<< back to Pathology Atlas menu
Pathology Atlas: Ovary
Low Malignant Potential
Malignancy
Normal Growth and Development
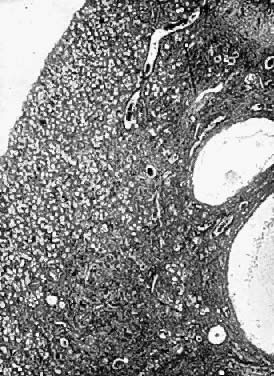 Photomicrograph (low power) of the cortex of the ovary of a human infant. The
cortex of the ovary has numerous primordial germ cells with relatively
little stroma. The ovarian stroma is more abundant in the medulla, where
the larger follicles are seen.
Photomicrograph (low power) of the cortex of the ovary of a human infant. The
cortex of the ovary has numerous primordial germ cells with relatively
little stroma. The ovarian stroma is more abundant in the medulla, where
the larger follicles are seen.
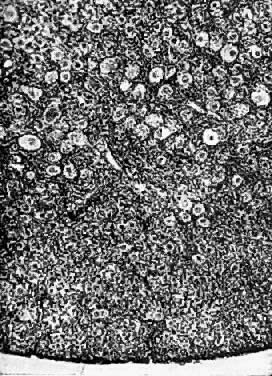 Photomicrograph (medium power) of the human ovary. The germinal epithelium
of the ovary rests upon the ovarian stroma. The primordial germ cells
embedded in the stroma are in the cortex of the ovary.
Photomicrograph (medium power) of the human ovary. The germinal epithelium
of the ovary rests upon the ovarian stroma. The primordial germ cells
embedded in the stroma are in the cortex of the ovary.
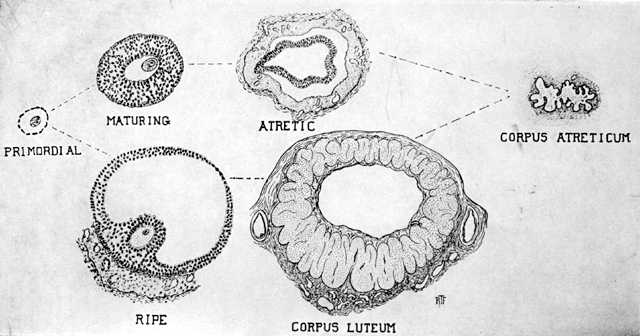 Life history of the ovarian follicle. Starting with the primordial follicle, we
have next the maturing follicle. Approximately 1 of 300 follicles
fully (as shown on lower line), ruptures, and a corpus luteum. The
other 299 become atretic. The final stage of both the artetic follicle
and the corpus luteum is the corpus atreticum, with eventual reabsorption
of this scar into the stroma of the ovary. (Frank.)
Life history of the ovarian follicle. Starting with the primordial follicle, we
have next the maturing follicle. Approximately 1 of 300 follicles
fully (as shown on lower line), ruptures, and a corpus luteum. The
other 299 become atretic. The final stage of both the artetic follicle
and the corpus luteum is the corpus atreticum, with eventual reabsorption
of this scar into the stroma of the ovary. (Frank.)
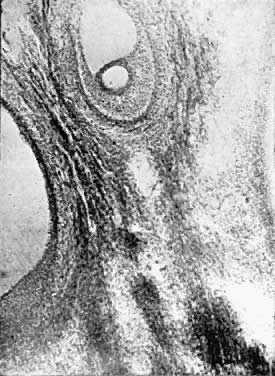 Photomicrograph (low power) of the graafian follicle of the human ovary. The
eccentric location of the primordial germ cell is seen in the graafian
follicle.
Photomicrograph (low power) of the graafian follicle of the human ovary. The
eccentric location of the primordial germ cell is seen in the graafian
follicle.
