This chapter should be cited as follows:
Kane SC, Wium L, et al., Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.413253
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 8
Maternal medical health and disorders in pregnancy
Volume Editor:
Dr Kenneth K Chen, Alpert Medical School of Brown University, USA
Originating Editor: Professor Sandra Lowe

Chapter
Screening for Hypertension and Proteinuria in Pregnancy
First published: February 2021
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
Hypertensive disorders of pregnancy remain an important cause of maternal mortality in both the developed and developing world.1,2 An estimated 40,000 women die each year from this condition. Deaths due to hypertension were ranked fourth as a direct cause of maternal death in the United Kingdom during 2009–2012, with a maternal mortality ratio (MMR) of 0.25.3 A reduction in MMR was observed in the UK during 2012–2014 when a MMR of 0.08 was reported, when only two women died from pre-eclampsia and eclampsia, translating to approximately one death per 1,000,000 pregnancies.4 A sizeable proportion of hypertensive deaths may be avoided with good obstetric care. Approximately 70% of deaths in South Africa and France are considered avoidable, while a Dutch audit found that 90% of women who died from hypertensive disorders of pregnancy received substandard care.1,5,6 Most hypertensive maternal deaths reported in South Africa are due to cerebral hemorrhage, therefore identification and treatment of severe hypertension is important.
The remarkable reduction in deaths reported in the UK indicates a considerable potential for reduction in other parts of the world. Interventions to prevent hypertensive disorders in pregnancy, including pre-eclampsia have been disappointing and an important factor associated with sub-standard care is poor detection. In a commentary on maternal deaths in the UK, Shennan et al. stated that hypertension and pre-eclampsia are only safe for the mother if identified and well managed.4
Assessment of urine for protein at booking, and thereafter as indicated by local guidelines, is a key step in ruling out or diagnosing severe conditions associated with high morbidity and mortality, particularly conditions such as pre-eclampsia and chronic renal disease. Pre-existing renal disease should be considered in women in whom proteinuria is documented before 20 weeks' gestation, while new-onset proteinuria after 20 weeks is a risk factor for pre-eclampsia, even in normotensive women.7 About one-third of women who present with proteinuria after 20 weeks will develop pre-eclampsia later in the pregnancy and adverse pregnancy and neonatal outcomes are more common as compared with women in whom hypertension was the first presenting sign of pre-eclampsia.8 It is important to distinguish between renal disease and pre-eclampsia because of different clinical management.
Physiological changes in pregnancy
Understanding the physiological changes in pregnancy is crucial when managing women with hypertensive disorders. Pregnancy is associated with significant hemodynamic and hormonal changes affecting the cardiovascular system. Maternal hemodynamic adaptation begins at 5–6 weeks’ gestation. Cardiac output increases gradually from 6 weeks’ gestation and reaches a plateau in the second and third trimester with levels increasing by 40% compared to pre-pregnancy. There is a simultaneous decrease in systemic vascular resistance (SVR) which is in turn responsible for the decrease in mean arterial blood pressure. Mean arterial blood pressure decreases significantly from the mid-follicular phase to 6 weeks’ gestation reaching a nadir between 16 and 20 weeks.9 After this period, blood pressure begins to rise slowly again, reaching pre-conception levels around term. The heart undergoes remodeling with an increase in left ventricular wall thickness and mass.10 Despite these changes, left ventricular contractile function is maintained and any changes in cardiac geometry are reversed within 3 months postpartum in normotensive women.
The fall in SVR also affects the renal vasculature. As a consequence of the renal vasodilatation, renal plasma flow and glomerular filtration rate (GFR) both increase compared to non-pregnant levels by 40–65% and 50–85%, respectively. Due to the increases in both GFR and glomerular capillary permeability to albumin, the fractional excretion of protein may increase up to 300 mg/day and the protein excretion also increases. In normal pregnancies the total protein concentration in urine does not increase above the upper normal limit. Uric acid excretion also increases due to increased GFR and/or decreased tubular reabsorption.11
‘SCREENING’ IN PREGNANCY
General principles
‘Screening’ may be defined as the systematic application of a test or enquiry to identify individuals at sufficient risk of a specific disorder to warrant further investigation or direct preventive action, amongst persons who have not sought medical attention on account of symptoms of that disorder.12 It has long been recognized that any screening program has both potential benefits and harms, and it was with a view to maximizing the former and minimizing the latter that Wilson and Jungner produced their 1968 WHO report titled Principles and practice of screening for disease.13 The principles enunciated in this classic report are presented in Box 1.
Box 1 Classic screening criteria13
- The condition sought should be an important health problem.
- There should be an accepted treatment for patients with recognized disease.
- Facilities for diagnosis and treatment should be available.
- There should be a recognizable latent or early symptomatic stage.
- There should be a suitable test or examination.
- The test should be acceptable to the population.
- The natural history of the condition, including development from latent to declared disease, should be adequately understood.
- There should be an agreed policy on whom to treat as patients.
- The cost of case-finding (including diagnosis and treatment of patients diagnosed) should be economically balanced in relation to possible expenditure on medical care as a whole.
- Case-finding should be a continuing process and not a “once and for all” project.
The explosion in genetic and genomic knowledge since then has prompted the WHO to revise these principles, to maintain their relevance in the face of these transformative technologies. The revised principles are presented in Box 2, and in many ways are more readily applicable to screening tests in pregnancy than the original criteria.
Box 2 Synthesis of emerging screening criteria proposed over the past 40 years14
- The screening program should respond to a recognized need.
- The objectives of screening should be defined at the outset.
- There should be a defined target population.
- There should be scientific evidence of screening program effectiveness.
- The program should integrate education, testing, clinical services and program management.
- There should be quality assurance, with mechanisms to minimize potential risks of screening.
- The program should ensure informed choice, confidentiality and respect for autonomy.
- The program should promote equity and access to screening for the entire target population.
- Program evaluation should be planned from the outset.
- The overall benefits of screening should outweigh the harm.
The requirement for screening programs to promote equity and access for the entire target population will necessitate varied approaches to screening in pregnancy depending on local resources and logistic considerations.
Identifying evolving concerns specific to pregnancy versus identifying hitherto unrecognized but pre-existing problems
All ‘routine’ examinations and investigations performed in pregnancy can be considered as screening tests, and should therefore adhere to the principles outlined above. Many such assessments aim to identify pre-existing maternal conditions, such as iron deficiency anemia or chronic hypertension, that could increase the chance of an adverse pregnancy outcome if left untreated. Such assessments may, of course, have benefits that extend beyond the end of the pregnancy, and highlight the opportunistic potential for pregnancy care to improve the health of mothers in the long term, especially those whose prior interactions with the healthcare system may have been limited.15
Other antenatal screening assessments aim to identify conditions unique to pregnancy when they are present, such as pre-eclampsia or fetal growth restriction, or predict the risk of such conditions at a sufficiently early gestation to permit initiation of prophylactic therapies and/or triaging of ongoing care to an appropriate facility or provider. Hypertension and proteinuria may, of course, be either pre-existing or of new onset in pregnancy, whereas the specific syndrome of pre-eclampsia is unique to the gestational state.
DEFINITIONS OF HYPERTENSION AND PROTEINURIA
Hypertension in pregnancy is diagnosed at blood pressure levels of >140/90 mmHg on two occasions 4 hours apart. A single reading indicative of severe hypertension (blood pressure >160/110 mmHg) is sufficient to establish a diagnosis. The concept of pre-hypertension has been proposed by the South African National Department of Health in their guideline on managing hypertension in pregnancy.1 The guideline recommends that women with borderline blood pressure levels of 135/85–139/89 mmHg have their blood pressure repeated within 30 minutes to 2 hours and, if still borderline, are asked to return to have their blood pressure checked within 3–7 days. The International Society for the Study of Hypertension in Pregnancy (ISSHP) has classified hypertension in pregnancy as follows:16
- Chronic hypertension – hypertension diagnosed before 20 weeks’ gestation
- Gestational hypertension – hypertension arising de novo after 20 weeks’ gestation in the absence of proteinuria and without biochemical or hematological abnormalities. It is usually not associated with fetal growth restriction.
- White-coat hypertension refers to elevated blood pressure levels recorded at a clinic (140/90 mmHg), but normal levels measured at home or work (< 135/85 mmHg). May be diagnosed before 20 weeks.
- Transient gestational hypertension – hypertension diagnosed during the second or third trimester but normalizes with repeated readings.
- Pre-eclampsia is diagnosed by hypertension after 20 weeks' gestation accompanied by proteinuria and/or evidence of acute kidney injury, liver dysfunction, neurological features, hemolysis or thrombocytopenia in the mother or fetal growth restriction.
Screening for proteinuria is best assessed using dipstix urinalysis and, if positive, quantified by a spot urine protein/creatinine ratio or 24-hour urine protein collection. A 24-hour urinary protein >300 mg/day or spot urine protein/creatinine ratio of >30 mg per mmol is abnormal.
