women between second and third month of pregnancy.130,131 Although it is mostly asymptomatic, the gingival growth may occasionally cause bleeding and cause discomfort with chewing, thus increasing the risk of periodontal disease.131 Pregnancy is associated with changes in salivation. The content of saliva is richer in electrolytes and becomes more acidic. There are changes in saliva microbiome, with increase in microorganism load.127,130,132 Although there is no increase in saliva volume,130 some women may report an increase in saliva production that may be caused by difficulty in swallowing, nausea, and vomiting.133 Only a small proportion of women develop ptyalism.133
As pregnancy advances, the growing uterus displaces the digestive organs, especially the stomach and intestines. The esophagus is also affected, with decreased lower esophageal sphincter tone. The pressure falls by 30–50%, mainly in the third trimester, reaching a nadir by 36 weeks’ gestation.134 The decrease in the ability of the lower esophageal sphincter to respond to increase in intragastric pressure leads to esophageal reflux.135 Along with the mechanical effects caused by the enlarging uterus, the elevated levels of progesterone contribute to gastric hypotonia and decreased motility, with delayed gastric emptying and increased gastrointestinal transit times.136,137 The effect of pregnancy on gastric acid secretion is unclear. A decrease in gastric pH has been reported during first and second trimesters under the influence of estrogen and increased levels of placental histaminases.136,138 While during this time the gastrin levels are normal, with progress of the pregnancy in the third trimester, the gastrin levels tend to rise. Increased levels of gastrin, likely placental in origin in the third trimester, are associated with increased gastric acidity towards the end of pregnancy.139,140
During pregnancy, serum transaminase and bilirubin levels are decreased slightly, whereas serum alkaline phosphatase levels are increased because of placental production.141 The decrease in the intestinal tone and motility in pregnancy are due to the actions of progesterone on intestinal smooth muscle and inhibition by plasma motilin.141 The resulting alterations in the transit time increase with advancing gestation. Similar changes are seen in the gallbladder. Muscle tone and motility decrease under the influence of progesterone leading to increase in the volume of gallbladder and decrease in the emptying rate, particularly in the second and third trimesters. The decrease in water absorption through the gallbladder mucosa along with decreased bile ability to solubilize cholesterol, leads to precipitation of crystals and gallbladder stones.142,143 The tendency of the gallbladder to retain bile salts leads to pruritus.
The liver is displaced superiorly by the gravid uterus, but the size remains unchanged. The hepatic blood flow is not significantly altered, maintained in the range of 25–35%.144 Liver function is not impaired during pregnancy.145 Under the influence of estrogen and progesterone, liver production of proteins, lipids, bilirubin, and enzymes is altered. The changes in the liver function tests are in the same direction as in individuals with liver disorders.145 Most of the routine liver function tests are only slightly altered during pregnancy compared to the non-pregnant state. Changes in liver function tests during pregnancy are summarized in Table 8.
8
Changes in liver function tests during pregnancy. (Adapted from Blackburn33)
Lab test | Effect of pregnancy | Time of change | Significance |
Albumin | ↓ 20–40% | Second trimester | Hemodilution; decrease proteins for biding, increase concentration of free substances |
Globulin | Slight ↑ | Third trimester | Facilitate transport of lipids, carbohydrates, iron and hormones to the placenta |
Total proteins | ↓ by 20% | Second trimester | Mainly related to decrease in albumin; increase in concentration of free proteins |
Bilirubin | N | – | – |
Aspartate aminotransferase (AST) | Slight ↓ in upper limit; may ↑ during labour | – | Do not change significantly during pregnancy, can be used to assess liver damage during pregnancy |
Alanine aminotransferase (ALT) | Slight ↓ in upper limit; may ↑ during labour | – | Do not change significantly during pregnancy, can be used to assess liver damage during pregnancy |
Gamma glutamyl transferase (GGT) | Slight ↓ in upper limit | ||
Alkaline phosphatase | ↑ 2 to 4-fold | Third trimester | Increased production by fetus and placenta |
Lactate dehydrogenase (LDH) | Slight ↑ | Third trimester | Associated with tissue injury |
PRACTICE RECOMMENDATIONS
Physiological adaptation of hematological system to pregnancy
- To meet the daily iron requirements, an average 3–4 mg of iron needs to be absorbed per day during pregnancy. This may increase to 6–7 mg daily in the late second to third trimester.
- Iron supplementation is provided as 325 mg of oral tablets, in the form of ferrous sulfate (65 mg of elemental iron), ferrous gluconate (35 mg) or ferrous fumarate (107 mg).