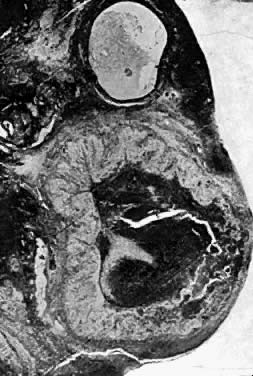 Photomicrograph (low power) of the corpus luteum of the human ovary. The
developing corpus luteum with the central hemmorrhagic area is contiguous
to a graafian follicle.
Photomicrograph (low power) of the corpus luteum of the human ovary. The
developing corpus luteum with the central hemmorrhagic area is contiguous
to a graafian follicle.
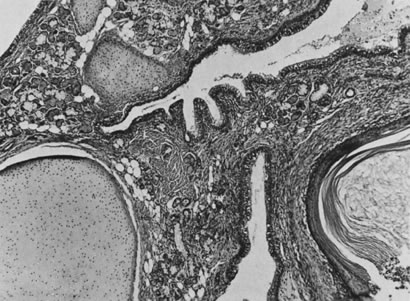
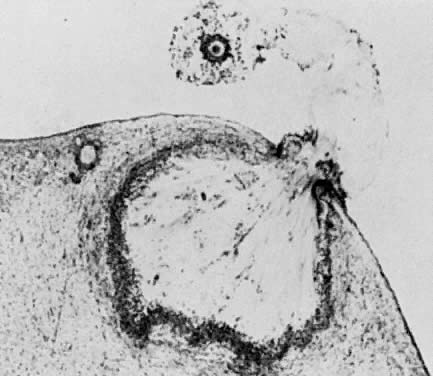 Photomicrograph of ovulation shows the expanded egg-cumulus complex leaving
the follicle through the stigma. The remaining cells in the follicle
wall ( i.e. granulosa, membrana and periantral, theca, the theca interna and externa) develop
into the corpus luteum.(From Blandau RJ: Growth of the ovarian follicle and ovulation. Prog Gynecol 5:58, 1970.)
Photomicrograph of ovulation shows the expanded egg-cumulus complex leaving
the follicle through the stigma. The remaining cells in the follicle
wall ( i.e. granulosa, membrana and periantral, theca, the theca interna and externa) develop
into the corpus luteum.(From Blandau RJ: Growth of the ovarian follicle and ovulation. Prog Gynecol 5:58, 1970.)
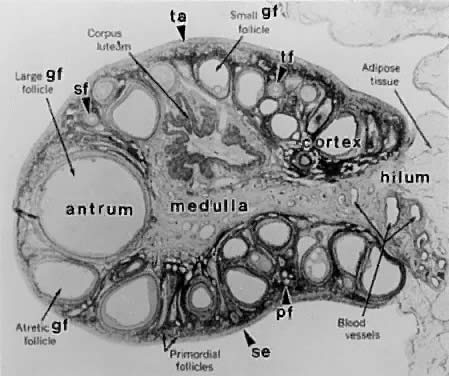 Photomicrograph of an adult primate ovary. Follicular and luteal units
are seen in the cortex and large blood vessels and nerves in the medulla. se, serous
or surface epithelium; ta, tunica albuginea; pf, primary
follicle; sf, secondary follicle; tf, tertiary follicle; gf, graafian
follicle.(From Bloom W, Fawcett DW: A Textbook of Histology. Philadelphia: WB Saunders, 1975.)
Photomicrograph of an adult primate ovary. Follicular and luteal units
are seen in the cortex and large blood vessels and nerves in the medulla. se, serous
or surface epithelium; ta, tunica albuginea; pf, primary
follicle; sf, secondary follicle; tf, tertiary follicle; gf, graafian
follicle.(From Bloom W, Fawcett DW: A Textbook of Histology. Philadelphia: WB Saunders, 1975.)