SCREENING FOR HYPERTENSION
Pre-pregnancy counseling
Pre-pregnancy counseling may be of significant benefit in women who are known to have chronic hypertension or who have had previous episodes of gestational hypertension. It is vital to identify these patients prior to attempting pregnancy, in order to optimize their blood pressure control before the start of pregnancy and to ensure correct treatment and lifestyle modifications. All women planning a pregnancy should be encouraged to attend their primary health care provider to identify hitherto unrecognized hypertension and start on the correct management. This also represents an opportunity for counseling regarding lifestyle modification, especially weight optimization, and to recommend early booking for women with risk factors for pre-eclampsia (including chronic hypertension, obesity, diabetes, systemic lupus erythematosus, antiphospholipid syndrome, etc.), so that aspirin prophylaxis can be commenced in a timely fashion (see below).
Women taking angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) should be informed of an increased risk of adverse fetal outcomes if these drugs are taken during pregnancy.17 Both these drugs form part of the first-line management of blood pressure control outside pregnancy and also optimize proteinuria. Pre-pregnancy counseling will identify women who are using these and other potentially unsuitable drugs such as diuretics and high dose beta blockers. In patients with reliable access to care, these drugs only need to be changed once a pregnancy is confirmed. Clinicians may choose to avoid these drugs in women who are at risk of unplanned pregnancies that may be detected late.
Screening for hypertension during pregnancy
WHO released new basic antenatal care (BANC plus) guidelines in 2016.18 In these guidelines, they recommend eight antenatal care contacts during all pregnancies. They increased the number of contacts from four visits to eight contacts. The term ‘contact’ is used as this does not have to be a hospital or clinic visit, but may occur in the community by a trained community health worker. These recommendations are based on evidence that more frequent contacts lead to an increased detection of hypertension in pregnancy, thereby potentially reducing the stillbirth rate. A 1-year prospective trial conducted in 2017 in Pretoria, South Africa proved an increase in detection of hypertension in pregnancy, however, there was an unchanged rate in stillbirths.19 An increased detection of hypertension in pregnancy may, however, have other maternal and fetal benefits. This was a small study and will need to be repeated in multiple centers.
WHO proposes a booking visit as early in pregnancy as possible (before 14 weeks) with follow-up contacts at: 20, 26, 30, 34, 36, 38, 40 weeks (Table 1). Blood pressure and urine should be tested at every contact.
1
WHO 'basic antenatal care (BANC) plus’ schedule of antenatal contacts.
Gestation | Contact |
Before 12 weeks | Booking |
20 weeks | Ultrasound |
26 weeks | Contact |
30 weeks | Contact |
34 weeks | Contact |
36 weeks | Contact |
38 weeks | Contact |
40 weeks | Contact |
Screening for hypertension during labor
Blood pressure should be routinely monitored four hourly during the first stage of labor and hourly during the second stage of labor. Blood pressure should be checked after delivery is complete.17
Screening for hypertension by healthcare staff outside maternity services
Hypertension is mostly asymptomatic or may present with atypical features. Concerning signs include headache, blurred vision, epigastric pain, nausea and vomiting and difficulty breathing.20 Blood pressure checks are advised in all women who are pregnant presenting to health care facilities outside of their antenatal care facility, even with unrelated or loosely related complaints.
Postpartum screening for chronic hypertension
Women who were managed for chronic hypertension, gestational hypertension or pre-eclampsia during their pregnancy should have daily blood pressure monitoring for the first 2 days post-delivery. They should have another blood pressure assessment at least once between days 3 and 5, aiming for blood pressures below 140/90 mmHg.17 These women should be offered a postnatal follow-up appointment at 6 weeks to monitor their blood pressure and proteinuria. If these are not controlled, further investigation is required, including a possible kidney biopsy for systemic causes of proteinuria.
Numerous studies have tried to predict recurrence of hypertensive disorders in a following pregnancy. Women with gestational hypertension have a 13–53% chance of recurrence.17,20,21 Recurrence of pre-eclampsia is discussed in the section of this chapter on pre-eclampsia.
Accurate blood pressure measurement
Technique
The most recommended method of measuring blood pressure during and outside of pregnancy, is described in the European Society of Cardiology guidelines for the management of cardiovascular disease during pregnancy.21 The patient should be in the seated position (or left lateral recumbent position during labor), with an appropriately sized cuff (the bladder length of which should be 80% of the patient's arm circumference, and the width at least 40%)22 placed around the upper arm at the level of the heart. Most health services have two different sizes of blood pressure cuff, at most, with the larger cuff used when the mid-upper arm circumference is >33 cm. Korotkoff V (disappearance of sounds) should be used to determine diastolic blood pressure.
Equipment
Mercury manometers are still seen as the gold standard of measuring blood pressure, although such devices are no longer available. Unfortunately, the best alternative – liquid-crystal sphygmomanometry – is not yet widely available.16 Aneroid devices are commonly employed in their place, but these require regular calibration given their tendency to be inaccurate.
Untrained health care workers may find it easier to use automated cuffs, which are preferable to aneroid devices if they have been shown to be reliable in pregnancy in the presence or absence of pre-eclampsia. A group of British researchers have developed and tested a vital signs device that is reliable in pregnancy and has been validated in low- to middle-income countries.23 The Microlife® CRADLE (Community blood pressure monitoring in Rural Africa & Asia. Detection of underLying pre-Eclampsia and shock) Vital Signs Alert (VSA) is a hand-held, upper-arm, semi-automated device measuring blood pressure and pulse. The device uses a traffic light early warning system to aid untrained health care workers in referrals to health care facilities.
Self-monitoring
Women may elect to monitor their blood pressure at home on a calibrated blood pressure manometer. There is only moderate evidence for this practice,24 although the ISSHP guidelines recommend its use for women with chronic high blood pressure and ‘white coat’ hypertension.16 The woman’s blood pressure device should be brought in and compared to a calibrated device in the health center to ensure accuracy of measurements. A list of validated home blood pressure assessment devices (not specific to pregnancy) is available at http://bhosc.ord/bp-monitors/bp-monitors/.
Ambulatory blood pressure monitoring
There is a limited evidence for the use of ambulatory blood pressure monitoring (ABPM) in women with suspected white coat hypertension.24 A hyperbaric index (HBI) is calculated as the amount of blood pressure excess during a given time period above a 90% tolerance limit, with units of mmHg multiplied by hours. A single study has shown a 93% sensitivity and 100% specificity for an HBI calculated from an ABPM over 48 hours.25 Other studies have not been able to reproduce these results.26,27
Initial management of new-onset hypertension in pregnancy
Most young adults diagnosed with hypertension are asymptomatic. A thorough history and clinical examination is warranted before special investigations are requested.28
History
A detailed history should include enquiry into a previous diagnosis of chronic medical conditions (hypertension, diabetes mellitus, premature cardiovascular disease, stroke); use of medication (including antiretroviral therapy, immune-modulatory drugs, glucocorticoids); a history of stimulants (caffeine, alcohol, tobacco products, or illicit drugs such as cocaine and amphetamines); and risk factors for pre-eclampsia (multiple pregnancy, prior pre-eclampsia, family history of pre-eclampsia).
Examination
The examination should focus on determining the presence of target organ damage and features of secondary hypertension. Features suggestive of target organ damage include:
- A pressure loaded apex suggested of left ventricular hypertrophy;
- Retinal changes suggestive hypertensive retinopathy;
- The presence of blood or protein on a urine dipstick.
Features suggestive of secondary causes include:
- A difference in pulse volumes (vasculopathy, e.g. Takayasu’s arteritis);
- Radio-femoral delay, low or abnormal arm to leg blood pressures (coarctation of the aorta);29
- Abdominal bruits (renal artery stenosis);29
- Flushing, sweating, tachycardia (phaeochromocytoma);
- Thin skinfold, central obesity, moon facies, ecchymoses (Cushings disease);29
- Elevated body mass index (obesity and insulin resistance).
The absence of these clinical signs does not exclude the possibility of secondary causes.
Routine investigations
Hypertension diagnosed before 20 weeks’ gestation warrants a set of basic laboratory tests for initial assessment. These include serum urea and electrolytes, calcium, thyroid-stimulating hormone (TSH), fasting blood glucose, hematocrit, urine microscopy and protein excretion, and an electrocardiogram.
Fetal assessment
New-onset hypertension in the second half of pregnancy is an indication for sonographic assessment of fetal size in the third trimester, given that fetal growth restriction is a common corollary of gestational hypertension, and is one of the criteria that permits the diagnosis of pre-eclampsia.
Further assessment of secondary causes of chronic hypertension
Pregnancy-related hypertension is usually diagnosed in the second half of pregnancy and typically resolves within 3 months of delivery. If hypertension is diagnosed for the first time in the first half of pregnancy, or does not resolve after delivery, it is likely that the woman has undiagnosed chronic hypertension. Hypertension before the age of 35 should not be seen as essential hypertension until secondary causes have been investigated.18 In the developing world antiretroviral therapy30 and obesity are among the most common causes of early onset hypertension. Rare causes of hypertension should not be missed.