- Folic acid supplementation (400–800 μg) is recommended daily during pregnancy.
- Hemoglobin or hematocrit should be routinely determined at the first prenatal visit in order to detect pre-existing anemia.
- Anemia in pregnancy is defined as a hemoglobin level below 11.0 g/dl during the first or third trimesters or below 10.5 g/dl during the second trimester.
- Gestational thrombocytopenia, present in approximately 5% of pregnancies, tends to present without a prior history and is usually asymptomatic and mild in severity.
- A platelet count between 70,000 and 150,000/mm3 has been described in about 8% of pregnancies and resolves by 4 weeks postpartum.
- The risk of venous thromboembolism (VTE) in pregnancy and the postpartum period is 0.7/1000 women, about 2- to 4-fold higher in the puerperium than in the non-pregnant women.
Physiological adaptation of cardiovascular system to pregnancy
- Pregnancy is a physiological state associated with a dramatic cardiac structural remodeling and an improved functional performance.
- Cardiac adjustments to a pregnancy state may mimic abnormalities of the cardiovascular system.
- Maternal cardiovascular changes need to be assessed throughout pregnancy.
- Women should be counseled on normal cardiovascular changes in pregnancy and on potential symptoms associated with those changes.
- Aortocaval compression is frequent from mid-pregnancy. Women should be counseled to use lateral recumbent position.
- Encourage women to maintain an active lifestyle and moderate exercise program throughout pregnancy.
- General anesthesia, spinal and epidural blocks abolish the sympathetic response and increase the risk of supine hypotension.
- Monitor for signs of cardiac failure in women with cardiac diseases, particularly towards the end of pregnancy, intrapartum and immediate postpartum period.
- Counsel women with cardiac diseases on signs of cardiac failure and discuss management of daily activities and exercise tolerance.
- During cesarean section and for other situations requiring a supine position, the uterus should be displaced (usually to the left) by placing a rigid wedge under the hip and/or tilting the table.
Physiological adaptation of respiratory system to pregnancy
- Abdominal respiration, increase in ventilation rate, and respiratory alkalosis are features of the normal adaptation of the respiratory system in pregnancy.
- Pregnant women should be counseled regarding the respiratory changes in pregnancy and how they can affect pre-existing respiratory diseases, daily activity and exercise tolerance.
- Discuss changes in upper airways that lead to nasal congestion, nose bleeds, snoring.
- Counsel asthmatic women on the influence of pregnancy on asthma manifestation, advise on medication, precipitating factors, and to seek medical advice when symptoms worsen. Advise on heightened adverse effects of respiratory infections during pregnancy and encourage flu vaccinations.
Physiological adaptation of the renal system to pregnancy
- The physiological dilatation should be taken into account when interpreting radiological studies undertaken for possible urinary tract obstruction.
- Ureteric compression leads to urine stasis, increasing the incidence of urinary tract infection, nephrolithiasis and pyelonephritis.
- Glomerular filtration rate increases by 40–50% in normal pregnancy.
- Creatinine clearance per 24-hour urine collection is the current gold standard for measurement of glomerular filtration rate in pregnant women.
- Pregnancy is a volume-expanded state characterized by net sodium retention of 900–1000 mEq and 1.1–1.6 l water mediated by a balance between natriuretic and anti-natriuretic forces.
- Renal excretion of amino acids, glucose, proteins, electrolytes, urea, creatinine, blood urea nitrogen and uric acid increases due to increased filtration fraction
- Proteinuria is common in pregnant women. A urine dipstick value of +1 is not evident of pathology. A protein excretion rate greater than 300 mg in 24 hours requires evaluation for preeclampsia or other hypertensive disorders of pregnancy as well as previously undiagnosed renal disease.
- Pregnant women who need an intervention for symptomatic urolithiasis can undergo placement of a ureteral stent or nephrostomy tube. Definitive treatment for urolithiasis is deferred until after delivery.
- Pyelonephritis is a common non-obstetric indication for admission during pregnancy that complicates 1–2% of pregnancies. It causes severe maternal and fetal morbidity and is associated with a 6–50% risk of preterm delivery. The physiological changes of pregnancy, such as decreased ureteral peristalsis and detrusor tone, mechanical compression of the ureters, and incomplete bladder emptying are predisposing factors for developing pyelonephritis in pregnant women.
Physiological adaptation of digestive system to pregnancy
- The anatomical alterations can confuse the diagnosis of acute abdominal surgical emergencies.
- Abdominal examination for signs of peritonism can be difficult and potentially inaccurate due to peritoneal desensitization.