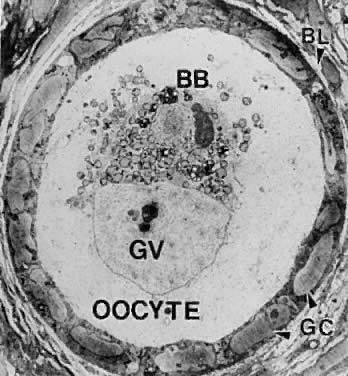 Electron micrograph of a human primordial follicle shows the flattened
granulosa cells (GC), the oocyte with its germinal vesicle (GV) or nucleus, the
Balbiani body (BB), with all the oocyte organelles gathered
at one pole of the GV, and basal lamina (BL).(From Erickson GF: The ovary: Basic principles and concepts. In Felig P, Baxter
JD, Frohman L (eds): Endocrinology and Metabolism. New York: McGraw-Hill, 1995.)
Electron micrograph of a human primordial follicle shows the flattened
granulosa cells (GC), the oocyte with its germinal vesicle (GV) or nucleus, the
Balbiani body (BB), with all the oocyte organelles gathered
at one pole of the GV, and basal lamina (BL).(From Erickson GF: The ovary: Basic principles and concepts. In Felig P, Baxter
JD, Frohman L (eds): Endocrinology and Metabolism. New York: McGraw-Hill, 1995.)
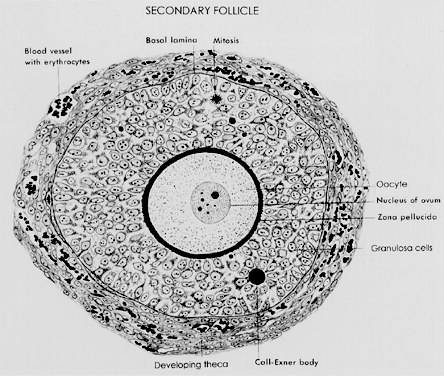 A typical healthy secondary follicle contains a fully grown oocyte surrounded
by the zona pellucida, five to eight layers of granulosa cells, a
basal lamina, and developing theca tissue with numerous blood vessels.(From Bloom W, Fawcett DW: A Textbook of Histology. Philadelphia: WB Saunders, 1975, with permission from Arnold Ltd.)
A typical healthy secondary follicle contains a fully grown oocyte surrounded
by the zona pellucida, five to eight layers of granulosa cells, a
basal lamina, and developing theca tissue with numerous blood vessels.(From Bloom W, Fawcett DW: A Textbook of Histology. Philadelphia: WB Saunders, 1975, with permission from Arnold Ltd.)
Back to Top
Follicular Cyst
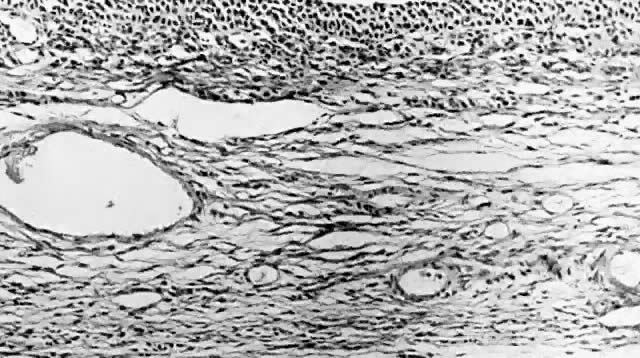 Follicle cyst. Inner lining composed of granulosa cells with outer layer
of luteinzed theca interna cells. (hematoxylin and eosin stain, x25)
Follicle cyst. Inner lining composed of granulosa cells with outer layer
of luteinzed theca interna cells. (hematoxylin and eosin stain, x25)
Back to Top
Teratoma
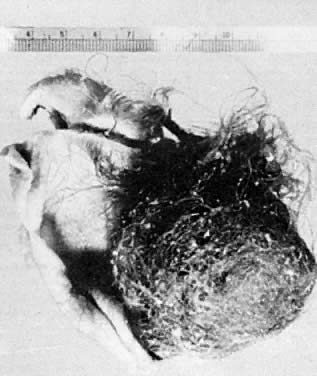 Benign cystic teratoma (dermoid cyst). Opened cyst containing hair and
sebaceous material.