More than 90% of young patients with hypertension have primary (essential) hypertension. Secondary causes should only be pursued if clinically suspected or in cases of true refractory hypertension.31 The following are causes of secondary hypertension, and should be considered when suggested by the patient’s history and examination:32
- Renal disease of any form
- Polycystic kidney disease, chronic kidney disease, nephritic syndrome, etc.
- Renovascular disease
- Fibromuscular dysplasia in the young woman
- Arterosclerosis in advanced age
- Primary aldosteronism
- Presenting with refractory hypertension, hypokalemia and hypernatremia
- Cushings disease
- Excess cortisol production
- Pheochromocytoma
- Catecholamine secreting tumor
- Miscellaneous causes
- Obstructive sleep apnea
- Coarctation of the aorta
- Other endocrine disorders
- Thyroid disease
- Acromegaly
- Hypercalcemia of any cause.
Evidence for the benefit of identifying secondary causes of hypertension during pregnancy is lacking.24 None of the international guidelines has a suggested approach to the work-up of chronic hypertension. In centers where special investigations are possible and a clinical suspicion of secondary causes warrant further investigation, an appropriate work-up would include:28,33
- Electrolytes particularly potassium (Conn’s Syndrome), sodium (Cushing’s disease), calcium (hypercalcemia). Follow with specialized testing if abnormal;
- Urea, creatinine, urinalysis for blood and protein, urine microscopy for casts (intrinsic renal disease);
- Renal ultrasound (intrinsic renal disease, obstruction);
- An echocardiogram:
- ECG changes suggestive of left ventricular hypertrophy;
- Suspicion of coarctation of the aorta;
- Polysomnograph (obstructive sleep apnea);
- Fasting plasma or 24-hour fractionated urinary metanephrines (low index of suspicion of phaeochromocytoma).34
Only tests appropriate to the specific patient should be performed: clinicians should refrain from sending patients for a batch of tests to look for secondary causes. True refractory hypertension is defined as a blood pressure that remains above goal in spite of concurrent use of three antihypertensive agents of different classes.33
Hypertension detected after 20 weeks should be seen as pregnancy-related hypertension until proven otherwise, i.e. by persistence after delivery. Only patients with a clinical suspicion of secondary hypertension or refractory hypertension warrant specialized investigation. The initial work-up would be the same as for patients with hypertension before 20 weeks’ gestation.
Admission
The care pathway for patients in whom new-onset hypertension has been identified will vary according to the location and model of care in question. In many centers, such patients are admitted to ‘day assessment units’,35 in which serial blood pressure measurements may be performed, in addition to routine investigations for pre-eclampsia and fetal well-being. Such day units allow for a diagnosis to be made (transient hypertension, gestational hypertension or pre-eclampsia) without an inpatient admission. Further detail on the inpatient versus outpatient management of women with confirmed gestational hypertension or pre-eclampsia may be found in the respective chapters.
Admission for work-up of hypertension should always be considered for patients who:
- Have refractory hypertension and require urgent improvement of their blood pressure;
- Live far away from the hospital and require numerous special investigations; or
- Have other signs of maternal or fetal compromise.
Summary screening pathways for hypertension in pregnancy
Hypertension identified for the first time prior to 20 weeks’ gestation
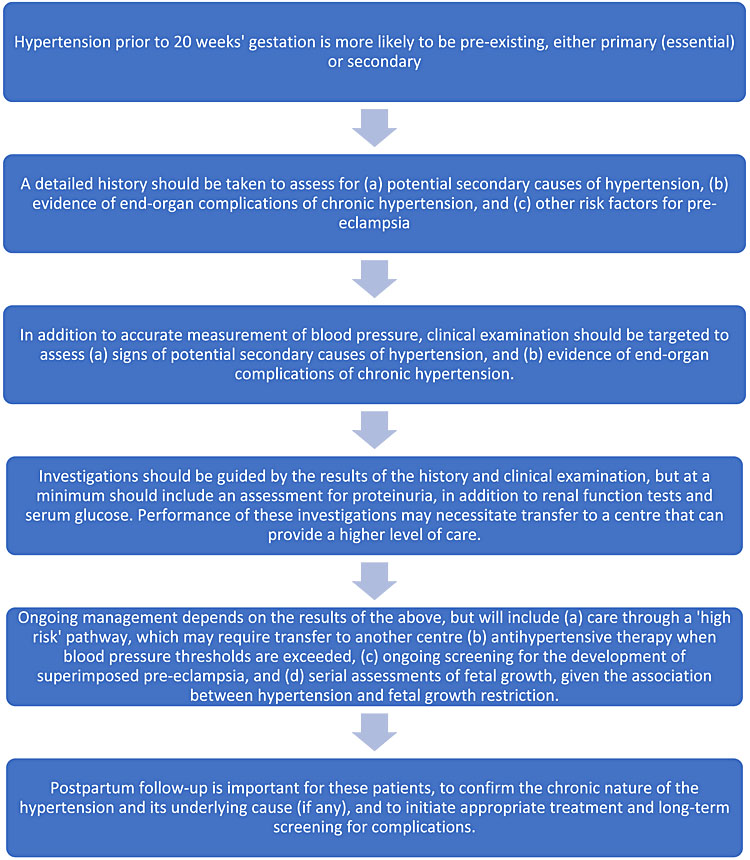
Hypertension identified for the first time after 20 weeks’ gestation
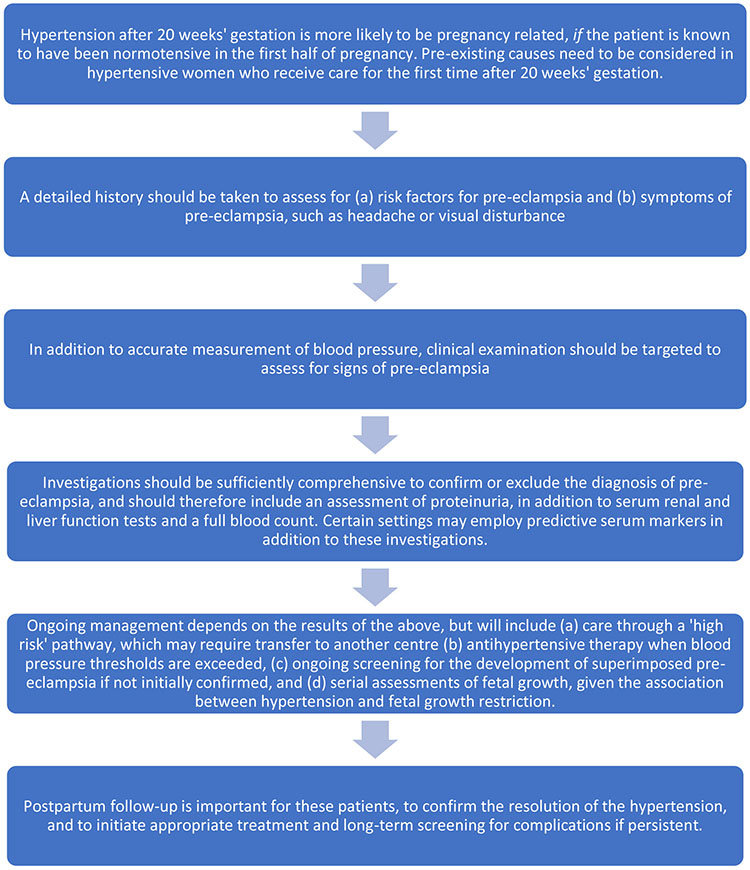
SCREENING FOR PROTEINURIA IN PREGNANCY
Proteinuria is an essential part of the diagnosis of pre-eclampsia. It is also a complication of intrinsic renal disease such as nephropathy related to diabetes, HIV or autoimmune disease. All these conditions are associated with a higher risk for pre-eclampsia in pregnancy. It can be very difficult to distinguish between primary renal disease and pre-eclampsia.
Proteinuria of more than 300 mg/24 hours in a pregnant woman is considered as abnormal and requires further investigation.36 Women with multiple pregnancies typically have a higher excretion of proteins; however, 300 mg/24 hours remains the upper acceptable limit of normal.37
The initial diagnosis of proteinuria in pregnancy should be quantified. If the onset is before 20 weeks’ gestation, this could point towards intrinsic kidney disease. Women with known chronic kidney disease who have more than 1 g/24 hours are at risk of adverse maternal and neonatal outcomes including preterm delivery, small for gestational age infants and admissions to neonatal ICU.38
Screening at antenatal visits
An assessment for proteinuria should be performed at the booking visit for all women. This can be done by sending a midstream urine sample for microbiological culture and sensitivity, or by dipsticking a clean catch of urine at the point of care. If proteinuria is present, urinary infection must be excluded as a cause. In the absence of infection, the presence of proteinuria necessitates further investigation, the nature of which would be determined by the patient’s history, examination findings, and gestation.
Guidelines differ in their recommendations regarding the role of screening for proteinuria at every antenatal contact or visit after the booking visit. An increased incidence of intrinsic renal disease has been reported in low- to middle-income countries such as South Africa.39,40 Routine screening for proteinuria is performed at every antenatal visit in these centers, by means of bedside urine dipstick testing. Similarly, in the UK, the NICE (National Institute for Health and Care Excellence) guidelines recommend screening for proteinuria at every antenatal visit, as a part of routine care for all patients41 (including those with mild to moderate hypertension).17 Women with severe hypertension (160/110 mmHg or greater) should be admitted and have daily assessment for proteinuria performed as part of their workup.