- The choice and position of surgical incisions can be affected.
- Reflux esophagitis and heartburn symptoms affect 50–80% of pregnant women.
- Nausea and vomiting are common, affecting up to 50% of pregnant women.
- Sensation of bloating and constipation is common in pregnancy.
- Increased risk of aspiration of gastric contents, especially during administration of general anesthesia. This risk is further elevated in obese gravida.
- Pregnant woman planned for general anesthesia require fasting 6–8 hours beforehand, an antacid, and a H2-receptor antagonist.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Akinbami AA, Ajibola SO, Rabiu KA, et al. Hematological profile of normal pregnant women in Lagos, Nigeria. Int J Womens Health 2013;5:227–32. | |
Magriples U, Boynton MH, Kershaw TS, et al. Blood pressure changes during pregnancy: impact of race, body mass index, and weight gain. Am J Perinatol. 2013;30(5):415–424. doi:10.1055/s-0032-1326987 ;[published correction appears in Am J Perinatol. 2013 Feb;30(2):161. | |
Brewster LM, et al. Ethnic differences in resistance artery contractility of normotensive pregnant women. American journal of physiology. Heart and circulatory physiology 299, H431-436, doi:10.1152/ajpheart.00919.2009 (2010). | |
Chandra S, Tripathi AK, Mishra S, et al. Physiological changes in hematological parameters during pregnancy. Indian J Hematol Blood Transfus 2012;28(3):144–6. | |
Stephansson O, Dickman PW, Johansson A, et al. Maternal hemoglobin concentration during pregnancy and risk of stillbirth. JAMA 2000;284(20):2611–7. | |
Thomsen JK, Fogh-Andersen N, Jaszczak P, et al. Atrial natriuretic peptide (ANP) decrease during normal pregnancy as related to hemodynamic changes and volume regulation. Acta Obstet Gynecol Scand 1993;72(2):103–10. | |
Ringholm L, Pedersen-Bjergaard U, Thorsteinsson B, et al. Atrial Natriuretic Peptide (ANP) in early pregnancy is associated with development of preeclampsia in type 1 diabetes. Diabetes Res Clin Pract 2011;93(3):e106–9. | |
Faupel-Badger JM, Hsieh CC, Troisi R, et al. Plasma volume expansion in pregnancy: implications for biomarkers in population studies. Cancer Epidemiol Biomarkers Prev 2007;16(9):1720–3. | |
Jansen AJ, van Rhenen DJ, Steegers EA, et al. Postpartum hemorrhage and transfusion of blood and blood components. Obstet Gynecol Surv 2005;60(10):663–71. | |
Akinlaja O. Hematological Changes in Pregnancy – The Preparation for Intrapartum Blood Loss. Obstet Gynecol Int J 2016;4(3). | |
Milman N. Iron and pregnancy – a delicate balance. Ann Hematol 2006;85(9):559–65. | |
Whittaker PG, Macphail S, Lind T. Serial hematologic changes and pregnancy outcome. Obstet Gynecol 1996;88(1):33–9. | |
World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva, World Health Organization. 2011. | |
Peña-Rosas JP, De-Regil LM, Dowswell T, et al. Daily oral iron supplementation during pregnancy. The Cochrane Database Syst Rev 2012;12:CD004736-CD. | |
Cunningham FG. Laboratory values in normal pregnancy. In: Protocols for High-Risk Pregnancies: An Evidence-Based Approach. 2010; 5th Ed, Blackwell Science Ltd, USA | |
Sharma S, Brugnara C, Betensky RA, et al. Reductions in red blood cell 2,3-diphosphoglycerate concentration during continuous renal replacment therapy. Clin J Am Soc Nephrol 2015;10(1):74–9. | |
Luppi P, Haluszczak C, Trucco M, et al. Normal pregnancy is associated with peripheral leukocyte activation. Am J Reprod Immunol 2002;47(2):72–81. | |
Edelstam G, Lowbeer C, Kral G, et al. New reference values for routine blood samples and human neutrophilic lipocalin during third-trimester pregnancy. Scand J Clin Lab Invest 2001;61(8):583–92. | |
Dhariwal SKNS, Singh A, Nema S. Evaluation of haematological indices, neutrophils and platelets in pregnant women attending tertiary care centre. Indian Journal of Pathology and Oncology 2016(3):297–304. | |