Benign cystic teratoma (dermoid cyst). Opened cyst containing hair and
sebaceous material.
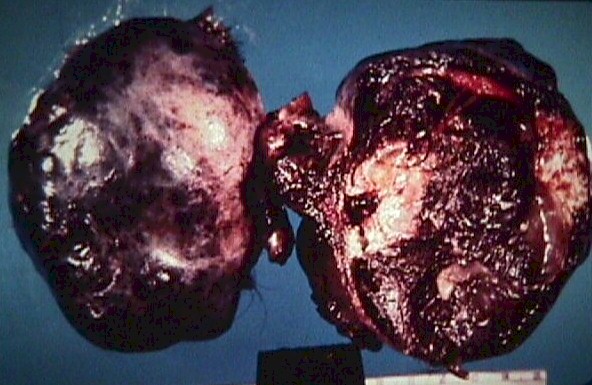 Infarction
of a Dermoid Cyst (From Operational Obstetrics &
Gynecology - 2nd Edition, The Health Care of Women in Military Settings,
CAPT Michael John Hughey, MC, USNR, NAVMEDPUB 6300-2C, Bureau of Medicine
and Surgery, Department of the Navy, 2300 E Street NW, Washington, D.C.
20372-5300, January 1, 2000. Original image courtesy Armed Forces Institute
of Pathology) Infarction
of a Dermoid Cyst (From Operational Obstetrics &
Gynecology - 2nd Edition, The Health Care of Women in Military Settings,
CAPT Michael John Hughey, MC, USNR, NAVMEDPUB 6300-2C, Bureau of Medicine
and Surgery, Department of the Navy, 2300 E Street NW, Washington, D.C.
20372-5300, January 1, 2000. Original image courtesy Armed Forces Institute
of Pathology)
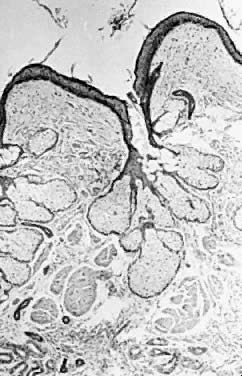 Benign cystic teratoma. Cyst lining composed of keratinizing squamous epithelium
with sebaceous glands, smooth muscle, and sweat glands in the
wall (hematoxylin and eosin stain, x60).
Benign cystic teratoma. Cyst lining composed of keratinizing squamous epithelium
with sebaceous glands, smooth muscle, and sweat glands in the
wall (hematoxylin and eosin stain, x60).
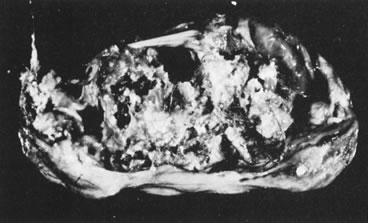 Sectioned surface of dermoid cyst.
Sectioned surface of dermoid cyst.
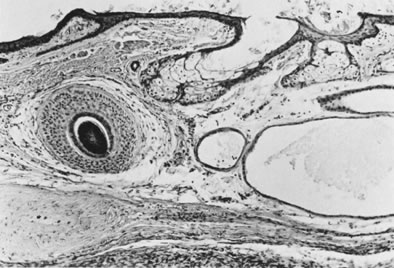 Dermoid cyst with hair follicle ( left ), sebaceous glands, and sweat glands (magnification, ×80).(Serov SF, Scully RE, Sobin LJ: Histological Typing of Ovarian Tumours. Geneva, World
Health Organization, 1973.)