In contrast, guidelines for antenatal care in Australia42 and the USA43 do not recommend screening for proteinuria at every visit for healthy patients, only in those with pre-existing renal or systemic conditions (such as systemic lupus erythematosus or type 1 diabetes mellitus), or those with new-onset hypertension (to assess for pre-eclampsia). Women who enter pregnancy with established proteinuria are clearly not candidates for screening, but should have quantification of proteinuria performed periodically during pregnancy, especially if a flare of disease activity is suspected.
Advantages and disadvantages of urine dipstick testing
Bedside urine dipstick tests have been shown to have a high rate of false positive as well as false negative results when compared to a 24-hour urine protein excretion.44 A systematic review of 14 studies evaluating the accuracy of urine dipsticks in pregnancy found a wide range of accuracy (sensitivity 22–100%; specificity 36–100%).45 A comparison of manual and automated urine dipstick tests a found poor interobserver as well as observer to machine relationship.46 In many settings, manual urine dipsticks are still the only available measure of proteinuria.
A positive reaction on the dipstick (1+) develops when there is approximately 30 mg/dL of protein in the urine; this is the equivalent of 300 mg per day. Table 2 demonstrates the correlation between urinary dipstick readings and proteinuria values.
2
Correlation between urine dipstick readings and proteinuria.
Dipstick reading | Quantified proteinuria (mg/dL) |
None | None |
Trace | 15–30 |
1+ | 30–100 |
2+ | 100–300 |
3+ | 300–1000 |
4+ | >1000 |
False positive tests may result from:
- Macroscopic hematuria
- Semen
- Very alkaline urine (pH >7)
- Quaternary ammonium compounds
- Detergents, disinfectants
- Drugs
- Radio-contrast agents
- Concentrated urine (SG >1.030).
False negative tests may result from:
- Dilute urine (SG <1.010)
- High salt concentration
- Highly acidic urine
- Non-albumin proteinuria.
Quantifying proteinuria
If a urine dipstick is used to detect proteinuria, any reading of 1+ or more should be formally quantified for proteinuria via a spot urinary protein : creatinine ratio or 24-hour urine protein excretion.17 Significant proteinuria is diagnosed if the urinary protein : creatinine ratio is greater than 30 mg/mmol or a 24-hour urinary protein collection is greater than 300 mg.
The NICE guidelines17 and ACOG guidelines24 suggest quantifying proteinuria with either a spot protein : creatinine ratio or a 24-hour urine protein excretion. The ISSHP and Australian guidelines advocate quantifying of proteinuria with a spot protein : creatinine ratio.16,48 A ratio of ≥30 mg/mmol (0.3 mg/mg) is abnormal.
There is disagreement in the literature about the accuracy of the urinary spot protein : creatinine ratio for the detection of proteinuria in pregnant women with hypertension. A systemic review of 13 studies concluded that the protein : creatinine ratio is a reasonable “rule-out” test for screening for proteinuria of more than 0.3 g/day.49 Lane et al. argued that the predicted protein excretion compared poorly with a 24-hour excretion of protein when more than 1 g/24 hours was present.50 The protein : creatinine ratio did, however, provide good threshold values for above or below protein excretion rates of 300 mg/day. There are numerous benefits for the use of a spot urine collection including time, convenience, and reduced workload on nursing staff. Therefore, while a protein : creatinine ratio is not as accurate for quantifying proteinuria, it may be used to rule out the presence of abnormal levels of proteinuria.51
Follow-up of proteinuria
Unlike blood pressure, where the risk of adverse events correlates with higher values, there is no clear relationship between the severity of pre-eclampsia and the degree of proteinuria. Serial quantifying measures will rarely influence management.52 Formal quantifying of proteinuria should not be repeated once the diagnosis of pre-eclampsia has been established. In patients with intrinsic renal disease, repeat collections of protein excretion should be reserved for when progression of disease is suspected.
Summary screening pathway for proteinuria in pregnancy

SCREENING FOR PRE-ECLAMPSIA
Screening for the diagnosis of pre-eclampsia is largely achieved through the identification of hypertension in generally asymptomatic patients, followed by an assessment for the presence of other systemic features of the disease (including but not exclusively proteinuria), the absence of which would confer a diagnosis of gestational hypertension assuming it is of new-onset and no other cause is evident.
The principles of screening for hypertension and proteinuria have been covered above. This section focuses on antenatal assessments that can aid in the prediction of those women destined to develop pre-eclampsia. The purpose of such predictive assessments is threefold:53
- To allow initiation of prophylactic therapies in those at high risk, specifically aspirin54 and calcium;55
- To ensure the model of ongoing antenatal care is commensurate with a patient’s level of risk; and
- To permit the efficient recruitment of truly high-risk patients to trials of novel prophylactic therapies.
A range of approaches to the prediction of pre-eclampsia has evolved, the composition and performance of which are outlined below. This section is limited to reviewing approaches to the prediction of pre-eclampsia in women yet to develop the condition; strategies to predict the clinical outcome of women with established disease – such as the fullPIERS56 and miniPIERS57 models – are covered in another chapter.
Risk-factor based screening
History-based risk factor assessment remains the basis for all approaches to screening for pre-eclampsia.58 Indeed, the isolated use of other biochemical or ultrasonographic predictors of pre-eclampsia, without applying them to an a priori risk generated by risk factor assessment, is generally of limited clinical utility.
Clinical guidelines commonly recommend that an assessment of a patient’s risk of pre-eclampsia be determined at the booking visit.16,59 The relative risk associated with more common risk factors is outlined in Table 3 (adapted from Duckitt and Harrington).60
3
Risk factors for pre-eclampsia ascertainable at the booking visit. Reproduced from Duckitt and Harrington,60 with permission.
Risk factor | Relative risk | 95% confidence interval |
Prior pre-eclampsia | 7.19 | 5.85–8.83 |
Antiphospholipid antibody syndrome | 9.72 | 4.34–21.75 |
Pre-existing diabetes | 3.56 | 2.54–4.99 |
Multiple pregnancy | 2.93 | 2.04–4.21 |
Nulliparity | 2.91 | 1.28–6.61 |
Family history of pre-eclampsia | 2.90 | 1.70–4.93 |
Raised blood pressure at booking | 1.38 | 1.01–1.87 |
Raised body mass index pre-pregnancy | 2.47 | 1.66–3.67 |
Maternal age ≥40 (multiparous women) | 1.96 | 1.34–2.87 |
Other risk factors include interpregnancy interval of ≥10 years, autoimmune disease, renal disease, chronic hypertension, and use of assisted reproductive technology. | ||
Use of these risk factors in one of a range of algorithms will permit prediction of a substantial proportion of pre-eclampsia – up to 89% of early-onset (that requiring delivery by 34 weeks) and 93% of later onset disease – but at a ‘false positive’ rate of 64%,61 i.e. almost two-thirds of women would be deemed at high risk of pre-eclampsia but would not go on to develop the condition. The potential burden of this approach is not congruent with the principles of screening (see above), and has prompted the development of screening approaches that have lower false-positive rates.
Predictive tests in ‘asymptomatic’ women
Considerable research effort has been expended in attempting to identify a biochemical or biophysical parameter that could be assessed in early pregnancy to improve the predictive performance of maternal risk factors alone. No single such parameter – neither uterine artery Doppler analysis,62,63 nor any one of a wide range of serum biomarkers64,65 – has demonstrated adequate utility in this population. The search for a single predictive test continues, and has recently expanded to the ‘-omics’66 and cell-free DNA in maternal blood,67 but an optimal candidate remains elusive,47 likely as a consequence of the many antecedent pathways that lead to a final common maternal manifestation of the syndrome of pre-eclampsia.68
The experience of combined first-trimester screening for aneuploidy, in which multivariate analysis has permitted the development of multiparametric algorithms that combine a range of parameters to achieve high sensitivity and low false-positive rates, has informed the development of similar multiparametric predictive tests for pre-eclampsia.69 Such approaches generally combine maternal risk factors, maternal mean arterial pressure, uterine artery Doppler analysis, and one or more serum biomarkers such as placental growth factor (PlGF) and pregnancy-associated plasma protein A (PAPP-A).70 These models have been developed at a range of gestations, including the second71 and third trimesters,72 but are likely to be of greatest utility in the first trimester, when the capacity to prevent disease (a key principle of screening) is greatest. A summary of some of these multiparametric models and their performance in the cohort in which they were developed is contained in Table 4.62
4
Detection rate (DR) of early-onset pre-eclampsia at a 10% false positive rate (* = 5% false positive rate in this study) for multiparametric predictive models. Reproduced from Khong et al.,62without modification under the auspices of the Creative Commons Licence (CC BY).