Regal JF, Gilbert JS, Burwick RM. The complement system and adverse pregnancy outcomes. Mol Immunol 2015;67(1):56–70. | |
Richani K, Soto E, Romero R, et al. Normal pregnancy is characterized by systemic activation of the complement system. J Matern Fetal Neonatal Med 2005;17(4):239–45. | |
Michimata T, Sakai M, Miyazaki S, Aoki K, et al. Decrease of T-helper 2 and T-cytotoxic 2 cells at implantation sites occurs in unexplained recurrent spontaneous abortion with normal chromosomal content. Human Reprod 2003;18(7):1523–8. | |
Duong C, Kidson-Gerber G, Peters N, et al. Trajectory of platelets in pregnancy – do low-risk women need an intrapartum full blood count prior to epidural? Aust N Z J Obstet Gynaecol 2015;55(5):511–4. | |
Valera MC, Parant O, Vayssiere C, et al. Physiologic and pathologic changes of platelets in pregnancy. Platelets 2010;21(8):587–95. | |
Shehata N, Burrows R, Kelton JG. Gestational thrombocytopenia. Clin Obstet Gynecol 1999;42(2):327–34. | |
de Boer K, ten Cate JW, Sturk A, et al. Enhanced thrombin generation in normal and hypertensive pregnancy. Am J Obstet Gynecol 1989;160(1):95–100. | |
Hellgren M. Hemostasis during normal pregnancy and puerperium. Semin Thromb Hemost 2003;29(2):125–30. | |
Clark P, Brennand J, Conkie JA, et al. Activated protein C sensitivity, protein C, protein S and coagulation in normal pregnancy. Thromb Haemost 1998;79(6):1166–70. | |
Hui C, Lili M, Libin C, et al. Changes in coagulation and hemodynamics during pregnancy: a prospective longitudinal study of 58 cases. Arch Gynecol Obstet 2012;285(5):1231–6. | |
McLean KC, Bernstein IM, Brummel-Ziedins KE. Tissue factor-dependent thrombin generation across pregnancy. Am J Obstet Gynecol 2012;207(2):135.e1–6. | |
Kline JA, Williams GW, Hernandez-Nino J. D-dimer concentrations in normal pregnancy: new diagnostic thresholds are needed. Clinical chemistry 2005;51(5):825–9. | |
Eichinger S. D-dimer testing in pregnancy. Pathophysiol Haemost Thromb 2003;33(5–6):327–9. | |
Blackburn ST. Maternal, fetal & neonatal physiology: a clinical perspective. 4th edn. Maryland, USA: Elsevier, 2013. | |
Rubler S, Damani PM, Pinto ER. Cardiac size and performance during pregnancy estimated with echocardiography. Am J Cardiol 1977;40(4):534–40. | |
Pelech AN. The physiology of cardiac auscultation. Pediatr Clin North Am 2004;51(6):1515–35, vii-viii. | |
Hill CC, Pickinpaugh J. Physiologic changes in pregnancy. Surg Clin North Am 2008;88(2):391–401, vii. | |
Bedard E, Dimopoulos K, Gatzoulis MA. Has there been any progress made on pregnancy outcomes among women with pulmonary arterial hypertension? Eur Heart J 2009;30(3):256–65. | |
Lechmanova M, Kittnar O, Mlcek M, et al. QT dispersion and T-loop morphology in late pregnancy and after delivery. Physiol Res 2002;51(2):121–9. | |
Sunitha M, Chandrasekharappa S, Brid SV. Electrocradiographic Qrs Axis, Q Wave and T-wave Changes in 2nd and 3rd Trimester of Normal Pregnancy. J Clin Diagn Res 2014;8(9):Bc17–21. | |
Blanchard DG, Shabetal R. Cardiac disease. In: Creasy & Resnik's Maternal-fetal medicine: Principles and practice, 6th edn. Philadelphia, USA: Saunders Elsevier, 2009. | |
Gei AF, Hankins GD. Cardiac disease and pregnancy. Obstet Gynecol Clin North Am 2001;28(3):465–512. | |
Caulin-Glaser T, Setaro JF. Pregnancy and cardiovascular disease. In: Medical complications during pregnancy, 6th edn. Philadelphia, USA: Saunders Elsevier, 2004. | |
Kametas NA, McAuliffe F, Hancock J, et al. Maternal left ventricular mass and diastolic function during pregnancy. Ultrasound Obstet Gynecol 2001;18(5):460–6. | |
Keser N. Echocardiography in pregnant women. Anadolu Kardiyol Derg 2006;6(2):169–73. | |