Dermoid cyst with hair follicle ( left ), sebaceous glands, and sweat glands (magnification, ×80).(Serov SF, Scully RE, Sobin LJ: Histological Typing of Ovarian Tumours. Geneva, World
Health Organization, 1973.)
 Mature teratoma (magnification, x50).(Serov SF, Scully RE, Sobin LH: Histological Typing of Ovarian Tumours. Geneva, World
Health Organization, 1973.)
Mature teratoma (magnification, x50).(Serov SF, Scully RE, Sobin LH: Histological Typing of Ovarian Tumours. Geneva, World
Health Organization, 1973.)
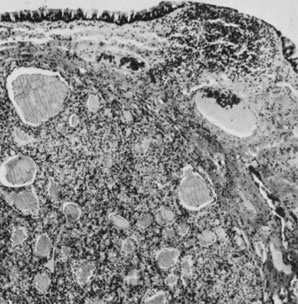 Struma ovarii in wall of mucinous cystadenoma (magnification, ×80). The
appearance is similar to that of a thyroid adenoma.
Struma ovarii in wall of mucinous cystadenoma (magnification, ×80). The
appearance is similar to that of a thyroid adenoma.
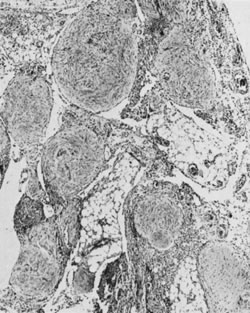 Mature glial implants of immature teratoma on omentum (magnification, ×50).(Robboy SJ, Scully RE: Ovarian teratoma with glial implants on the peritoneum. Hum
Pathol 1:643, 1970.)
Mature glial implants of immature teratoma on omentum (magnification, ×50).(Robboy SJ, Scully RE: Ovarian teratoma with glial implants on the peritoneum. Hum
Pathol 1:643, 1970.)
Back to Top
Immature Teratoma
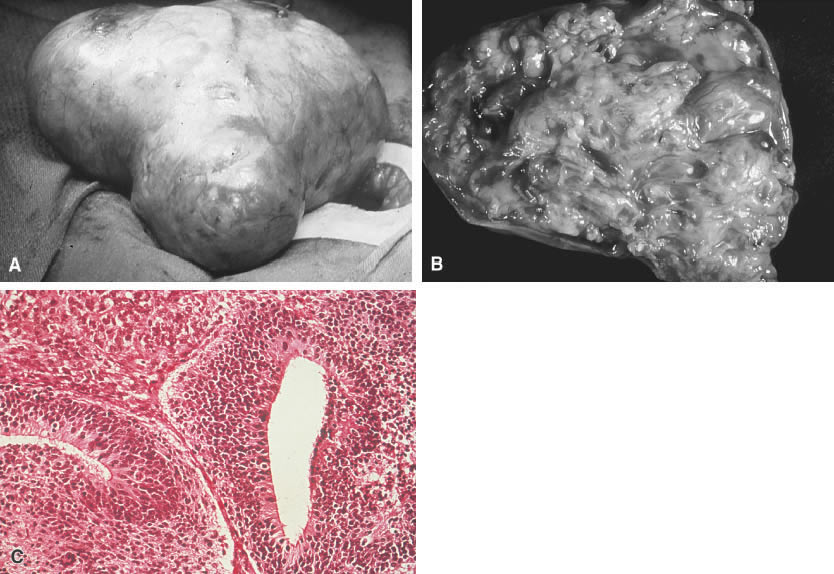 Immature teratoma. A. An immature teratoma is encapsulated and grossly similar to its benign
counterpart, the mature teratoma. The main difference is the immature
teratoma is solid rather than cystic. B. An immature teratoma reveals a variegated appearance with areas of necrosis
and cystic changes. C. An immature teratoma reveals mixtures of mature and immature tissues reminiscent
of the developing stages of embryonic tissues. The most common
pattern is seen here (e.g., an immature neural epithelium [neuroepithelial
rosettes]).