Predictive model | Parameters | DR% |
Parra-Cordero73 | BMI, smoking, lowest UtA-PI, PlGF | 47 |
Odibo74 | HTN, mean UtA-PI, PAPP-A, PP-13 | 68 |
Poon75 | Maternal history, UtA-PI, PAPP-A | 71.9 |
Scazzocchio76 | Ethnicity, BMI, parity, previous PE, age, HTN, | 81 |
Poon77 | Ethnicity, BMI, parity, previous PE, age, HTN, DM, | 89 |
Crovetto78 | Maternal characteristics, MAP, UtA-PI, sFlt-1 | 91.2 |
Poon79 | Maternal characteristics, lowest UtA-PI, MAP, PlGF | 92.3 |
Poon70 | Maternal factors, UtA PI, MAP, PAPP-A, PlGF | 93.1* |
Poon80 | Ethnicity, BMI, parity, previous PE, age, HTN, DM, | 95 |
Akolekar81 | Maternal factors, MAP, UtA-PI, PAPP-A, PlGF, PP13, | 95.2 |
Akolekar82 | UtA-PI, MAP, PAPP-A, PlGF | 96.3 |
BMI, body mass index; UtA-PI, uterine artery pulsatility index; PlGF, placental growth factor; PAPP-A, pregnancy-associated plasma protein A; PP-13, placental protein 13; HTN, hypertension; PE, pre-eclampsia; MAP, mean arterial pressure; DM, diabetes mellitus; sFlt-1, soluble fms-like tyrosine kinase 1; sEng, soluble endoglin; PTX3, pentraxin-related protein 3.
The performance of these tests is such that some,16,83 but not all,84 professional guidelines recommend early pregnancy screening for pre-eclampsia using these multiparametric approaches, if they can be integrated into the local health system. The shift in guidance toward recommending such tests has largely arisen from the publication of the ASPRE study, which demonstrated a significant reduction in preterm pre-eclampsia in women screened to be high risk by a multiparametric model and administered aspirin when compared to an unscreened population (1.6% vs. 4.3%).85 The Fetal Medicine Foundation has made its predictive algorithm – incorporating maternal risk factors + mean arterial pressure ± mean uterine artery pulsatility index ± serum levels of PlGF or PAPP-A – available for use without charge on its website: https://fetalmedicine.org/research/assess/preeclampsia/first-trimester (accessed April 2019). FIGO recommends the universal application of this algorithm in the first trimester, and advises that maternal risk factors and mean arterial pressure should be used to assess the risk of pre-eclampsia in the first trimester where facilities for serum biomarker and uterine artery Doppler assessment are not available, rather than maternal risk factors alone.83
Nevertheless, these multiparametric predictive tests have a range of limitations that merit consideration.86 Their predictive utility is maximal for early-onset pre-eclampsia, whereas their performance in predicting later disease – which accounts for a majority of cases – is much poorer. The tests are complicated and expensive, requiring specialist laboratory and ultrasound equipment and expertise, and will thus be of no benefit in most low-income settings, where the incidence of pre-eclampsia is higher.87 Even in high-income settings, the economic justification for these tests may be challenging, given that the primary prophylactic agent employed in women at high risk is aspirin – a safe, cheap and well-tolerated drug that has potential benefits beyond the prevention of hypertensive disease, including lower rates of preterm birth, fetal growth restriction and perinatal death.88 Such observations have prompted repeated calls for universal aspirin prophylaxis to be considered by health authorities,89,90 in place of these screening tests.
Finally, it is important to note that test performance in one population cannot be assumed to be the same in others. Although similar results can be obtained when the validation population is similar to that in which the multiparametric algorithm was derived,91 quite varied results can occur in other populations, as outlined in Table 5.
5
External validation of multiparametric models for the prediction of early pre-eclampsia (< 34 weeks). Reproduced from Khong et al.62 without modification under the auspices of the Creative Commons Licence (CC BY).
| Study | Population | Incidence of early | Predictive models tested | Detection rate (%) at 10% false positive rate | |
| Reported | Observed | ||||
| Oliveira92 | 2962 American women | 1–1.2% | Parra-Cordero73 | 47 | 29 |
| Scazzocchio76 | 81 | 43 | |||
| Poon80 | 95 | 52 | |||
| Odibo74 | 68 | 80 | |||
| Park93 | 3066 Australian women | 0.4% | Poon80 | 95 | 91.7 |
In light of the above, it is imperative that individual institutions and health jurisdictions develop locally applicable approaches to the identification in early pregnancy of women at high risk of pre-eclampsia that reflect local resources and models of care.83
Predictive tests in women with suspected pre-eclampsia
It is not uncommon for maternity clinicians to suspect a diagnosis of pre-eclampsia in the course of routine antenatal care, often on account of borderline hypertension or symptoms such as headache. Most such patients will not go on to develop pre-eclampsia, although the process of excluding the evolution of this disease commonly involves repeated visits to an antenatal ‘day assessment’ unit or similar, wherein serial measurements of blood pressure are taken, along with blood and urine tests. This process is time and resource intensive for health services and patients alike, and has prompted the development of ‘rule-in/rule-out’ tests that can potentially result in an efficient and cost-effective means of determining those at low versus high risk.
In contrast to the multiparametric approaches described above, the focus in this population has been on the utility of a single blood test for angiogenic factors, most commonly for placental growth factor (PlGF) alone, or the ratio of soluble fms-like tyrosine kinase 1 (sFlt-1) to PlGF. The results of the largest cohort and randomized trial studies of these tests are presented in Table 6.
6
Summary of large cohort studies of ‘rule-in/rule-out’ tests for pre-eclampsia in patients with a suspected diagnosis of the same.
Study | Test and population | Result |
PELICAN94 | PlGF in 625 women with suspected pre-eclampsia in the UK at 20–35 weeks; all results concealed |
|
PROGNOSIS95 | sFlt/PlGF ratio in 550 women with suspected pre-eclampsia in 14 countries at 24–36+6 weeks; all results concealed |
|
MAPPLE96 | PlGF in 396 women with suspected pre-eclampsia in 4 countries; results revealed and clinical outcomes compared with PELICAN cohort |
|
PARROT97 | Randomized controlled trial of 1023 women with suspected pre-eclampsia in UK: 576 assigned to revealed testing group, 447 assigned to usual care and concealed testing |
|
sFlt, soluble fms-like tyrosine kinase 1; PlGF, placental growth factor; PPV, positive predictive value; NPV, negative predictive value; CI, confidence interval; RR, relative risk.
The primary utility of these tests is in ‘ruling out’ the development of pre-eclampsia in the ensuing week or month, as the positive predictive values of these tests are not sufficiently high to permit clinical utility in ‘ruling in’ the development of the disease. Health technology assessments98 have informed the 2019 UK National Institute for Health and Care Excellence (NICE) guidance that recommends the use of these tests to ‘rule out’ pre-eclampsia, along with standard clinical assessment and follow-up.99 In contrast, international guidelines still recommend that such testing only be performed in the research context,16 although the PARROT97 study provides randomized controlled trial-level evidence of neonatal safety that was previously a source of concern.
Again, it is essential that individual health jurisdictions assess the performance and economic implications of these tests in their own populations prior to their introduction.
Management of the ‘screen positive’ patient – frequency of visits, prophylaxis
A key principle of screening is having a defined care pathway for those deemed to be at ‘high risk’, regardless of the process by which such risk is ascertained. This pathway will vary depending on local resources and models of care, and may also depend on the magnitude of the risk estimate. In the context of screening for pre-eclampsia, a ‘screen positive’ result should optimally prompt two actions:
- Initiation of prophylactic therapies (see below); and
- Triage to a ‘higher risk’, more intensive model of antenatal care, within which frequent assessment of blood pressure and other clinical features of pre-eclampsia can be performed, with ready access to facilities for additional testing and evaluation. This would generally occur at least fortnightly after 24 weeks’ gestation, with more frequent assessments or inpatient admission indicated by the clinical course.
Aspirin has been shown to have a modest but consistent beneficial effect in reducing the incidence of pre-eclampsia by about 10%.100 There is ongoing debate as to the optimal dose, gestation of initiation and time of administration of ‘low dose’ aspirin for this purpose,101 which is reflected in the disparate recommendations made in various guidelines.16,17 Although subgroup analyses in recent systematic reviews have suggested that aspirin is only effective when commenced ≤16 weeks’ gestation and at doses of ≥100 mg,102 such analyses have significant limitations,101,103 and do not align with the findings of recent individual patient data (IPD) meta-analyses, in which beneficial effect is still observed whether treatment is started before or after 16 weeks,104 and with doses of <100 mg.105 It has been proposed that aspirin is more effective in preventing pre-eclampsia when taken in the evening,106 although further studies are required to confirm this finding.
The placebo-controlled ASPRE study85 used 150 mg of aspirin nocte in the treatment arm, in which the incidence of preterm pre-eclampsia was reduced to 1.6% from 4.3% in the control group. The latest guidelines from the International Society for the Study of Hypertension in Pregnancy recommend that 100–150 mg of aspirin be taken by at-risk women preferably from before 16 weeks’ gestation,16 although they acknowledge the uncertainty surrounding these parameters. The NICE guidelines recommend 75 mg of aspirin daily for this population,17 while ACOG – reflecting the position of the United States Preventive Services Task Force – recommends 81 mg daily.107 Further research will hopefully address these uncertainties and allow an alignment of recommendations.