Monga M. Maternal cardiovascular, respiratory and renal adaptation to pregnancy. In Creasy & Resnik's Maternal-fetal medicine: Principles and practice, 6th Ed. 2009, Saunders Elsevier, Philadelphia, USA. | |
Pacheco L, Costantine MM, Hankins GDV. Physiologic changes during pregnancy. In: Clinical Pharmacology During Pregnancy, 2013:5–14, Academic Press, San Diego, USA. | |
Capeless EL, Clapp JF. When do cardiovascular parameters return to their preconception values? Am J Obstet Gynecol 1991;165(4 Pt 1):883–6. | |
Bridges EJ, Womble S, Wallace M, et al. Hemodynamic monitoring in high-risk obstetrics patients, I. Expected hemodynamic changes in pregnancy. Critical care nurse 2003;23(4):53–62. | |
Robson SC, Hunter S, Boys RJ, et al. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol 1989;256(4 Pt 2):H1060–5. | |
Ouzounian JG, Elkayam U. Physiologic changes during normal pregnancy and delivery. Cardiol Clin 2012;30(3):317–29. | |
Ueland K, Hansen JM. Maternal cardiovascular dynamics. II. Posture and uterine contractions. Am J Obstet Gynecol 1969;103(1):1–7. | |
Kametas NA, McAuliffe F, Krampl E, et al. Maternal cardiac function in twin pregnancy. Obstet Gynecol 2003;102(4):806–15. | |
Costantine MM. Physiologic and pharmacokinetic changes in pregnancy. Frontiers Pharmacol 2014;5:65. | |
Carlin A, Alfirevic Z. Physiological changes of pregnancy and monitoring. Best Pract Res Clin Obstet Gynaecol 2008;22(5):801–23. | |
Cunningham F. Williams Obstetrics, 23rd edn. New York, USA: McGraw-Hill 2009. | |
ACOG committee opinion. Exercise during pregnancy and the postpartum period. Number 267, January 2002. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet 2002;77(1):79–81. | |
Fu Q, Levine BD. Autonomic circulatory control during pregnancy in humans. Semin Reprod Med 2009;27(4):330–7. | |
Carbillon L, Uzan M, Uzan S. Pregnancy, vascular tone, and maternal hemodynamics: a crucial adaptation. Obstet Gynecol Surv 2000;55(9):574–81. | |
Schrier RW. Systemic arterial vasodilation, vasopressin, and vasopressinase in pregnancy. J Am Soc Nephrol 2010;21(4):570–2. | |
Frederiksen MC. Physiologic changes in pregnancy and their effect on drug disposition. Semin Perinatol 2001;25(3):120–3. | |
Varga I, Rigo J, Jr, Somos P, et al. Analysis of maternal circulation and renal function in physiologic pregnancies; parallel examinations of the changes in the cardiac output and the glomerular filtration rate. J Matern Fetal Neonatal Med 2000;9(2):97–104. | |
Curry R, Swan L, Steer PJ. Cardiac disease in pregnancy. Curr Opin Obstet Gynecol 2009;21(6):508–13. | |
Morris SN, Johnson NR. Exercise during pregnancy: a critical appraisal of the literature. The Journal of Reproductive Medicine 2005;50(3):181–8. | |
Hytten FE, Paintin DB. Increase in plasma volume during normal pregnancy. The Journal of Obstetrics and Gynaecology of the British Empire 1963;70:402–7. | |
Clapp JF, 3rd. Exercise and fetal health. Journal of Developmental Physiology 1991;15(1):9–14. | |
Brooks VL, Dampney RA, Heesch CM. Pregnancy and the endocrine regulation of the baroreceptor reflex. Am J Physiol Regul Integr Comp Physiol 2010;299(2):R439–51. | |
Whitty JE, Dombrowski MP. Respiratory diseases in pregnancy. In: Creasy & Resnik's Maternal-fetal medicine: Principles and practice, 6th Ed, 2009, Saunders Elsevier, Philadelphia, USA. | |
Mandel J, Weinberger SE. Pulmonary diseases. In: Medical complications of pregnancy, 6th edn. Philadelphia, USA: Saunders Elsevier, 2004. | |
Hegewald MJ, Crapo RO. Respiratory physiology in pregnancy. Clin Chest Med 2011;32(1):1–13. | |
Blood-Siegfried J, Rende EK. The long-term effects of prenatal nicotine exposure on neurologic development. J Midwifery Womens Health 2010;55(2):143–52. | |
Torgersen KL, Curran CA. A systematic approach to the physiologic adaptations of pregnancy. Critical Care Nursing Quarterly 2006;29(1):2–19. | |