Immature teratoma. A. An immature teratoma is encapsulated and grossly similar to its benign
counterpart, the mature teratoma. The main difference is the immature
teratoma is solid rather than cystic. B. An immature teratoma reveals a variegated appearance with areas of necrosis
and cystic changes. C. An immature teratoma reveals mixtures of mature and immature tissues reminiscent
of the developing stages of embryonic tissues. The most common
pattern is seen here (e.g., an immature neural epithelium [neuroepithelial
rosettes]).
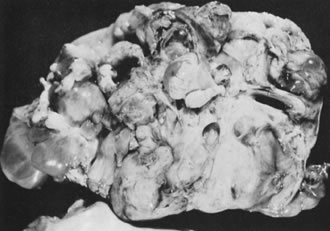 Sectioned surface of immature teratoma.
Sectioned surface of immature teratoma.
 Immature teratoma (magnification, ×120). Immature glial with central
area of necrosis contains neuroepithelial rosettes.(Scully RE: Recent progress in ovarian cancer. Hum Pathol 1:73, 1970.)
Immature teratoma (magnification, ×120). Immature glial with central
area of necrosis contains neuroepithelial rosettes.(Scully RE: Recent progress in ovarian cancer. Hum Pathol 1:73, 1970.)
Back to Top
Serous Cystoma
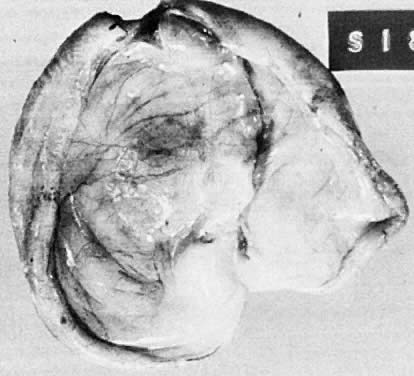 Simple serous cystoma. Opened cyst demonstrating uniloculation with a smooth
surface.
Simple serous cystoma. Opened cyst demonstrating uniloculation with a smooth
surface.
Back to Top
Mucinous Cystadenoma
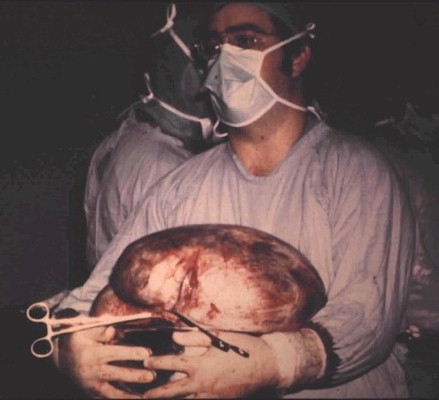 70
pound mucinous cystadenoma (From Operational Obstetrics
& Gynecology - 2nd Edition, The Health Care of Women in Military Settings,
CAPT Michael John Hughey, MC, USNR, NAVMEDPUB 6300-2C, Bureau of Medicine
and Surgery, Department of the Navy, 2300 E Street NW, Washington, D.C.
20372-5300, January 1, 2000. Original image courtesy Michael John Hughey,
MD. All rights reserved.) 70
pound mucinous cystadenoma (From Operational Obstetrics
& Gynecology - 2nd Edition, The Health Care of Women in Military Settings,
CAPT Michael John Hughey, MC, USNR, NAVMEDPUB 6300-2C, Bureau of Medicine
and Surgery, Department of the Navy, 2300 E Street NW, Washington, D.C.
20372-5300, January 1, 2000. Original image courtesy Michael John Hughey,
MD. All rights reserved.)