The other prophylactic therapies that have shown benefit and should be recommended include:
- Calcium 1.2–2.5 g daily, in women with low calcium intake (< 600 mg/day);55 and
- Aerobic and strength/flexibility training exercise for 50 minutes per day, 3 times per week.108
Patients with previous early-onset pre-eclampsia that necessitated an extremely preterm delivery, and those with a history of pregnancy complications arising from antiphospholipid antibody syndrome (which has the added complexity of requiring low molecular weight heparin therapy in addition to aspirin), are at especially high risk for pre-eclampsia and require care in a multidisciplinary environment where available. Similarly, patients with pre-existing endocrine, cardiovascular or other autoimmune diseases (such as type 1 diabetes, essential hypertension and systemic lupus erythematosus) are at risk of a range of adverse pregnancy outcomes, and also require multidisciplinary care in pregnancy, ideally including input from an internist specializing in pregnancy (an obstetric physician).109
Centers in which ‘rule out’ testing has been introduced also need clear care pathways both for those deemed to be at higher risk, and those whose risk is assessed to be low. Figure 1 reproduces the algorithm used in the PARROT97 trial, the components of which would require modification for the resources of individual care settings.
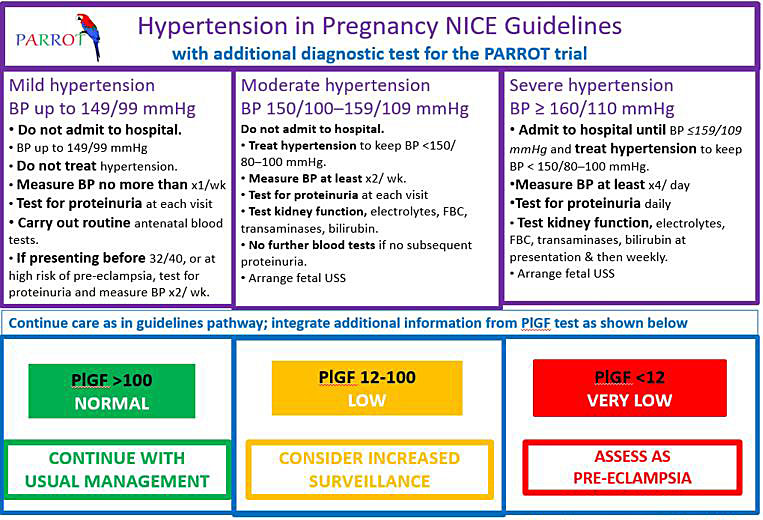
1
Clinical management algorithm in the PARROT trial for placental growth factor (PlGF) testing in women with suspected pre-eclampsia. Reproduced from Duhig et al.,97 without modification under the auspices of the Creative Commons Licence (CC BY).
PRACTICE RECOMMENDATIONS FOR HIGH-RESOURCE SETTINGS
- Pre-conceptional counseling may be beneficial for women with risk factors for pre-eclampsia.
- Blood pressure should be measured at every antenatal visit.
- Prevention and treatment of severe acute hypertension is important in reducing maternal mortality due to hypertensive disease in pregnancy.
- All women should be risk-assessed for pre-eclampsia at the booking visit, using the algorithm recommended by the International Federation of Gynecology and Obstetrics [FIGO] (available at https://fetalmedicine.org/research/assess/pre-eclampsia/first-trimester).
- Aspirin, started before 16 weeks, will reduce the risk of pre-eclampsia by around 10%. Calcium supplementation also results in a significant reduction in the incidence of pre-eclampsia, especially in those with low baseline calcium intakes.
- Proteinuria detected on dipstix should be quantified by either a spot urinary protein creatine ratio or 24-hour urine protein excretion.
- Pre-eclampsia is a risk factor for chronic hypertension and other long-term cardiovascular disease.
PRACTICE RECOMMENDATIONS FOR LOW-RESOURCE SETTINGS
- Pre-conceptional counseling for women with risk factors for pre-eclampsia is less commonly available in low-resource settings. Primary health care workers should be aware of these risk factors, and encourage women who possess them to seek care early in pregnancy.
- Blood pressure should be measured at every antenatal contact, whether in a health care facility or in the community. This may most easily be achieved with automated devices that indicate when referral to a higher level of care is indicated.
- Prevention and treatment of severe hypertension is essential to mitigate the risks of hypertensive disorders of pregnancy, regardless of the setting in which it is identified. For this reason, health authorities should ensure that the antihypertensive agents considered by WHO to be essential medicines are readily available.
- All women should be risk-assessed for pre-eclampsia at the booking visit, using the FIGO-recommended algorithm (available at https://fetalmedicine.org/research/assess/pre-eclampsia/first-trimester). This algorithm allows for calculation of risk even without serum biomarkers or uterine artery Doppler assessment.
- Aspirin and calcium reduce the risk of pre-eclampsia, and should be prescribed to those at high risk, ideally prior to the second trimester.
- Urine should be assessed for the presence of protein at the booking visit, and thereafter according to local guidelines. When its presence is suggested by dipstick testing, an attempt should be made to quantify proteinuria by whatever means is available.
- Pre-eclampsia is a risk factor for chronic hypertension and other long-term cardiovascular disease, and should be integrated into local risk assessment strategies for use by community-based primary health care workers .
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Pattinson RC. Saving Mothers 2017: Report on Confidential Enquiries into Maternal Deaths in South Africa. Pretoria, South Africa: Department of Health; 2019. | |
Nyflot LT, Ellingsen L, Yli BM, et al. Maternal deaths from hypertensive disorders: lessons learnt. Acta Obstetricia et Gynecologica Scandinavica 2018;Epub before print 2018/04/18 (doi: 10.1111/aogs.13357). | |
Knight M, Kenyon S, Brocklehurst P, et al. Saving Lives, Improving Mothers’ Care Lessons learned to inform future maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2009–2012. Available online at: https://wwwnpeuoxacuk/. 2014. | |
Shennan AH, Green M, Chappell LC. Maternal deaths in the UK: pre-eclampsia deaths are avoidable. Lancet 2017;389(10069):582–4. | |
Conti-Ramsden F, Knight M, Green M, et al. Reducing maternal deaths from hypertensive disorders: learning from confidential inquiries. BMJ (Clinical Research Edn.) 2019;364:l230. | |
Schutte JM, Steegers EA, Schuitemaker NW, et al. Rise in maternal mortality in the Netherlands. BJOG: an International Journal of Obstetrics and Gynaecology 2010;117(4):399–406. | |
Ekiz A, Kaya B, Polat I, et al. The outcome of pregnancy with new onset proteinuria without hypertension: retrospective observational study. The Journal of Maternal-Fetal & Neonatal Medicine: the Official Journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 2016;29(11):1765–9. | |
Sarno L, Maruotti GM, Saccone G, et al. Pregnancy outcome in proteinuria-onset and hypertension-onset preeclampsia. Hypertension in Pregnancy: Official Journal of the International Society for the Study of Hypertension in Pregnancy 2015;34(3):284–90. | |
Foo L, Tay J, Lees CC, et al. Hypertension in pregnancy: natural history and treatment options. Current Hypertension Reports 2015;17(5):36. | |
Soma-Pillay P, Louw MC, Adeyemo AO, et al. Cardiac diastolic function after recovery from pre-eclampsia. Cardiovasc J Afr 2018;29(1):26–31. | |
Cheung KL, Lafayette RA. Renal physiology of pregnancy. Adv Chronic Kidney Dis 2013;20(3):209–14. | |
Wald NJ. The definition of screening. J Med Screen 2001;8(1):1. | |
Wilson J, Jungner G. Principles and practice of screening for disease. World Health Organisation, Geneva, 1968 Public health papers. 1968;34. | |
Andermann A, Blancquaert I, Beauchamp S, et al. Revisiting Wilson and Jungner in the genomic age: a review of screening criteria over the past 40 years. Bulletin of the World Health Organization 2008;86:317–9. | |
Landeen LB, Bogue R, Schuneman M. Preconception and prenatal care–useful tools for providers of women's health. South Dakota Medicine: the Journal of the South Dakota State Medical Association 2015;Spec No:36–43. | |
Brown MA, Magee LA, Kenny LC, et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens 2018;13:291–310. | |
National Institute for Health and Clinical Excellence. Hypertension in Pregnancy: The Management of Hypertensive Disorders During Pregnancy 2010. | |
World Health Organization. WHO recommendations on antenatal care for a positive pregnancy experience: World Health Organization; 2016. p. | |
Hlongwane TV, V, Nkosi, BSS, Pattinson, RC. The effect of implementing basic antenatal care (BANC) plus on workload, detecting hypertension and perinatal mortality (conference abstract). 37th Conference on Priorities in Perinatal Care in Southern Africa; South Africa 2018. | |
Mol BWJ, Roberts CT, Thangaratinam S, et al. re-eclampsia. Lancet 2016;387(10022):999–1011. | |
Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. European Heart Journal 2018;39(34):3165–241. | |
Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018;71(6):1269–324. | |
Nathan HL, Boene H, Munguambe K, et al. The CRADLE vital signs alert: qualitative evaluation of a novel device designed for use in pregnancy by healthcare workers in low-resource settings. Reprod Health 2018;15(1):5. | |
ACOG Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstetrics and Gynecology 2013;122(5):1122–31. | |
Hermida RC, Ayala DE, Mojon A, et al. Blood pressure excess for the early identification of gestational hypertension and preeclampsia. Hypertension 1998;31(1):83–9. | |
Vollebregt KC, Gisolf J, Guelen I, et al. Limited accuracy of the hyperbaric index, ambulatory blood pressure and sphygmomanometry measurements in predicting gestational hypertension and preeclampsia. Journal of Hypertension 2010;28(1):127–34. | |
Brown MA, Bowyer L, McHugh L, et al. Twenty-four-hour automated blood pressure monitoring as a predictor of preeclampsia. American Journal of Obstetrics and Gynecology 2001;185(3):618–22. | |
Mangena P, Saban S, Hlabyago KE, et al. An approach to the young hypertensive patient. S Afr Med J 2016;106(1):36–8. | |
McGee SR. Evidence-based physical diagnosis. 4th edn. Philadelphia, PA: Elsevier; 2018. xvi, 736 pages. p. 89–94. | |
Dimala CA, Atashili J, Mbuagbaw JC, et al. Prevalence of Hypertension in HIV/AIDS Patients on Highly Active Antiretroviral Therapy (HAART) Compared with HAART-Naive Patients at the Limbe Regional Hospital, Cameroon. PloS one 2016;11(2):e0148100. | |
Rimoldi SF, Scherrer U, Messerli FH. Secondary arterial hypertension: when, who, and how to screen? European Heart Journal 2013;35(19):1245–54. | |
Kasper DL. Harrison's principles of internal medicine. 19th edn./eds, Dennis L. Kasper, MD, William Ellery Channing. edn. New York: McGraw Hill Education; 2015. p. 298–302. | |
Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. European Heart Journal 2018;39(33):3021–104. | |
Lenders JW, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA: the Journal of the American Medical Association 2002;287(11):1427–34. | |
Saleh MM, Selinger M. Evaluation of the role of day assessment unit in the management of pregnancy induced hypertension. Journal of Obstetrics and Gynaecology: the Journal of the Institute of Obstetrics and Gynaecology 2005;25(7):651–5. | |
Higby K, Suiter CR, Phelps JY, et al. Normal values of urinary albumin and total protein excretion during pregnancy. American Journal of Obstetrics and Gynecology 1994;171(4):984–9. | |
Osmundson SS, Lafayette RA, Bowen RA, et al. Maternal proteinuria in twin compared with singleton pregnancies. Obstetrics and Gynecology 2014;124(2 Pt 1):332–7. | |
Piccoli GB, Cabiddu G, Attini R, et al. Risk of Adverse Pregnancy Outcomes in Women with CKD. J Am Soc Nephrol 2015;26(8):2011–22. | |
Mills KT, Xu Y, Zhang W, et al. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int 2015;88(5):950–7. | |
Nathan HL, Seed PT, Hezelgrave NL, et al. Maternal and perinatal adverse outcomes in women with pre-eclampsia cared for at facility-level in South Africa: a prospective cohort study. J Glob Health 2018;8(2):020401. | |
National Institute for Health Care Excellence. Antenatal Care for Uncomplicated Pregnancies. NICE clinical guidelines Updated edition London; 2008. | |
Expert Advisory Committee. Clinical Practice Guidelines: Pregnancy Care. Canberra: Australian Government Department of Health; 2018. | |
Zolotor AJ, Carlough MC. Update on prenatal care. Am Fam Physician 2014;89(3):199–208. | |
Waugh JJ, Clark TJ, Divakaran TG, et al. Accuracy of urinalysis dipstick techniques in predicting significant proteinuria in pregnancy. Obstetrics and Gynecology 2004;103(4):769–77. | |
Henderson JT, Thompson JH, Burda BU, et al. Preeclampsia Screening: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA: the Journal of the American Medical Association 2017;317(16):1668–83. | |
Ahmed AI, Baz H, Lotfy S. Urinalysis: The Automated Versus Manual Techniques; Is It Time To Change? Clin Lab. 2016;62(1–2):49–56. | |
Townsend R, Khalil A, Premakumar Y, et al. Prediction of pre-eclampsia: review of reviews. Ultrasound in Obstetrics & Gynecology: the Official Journal of the International Society of Ultrasound in Obstetrics and Gynecology 2018;Epub date 2018/09/30 (doi: 10.1002/uog.20117). | |
Lowe SA, Bowyer L, Lust K, et al. The SOMANZ Guidelines for the Management of Hypertensive Disorders of Pregnancy 2014. The Australian & New Zealand Journal of Obstetrics & Gynaecology 2015;55(1):11–6. | |
Cote AM, Brown MA, Lam E, et al. Diagnostic accuracy of urinary spot protein:creatinine ratio for proteinuria in hypertensive pregnant women: systematic review. BMJ (Clinical Research Edn.) 2008;336(7651):1003–6. | |
Lane C, Brown M, Dunsmuir W, et al. Can spot urine protein/creatinine ratio replace 24 h urine protein in usual clinical nephrology? Nephrology (Carlton) 2006;11(3):245–9. | |
Bramham K, Hall M, Nelson-Piercy C. Renal disease in pregnancy. Cambridge University Press; 2018. | |
Chappell LC, Shennan AH. Assessment of proteinuria in pregnancy. BMJ (Clinical Research Edn.) 2008;336(7651):968–9. | |
Kane SC, Da Silva Costa F, Brennecke SP. New directions in the prediction of pre-eclampsia. The Australian & New Zealand Journal of Obstetrics & Gynaecology 2014;54(2):101–7. | |
Duley L, Henderson-Smart DJ, Meher S, et al. Antiplatelet agents for preventing pre-eclampsia and its complications (Review). Cochrane Database of Systematic Reviews 2007(2):121. | |
Hofmeyr GJ, Lawrie TA, Atallah AN, et al. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. The Cochrane Database of Systematic Reviews 2010(8):Cd001059. | |
Almeida ST, Katz L, Coutinho I, et al. Validation of fullPIERS model for prediction of adverse outcomes among women with severe pre-eclampsia. International Journal of Gynaecology and Obstetrics: the Official Organ of the International Federation of Gynaecology and Obstetrics 2017;138(2):142–7. | |
Payne BA, Hutcheon JA, Ansermino JM, et al. A risk prediction model for the assessment and triage of women with hypertensive disorders of pregnancy in low-resourced settings: the miniPIERS (Pre-eclampsia Integrated Estimate of RiSk) multi-country prospective cohort study. PLoS Med 2014;11(1):e1001589. | |
Al-Rubaie Z, Askie LM, Ray JG, et al. The performance of risk prediction models for pre-eclampsia using routinely collected maternal characteristics and comparison with models that include specialised tests and with clinical guideline decision rules: a systematic review. BJOG: An International Journal of Obstetrics and Gynaecology 2016;123(9):1441–52. | |
Al-Rubaie ZTA, Askie LM, Hudson HM, et al. Assessment of NICE and USPSTF guidelines for identifying women at high risk of pre-eclampsia for tailoring aspirin prophylaxis in pregnancy: An individual participant data meta-analysis. European Journal of Obstetrics, Gynecology and Reproductive Biology 2018;229:159–66. | |
Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ (Clinical Research Edn.) 2005;330(7491):565-. | |
Poon LCY, Kametas NA, Chelemen T, et al. Maternal risk factors for hypertensive disorders in pregnancy: a multivariate approach. Journal of Human Hypertension 2010;24(2):104–10. | |
Khong SL, Kane SC, Brennecke SP, et al. First-trimester uterine artery Doppler analysis in the prediction of later pregnancy complications. Disease Markers 2015;2015:679730. | |
Velauthar L, Plana MN, Kalidindi M, et al. First-trimester uterine artery Doppler and adverse pregnancy outcome: a meta-analysis involving 55 974 women. Ultrasound in Obstetrics & Gynecology 2014;43(5):500–7. | |
Anderson UD, Olsson MG, Kristensen KH, et al. Review: Biochemical markers to predict preeclampsia. Placenta 2012;33 Suppl:S42–7. | |
Grill S, Rusterholz C, Zanetti-Dallenbach R, et al. Potential markers of preeclampsia–a review. Reprod Biol Endocrinol 2009;7:70. | |
Bahado-Singh RO, Akolekar R, Mandal R, et al. First-trimester metabolomic detection of late-onset preeclampsia. American Journal of Obstetrics and Gynecology 2013;208(1):58.e1–7. | |
Martin A, Krishna I, Martina B, et al. Can the quantity of cell-free fetal DNA predict preeclampsia: a systematic review. Prenatal Diagnosis 2014;34(7):685–91. | |