Tsai CH, de Leeuw NK. Changes in 2,3-diphosphoglycerate during pregnancy and puerperium in normal women and in beta-thalassemia heterozygous women. Am J Obstet Gynecol 1982;142(5):520–3. | |
Baldwin GR, Moorthi DS, Whelton JA, et al. New lung functions and pregnancy. Am J Obstet Gynecol 1977;127(3):235–9. | |
Leboulanger N, Louvet N, Rigouzzo A, et al. Pregnancy is associated with a decrease in pharyngeal but not tracheal or laryngeal cross-sectional area: a pilot study using the acoustic reflection method. Int J Obstet Anesth 2014;23(1):35–9. | |
Bobrowski RA. Pulmonary physiology in pregnancy. Clin Obstet Gynecol 2010;53(2):285–300. | |
Freeman M, Ghidini A, Spong CY, et al. Does air travel affect pregnancy outcome? Arch Gynecol Obstet 2004;269(4):274–7. | |
Reyes-Zuniga M, Torre-Bouscoulet L. Obstructive Sleep Apnea and Perinatal Risk. Revista de investigacion clinica; organo del Hospital de Enfermedades de la Nutricion 2016;68(6):281–5. | |
Gardner MO, Doyle NM. Asthma in pregnancy. Obstet Gynecol Clin North Am 2004;31(2):385–413, vii. | |
Elkus R, Popovich J, Jr. Respiratory physiology in pregnancy. Clin Chest Med 1992;13(4):555–65. | |
McAuliffe F, Kametas N, Costello J, et al. Respiratory function in singleton and twin pregnancy. BJOG 2002;109(7):765–9. | |
Harirah HM, Donia SE, Nasrallah FK, et al. Effect of gestational age and position on peak expiratory flow rate: a longitudinal study. Obstet Gynecol 2005;105(2):372–6. | |
Murphy VE, Gibson PG. Asthma in pregnancy. Clin Chest Med 2011;32(1):93–110. | |
Wise RA, Polito AJ, Krishnan V. Respiratory physiologic changes in pregnancy. Immunol Allergy Clin North Am 2006;26(1):1–12. | |
Delivoria-Papadopoulos M, McGoven JE. In: Fetal and neonatal physiology, 2nd edn. Philadelphia, USA: Sanders Elsevier, 1998. | |
Templeton A, Kelman GR, Maternal blood-gases, (PAO2–PaO2), physiological shunt and VD/VT in normal pregnancy. Br J Anaesth 1976;48:1001 | |
Torgersen K. Complications of the childbearing experience. In: Nettina S. (ed.) Lippincott Manual for Nursing Practice, 8th edn. Philadelphia, 2006;1259–306. | |
Rasmussen PE, Nielsen FR. Hydronephrosis during pregnancy: a literature survey. Eur J Obstet Gynecol Reprod Biol 1988;27(3):249–59. | |
Cheung KL, Lafayette RA. Renal physiology of pregnancy. Adv Chronic Kidney Dis 2013;20(3):209–14. | |
Chapman AB, Abraham WT, Zamudio S, et al. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int 1998;54(6):2056–63. | |
Bailey RR, Rolleston GL. Kidney length and ureteric dilatation in the puerperium. The Journal of Obstetrics and Gynaecology of the British Commonwealth 1971;78(1):55–61. | |
Roy C, Saussine C, Jahn C, et al. Fast imaging MR assessment of ureterohydronephrosis during pregnancy. Magn Reson Imaging 1995;13(6):767–72. | |
Jeyabalan A, Lain KY. Anatomic and functional changes of the upper urinary tract during pregnancy. Urol Clin North Am 2007;34(1):1–6. | |
Jeyabalan A, Conrad KP. Renal function during normal pregnancy and preeclampsia. Front Biosci 2007;12:2425–37. | |
Christensen T, Klebe JG, Bertelsen V, et al. Changes in renal volume during normal pregnancy. Acta Obstet Gynecol Scand 1989;68(6):541–3. | |
Faundes A, Bricola-Filho M, Pinto e Silva JL. Dilatation of the urinary tract during pregnancy: proposal of a curve of maximal caliceal diameter by gestational age. Am J Obstet Gynecol 1998;178(5):1082–6. | |
Mandal D, Saha MM, Pal DK. Urological disorders and pregnancy: An overall experience. Urology annals 2017;9(1):32–6. | |
Shrotri KN, Morrison ID, Shrotri NC. Urological conditions in pregnancy: a diagnostic and therapeutic challenge. J Obstet Gynaecol 2007;27(7):648–54. | |
Beydoun SN. Morphologic changes in the renal tract in pregnancy. Clin Obstet Gynecol 1985;28(2):249–56. | |