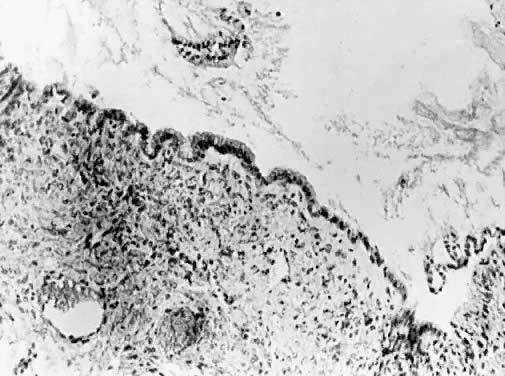 Mucinous cystadenoma. Cyst lining composed of a single layer of columnar
cells with basal nuclei and cystoplasmic vacuoles containing mucin (hematoxylin
and eosin stain, x125).
Mucinous cystadenoma. Cyst lining composed of a single layer of columnar
cells with basal nuclei and cystoplasmic vacuoles containing mucin (hematoxylin
and eosin stain, x125).
Back to Top
Brenner Tumor
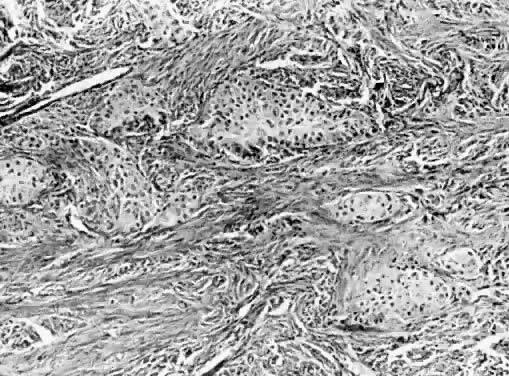 Brenner tumor. Islands of uniform epithelial cells in dense fibrous stroma (hematoxylin
and eosin stain, x125).
Brenner tumor. Islands of uniform epithelial cells in dense fibrous stroma (hematoxylin
and eosin stain, x125).
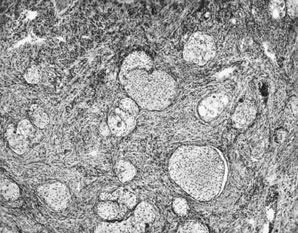 Benign transitional cell (Brenner) tumor. Rounded nests of transitional-type
epithelium are embedded in a fibrotic stroma resembling ovarian
stroma.
Benign transitional cell (Brenner) tumor. Rounded nests of transitional-type
epithelium are embedded in a fibrotic stroma resembling ovarian
stroma.
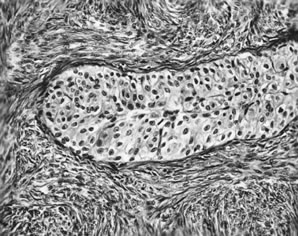 Benign transitional cell (Brenner) tumor. Ovoid nuclei with longitudinal
grooves characterize the transitional cell type.
Benign transitional cell (Brenner) tumor. Ovoid nuclei with longitudinal
grooves characterize the transitional cell type.
Back to Top
Fibroma
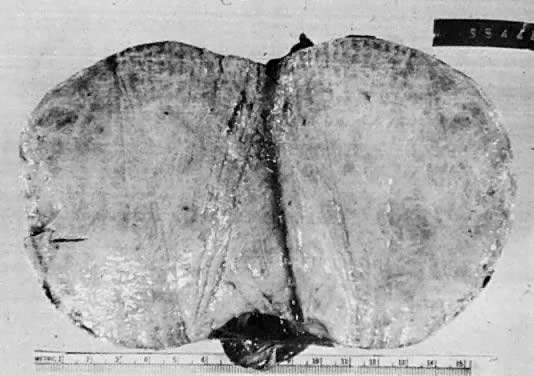 Fibroma. Solid circumscribed tumor composed of firm, uniform white tissue.
Fibroma. Solid circumscribed tumor composed of firm, uniform white tissue.