Baschat AA. First-trimester screening for pre-eclampsia: moving from personalized risk prediction to prevention. Ultrasound in Obstetrics & Gynecology 2015;45(2):119–29. | |
Cuckle HS. Screening for pre-eclampsia – lessons from aneuploidy screening. Placenta 2011;32 Suppl:S42–8. | |
Poon LC, Kametas NA, Maiz N, et al. First-trimester prediction of hypertensive disorders in pregnancy. Hypertension 2009;53(5):812–8. | |
Al-Amin A, Rolnik DL, Black C, et al. Accuracy of second trimester prediction of preterm preeclampsia by three different screening algorithms. The Australian & New Zealand Journal of Obstetrics & Gynaecology 2018;58(2):192–6. | |
Andrietti S, Silva M, Wright A, et al. Competing-risks model in screening for pre-eclampsia by maternal factors and biomarkers at 35–37 weeks' gestation. Ultrasound in Obstetrics & Gynecology: the Official Journal of the International Society of Ultrasound in Obstetrics and Gynecology 2016;48(1):72–9. | |
Parra-Cordero M, Rodrigo R, Barja P, et al. Prediction of early and late pre-eclampsia from maternal characteristics, uterine artery Doppler and markers of vasculogenesis during first trimester of pregnancy. Ultrasound in Obstetrics and Gynaecology 2013(5):538. | |
Odibo AO, Zhong Y, Goetzinger KR, et al. First-trimester placental protein 13, PAPP-A, uterine artery Doppler and maternal characteristics in the prediction of pre-eclampsia. Placenta 2011;32:598–602. | |
Poon LC, Maiz N, Valencia C, et al. First-trimester maternal serum pregnancy-associated plasma protein-A and pre-eclampsia. Ultrasound in Obstetrics & Gynecology: the Official Journal of the International Society of Ultrasound in Obstetrics and Gynecology 2009;33(1):23–33. | |
Scazzocchio E, Figueras F, Crispi F, et al. Performance of a first-trimester screening of preeclampsia in a routine care low-risk setting. American Journal of Obstetrics and Gynecology 2013;208(3):10. | |
Poon LCY, Karagiannis G, Leal A, et al. Hypertensive disorders in pregnancy: screening by uterine artery Doppler imaging and blood pressure at 11–13 weeks. Ultrasound in Obstetrics & Gynecology 2009;34(5):497–502. | |
Crovetto F, Figueras F, Triunfo S, et al. First trimester screening for early and late preeclampsia based on maternal characteristics, biophysical parameters and angiogenic factors. Prenatal diagnosis 2014. | |
Poon LC, Akolekar R, Lachmann R, et al. Hypertensive disorders in pregnancy: screening by biophysical and biochemical markers at 11–13 weeks. Ultrasound in Obstetrics & Gynecology: the Official Journal of the International Society of Ultrasound in Obstetrics and Gynecology 2010;35(6):662–70. | |
Poon LCY, Stratieva V, Piras S, et al. Hypertensive disorders in pregnancy: combined screening by uterine artery Doppler, blood pressure and serum PAPP-A at 11–13 weeks. Prenatal Diagnosis 2010;30(3):216–23. | |
Akolekar R, Syngelaki A, Sarquis R, et al. Prediction of early, intermediate and late pre-eclampsia from maternal factors, biophysical and biochemical markers at 11–13 weeks. Prenatal Diagnosis 2011;31(1):66–74. | |
Gallo DM, Poon LC, Akolekar R, et al. Prediction of preeclampsia by uterine artery Doppler at 20–24 weeks' gestation. Fetal Diagnosis and Therapy 2013;34(4):241–7. | |
Poon LC, Shennan A, Hyett JA, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. International Journal of Gynaecology and Obstetrics: the Official Organ of the International Federation of Gynaecology and Obstetrics 2019;145 Suppl 1:1–33. | |
ACOG Committee on Obstetric Practice. Committee Opinion No. 638: First-Trimester Risk Assessment for Early-Onset Preeclampsia. Obstetrics and Gynecology 2015;126(3):e25–7. | |
Rolnik DL, Wright D, Poon LC, et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N Engl J Med 2017;377(7):613–22. | |
Kane SC. First trimester screening for pre-eclampsia. Obstet Med 2016;9(3):106–12. | |
Duley L. The Global Impact of Pre-eclampsia and Eclampsia. Seminars in Perinatology 2009;33(3):130–7. | |
Roberge S, Nicolaides KH, Demers S, et al. Prevention of perinatal death and adverse perinatal outcome using low-dose aspirin: a meta-analysis. Ultrasound in Obstetrics & Gynecology: the Official Journal of the International Society of Ultrasound in Obstetrics and Gynecology 2013;41(5):491–9. | |
Ayala NK, Rouse DJ. A Nudge Toward Universal Aspirin for Preeclampsia Prevention. Obstetrics and Gynecology 2019;133(4):725–8. | |
Mone F, Mulcahy C, McParland P, et al. Should we recommend universal aspirin for all pregnant women? American Journal of Obstetrics and Gynecology 2017;216(2):141.e1-.e5. | |
Scazzocchio E, Crovetto F, Triunfo S, et al. Validation of a first-trimester screening model for pre-eclampsia in an unselected population. Ultrasound in Obstetrics & Gynecology: the Official Journal of the International Society of Ultrasound in Obstetrics and Gynecology 2017;49(2):188–93. | |
Oliveira N, Magder LS, Blitzer MG, et al. First-trimester prediction of pre-eclampsia: external validity of algorithms in a prospectively enrolled cohort. Ultrasound in Obstetrics & Gynecology 2014;44(3):279–85. | |
Park FJ, Leung CH, Poon LC, et al. Clinical evaluation of a first trimester algorithm predicting the risk of hypertensive disease of pregnancy. The Australian & New Zealand Journal of Obstetrics & Gynaecology 2013;53(6):532–9. | |
Chappell LC, Duckworth S, Seed PT, et al. Diagnostic Accuracy of Placental Growth Factor in Women With Suspected Preeclampsia. Circulation 2013;128(19):2121–31. | |
Zeisler H, Llurba E, Chantraine F, et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N Engl J Med 2016;374(1):13–22. | |
Sharp A, Chappell LC, Dekker G, et al. Placental Growth Factor informed management of suspected pre-eclampsia or fetal growth restriction: The MAPPLE cohort study. Pregnancy Hypertens 2018;14:228–33. | |
Duhig KE, Myers J, Seed PT, et al. Placental growth factor testing to assess women with suspected pre-eclampsia: a multicentre, pragmatic, stepped-wedge cluster-randomised controlled trial. Lancet 2019. | |
Frampton GK, Jones J, Rose M, et al. Placental growth factor (alone or in combination with soluble fms-like tyrosine kinase 1) as an aid to the assessment of women with suspected pre-eclampsia: systematic review and economic analysis. Health technology assessment (Winchester, England) 2016;20(87):1–160. | |
National Institute for Health Care Excellence. PlGF‐based testing to help diagnose suspected pre‐eclampsia (Triage PlGF test, Elecsys immunoassay sFlt‐1/PlGF ratio, DELFIA Xpress PlGF 1‐2‐3 test, and BRAHMS sFlt‐1 Kryptor/BRAHMS PlGF plus Kryptor PE ratio). 2016. | |
Askie LM, Duley L, Henderson-Smart DJ, et al. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet 2007;369(9575):1791–8. | |
Askie L, Duley L. Associations between the timing and dosing of aspirin prophylaxis and term and preterm pre-eclampsia. BMJ Evid Based Med 2018; Epub date 2018/06/09 (doi: 10.1136/bmjebm-2018-110931). | |
Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis. American Journal of Obstetrics and Gynecology 2018;218(3):287–93 e1. | |
Tong S, Mol BW, Walker SP. Preventing preeclampsia with aspirin: does dose or timing matter? American Journal of Obstetrics and Gynecology 2017;216(2):95–7. | |
Meher S, Duley L, Hunter K, et al. Antiplatelet therapy before or after 16 weeks' gestation for preventing preeclampsia: an individual participant data meta-analysis. American Journal of Obstetrics and Gynecology 2017;216(2):121–8 e2. | |
Seidler AL, Askie L, Ray JG. Optimal aspirin dosing for preeclampsia prevention. American Journal of Obstetrics and Gynecology 2018;219(1):117–8. | |
Ayala DE, Ucieda R, Hermida RC. Chronotherapy with low-dose aspirin for prevention of complications in pregnancy. Chronobiology International 2013;30(1–2):260–79. | |
American Congress of Obstetricians and Gynecologists. ACOG Committee Opinion No. 743: Low-Dose Aspirin Use During Pregnancy. Obstetrics and Gynecology 2018;132(1):e44-e52. | |
Davenport MH, Ruchat SM, Poitras VJ, et al. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: a systematic review and meta-analysis. Br J Sports Med 2018;52(21):1367–75. | |
Rosene-Montella K, Lowe S, Nelson-Piercy C. The growing importance of medical problems in pregnancy. Obstetric Medicine 2010;3(1):1–. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards programme CLICK HERE)