Mikhail MS, Anyaegbunam A. Lower urinary tract dysfunction in pregnancy: a review. Obstet Gynecol Surv 1995;50(9):675–83. | |
Dunlop W. Serial changes in renal haemodynamics during normal human pregnancy. BJOG 1981;88(1):1–9. | |
Odutayo A, Hladunewich M. Obstetric nephrology: renal hemodynamic and metabolic physiology in normal pregnancy. Clin J Am Soc Nephrol 2012;7(12):2073–80. | |
Sturgiss SN, Dunlop W, Davison JM. Renal haemodynamics and tubular function in human pregnancy. Bailliere's clinical obstetrics and gynaecology 1994;8(2):209–34. | |
Bucht H. Studies on renal function in man; with special reference to glomerular filtration and renal plasma flow in pregnancy. Scand J Clin Lab Invest 1951;3(3):1–64. | |
Conrad KP. Maternal vasodilation in pregnancy: the emerging role of relaxin. Am J Physiol Regul Integr Comp Physiol 2011;301(2):R267–75. | |
Ogueh O, Clough A, Hancock M, et al. A longitudinal study of the control of renal and uterine hemodynamic changes of pregnancy. Hypertens Pregnancy 2011;30(3):243–59. | |
Irani RA, Xia Y. Renin angiotensin signaling in normal pregnancy and preeclampsia. Semin Nephrol 2011;31(1):47–58. | |
Irani RA, Xia Y. The functional role of the renin-angiotensin system in pregnancy and preeclampsia. Placenta 2008;29(9):763–71. | |
De Alvarez RR. Renal glomerulotubular mechanisms during normal pregnancy. I. Glomerular filtration rate, renal plasma flow, and creatinine clearance. Am J Obstet Gynecol 1958;75(5):931–44. | |
Roberts M, Lindheimer MD, Davison JM. Altered glomerular permselectivity to neutral dextrans and heteroporous membrane modeling in human pregnancy. Am J Physiol 1996;270(2 Pt 2):F338–43. | |
Soma-Pillay P, Catherine, N-P, Tolppanen H, et al. Physiological changes in pregnancy. Cardiovascular Journal of Africa 2016;27(2):89–94. | |
Davison JM. Kidney function in pregnant women. Am J Kidney Dis 1987;9(4):248–52. | |
Cornelis T, Odutayo A, Keunen J, et al. The kidney in normal pregnancy and preeclampsia. Semin Nephrol 2011;31(1):4–14. | |
Erman A, Neri A, Sharoni R, et al. Enhanced urinary albumin excretion after 35 weeks of gestation and during labour in normal pregnancy. Scand J Clin Lab Invest 1992;52(5):409–13. | |
Hayashi M, Ueda Y, Hoshimoto K, et al. Changes in urinary excretion of six biochemical parameters in normotensive pregnancy and preeclampsia. Am J Kidney Dis 2002;39(2):392–400. | |
Yoshimatsu J, Matsumoto H, Goto K, et al. Relationship between urinary albumin and serum soluble fms-like tyrosine kinase 1 (sFlt-1) in normal pregnancy. Eur J Obstet Gynecol Reprod Biol 2006;128(1–2):204–8. | |
Davison JM, Hytten FE. The effect of pregnancy on the renal handling of glucose. BJOG 1975;82(5):374–81. | |
Davison JM, Shiells EA, Philips PR, et al. Serial evaluation of vasopressin release and thirst in human pregnancy. Role of human chorionic gonadotrophin in the osmoregulatory changes of gestation. J Clin Invest 1988;81(3):798–806. | |
Davison JM, Barron WM, Lindheimer MD. Metabolic clearance rates of vasopressin increase markedly in late gestation: possible cause of polyuria in pregnant women. Transactions of the Association of American Physicians 1987;100:91–8. | |
Brown MA, Sinosich MJ, Saunders DM, et al. Potassium regulation and progesterone-aldosterone interrelationships in human pregnancy: a prospective study. Am J Obstet Gynecol 1986;155(2):349–53. | |
Moon RJ, Harvey NC, Cooper C. Endocrinology in pregnancy: Influence of maternal vitamin D status on obstetric outcomes and the fetal skeleton. EEur J Endocrinol 2015;173(2):R69–83. | |
Gustafsson MK, Romundstad PR, Stafne SN, et al. Alterations in the vitamin D endocrine system during pregnancy: A longitudinal study of 855 healthy Norwegian women. PloS One 2018;13(4):e0195041. | |
Podymow T, August P, Akbari A. Management of renal disease in pregnancy. Obstet Gynecol Clin North Am 2010;37(2):195–210. | |
Parry E, Shields R, Turnbull AC. Transit time in the small intestine in pregnancy. The Journal of Obstetrics and Gynaecology of the British Commonwealth 1970;77(10):900–1. | |
Kelly TF, T.J. Gastrointestinal disease in pregnancy. In: Creasy & Resnik's Maternal-fetal medicine: Principles and practice, 6th Ed. 2009, Saunders Elsevier, Philadelphia, USA | |
George A, Shamim S, Johnson M, et al. Periodontal treatment during pregnancy and birth outcomes: a meta-analysis of randomised trials. Int J Evid Based Healthc 2011;9(2):122–47. | |
Polyzos NP, Polyzos IP, Mauri D, et al. Effect of periodontal disease treatment during pregnancy on preterm birth oncidence: a metaanalysis of randomized trials. Am J Obstet Gynecol 2009;200(3):225–32. | |
Uppal A, Uppal S, Pinto A et al. The effectiveness of periodontal disease treatment during pregnancy in reducing the risk of experiencing preterm birth and low birth weight: a meta-analysis. J Am Dent Assoc 2010;141(12):1423–34. | |
Fogacci MF, Vettore MV, Leao AT. The effect of periodontal therapy on preterm low birth weight: a meta-analysis. Obstet Gynecol 2011;117(1):153–65. | |
Geisinger ML, Geurs NC, Bain JL, et al. Oral health education and therapy reduces gingivitis during pregnancy. J Clin Periodontol 2014;41(2):141–8. | |
Muallem MM, Rubeiz NG. Physiological and biological skin changes in pregnancy. Clin Dermatol 2006;24(2):80–3. | |
Barak S, Oettinger-Barak O, Oettinger M, et al. Common oral manifestations during pregnancy: a review. Obstet Gynecol Surv 2003;58(9):624–8. | |
Vinturache AE, Gyamfi-Bannerman C, Hwang J, et al. Maternal microbiome – A pathway to preterm birth. Semin Fetal Neonatal Med 2016;21(2):94–9. | |
Thaxter Nesbeth KA, Samuels LA, Nicholson Daley C, et al. Ptyalism in pregnancy – a review of epidemiology and practices. Eur J Obstet Gynecol Reprod Biol 2016;198:47–9. | |
Richter JE. Review article: the management of heartburn in pregnancy. Aliment Pharmacol Ther 2005;22(9):749–57. | |
Hey VM, Cowley DJ, Ganguli PC, et al. Gastro-oesophageal reflux in late pregnancy. Anaesthesia 1977;32(4):372–7. | |
Van Thiel DH, Gavaler JS, Joshi SN, et al. Heartburn of pregnancy. Gastroenterology 1977;72(4 Pt 1):666–8. | |
Tan EK, Tan EL. Alterations in physiology and anatomy during pregnancy. Best Pract Res Clin Obstet Gynaecol 2013;27(6):791–802. | |
Quartarone G. Gastroesophageal reflux in pregnancy: a systematic review on the benefit of raft forming agents. Minerva Ginecol 2013;65(5):541–9. | |
Adamopoulos DA, Dessypris A, Xanthopoulos J, et al. Gastrin secretion in the menstrual cycle and pregnancy. Hepato-gastroenterology 1982;29(1):24–6. | |
Frick G, Bremme K, Sjogren C, et al. Plasma levels of cholecystokinin and gastrin during the menstrual cycle and pregnancy. Acta Obstet Gynecol Scand 1990;69(4):317–20. | |
Ruiz-Extremera A, Lopez-Garrido MA, Barranco E, et al. Activity of hepatic enzymes from week sixteen of pregnancy. Am J Obstet Gynecol 2005;193(6):2010–6. | |
Lindseth G, Bird-Baker MY. Risk factors for cholelithiasis in pregnancy. Res Nurs Health 2004;27(6):382–91. | |
Everson GT. Gastrointestinal motility in pregnancy. Gastroenterol Clin North Am 1992;21(4):751–76. | |
Mandic-Markovic VD, Mikovic ZM, Djukic MK, et al. Doppler parameters of the maternal hepatic artery blood flow in normal pregnancy: maternal hepatic artery blood flow in normal pregnancy. Eur J Obstet Gynecol Reprod Biol 2014;181:275–9. | |
Bacq Y, Zarka O, Brechot JF, et al. Liver function tests in normal pregnancy: a prospective study of 103 pregnant women and 103 matched controls. Hepatology 1996;23(5):1030–4. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards programme CLICK HERE)