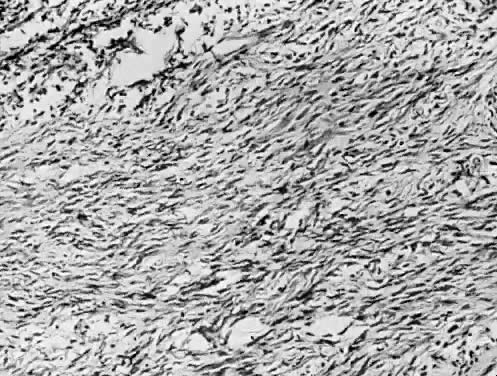 Ovarian fibroma. Spindle-shaped cells in a collagenous stroma (hematoxylin
and eosin stain, x125).
Ovarian fibroma. Spindle-shaped cells in a collagenous stroma (hematoxylin
and eosin stain, x125).
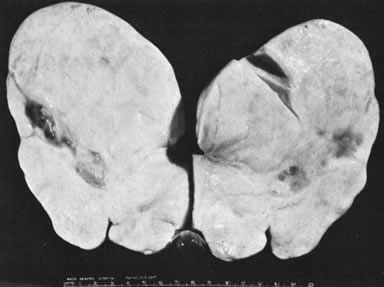 Sectioned surface of fibroma with areas of cystic degeneration.(Scully RE: Sex cord-stromal tumors. In Blaustein A [ed]: Pathology
of the Female Genital Tract, pp 505–526. New York, Springer-Verlag, 1977.)
Sectioned surface of fibroma with areas of cystic degeneration.(Scully RE: Sex cord-stromal tumors. In Blaustein A [ed]: Pathology
of the Female Genital Tract, pp 505–526. New York, Springer-Verlag, 1977.)
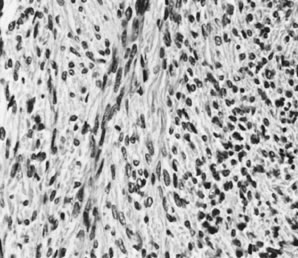 Fibroma (magnification, ×325).(Serov SF, Scully RE, Sobin LH: Histological Typing of Ovarian Tumours. Geneva, World
Health Organization, 1973.)
Fibroma (magnification, ×325).(Serov SF, Scully RE, Sobin LH: Histological Typing of Ovarian Tumours. Geneva, World
Health Organization, 1973.)
Back to Top
Thecoma
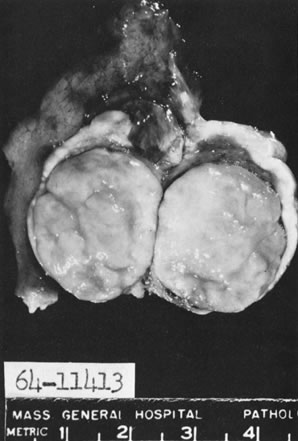 Sectioned surface of thecoma within ovary
with attached fallopian tube.(Scully RE:
Sex cord-stromal tumors. In Blaustein A [ed]: Pathology of the Female
Genital Tract. New York, Springer-Verlag, 1977.)
Sectioned surface of thecoma within ovary
with attached fallopian tube.(Scully RE:
Sex cord-stromal tumors. In Blaustein A [ed]: Pathology of the Female
Genital Tract. New York, Springer-Verlag, 1977.)
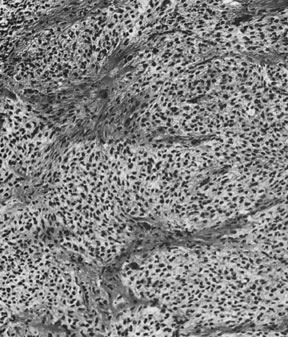 Thecoma (magnification, ×130).(Morris JM, Scully RE: Endocrine Pathology of the Ovary. St. Louis, CV
Mosby, 1958.)
Thecoma (magnification, ×130).(Morris JM, Scully RE: Endocrine Pathology of the Ovary. St. Louis, CV
Mosby, 1958.)
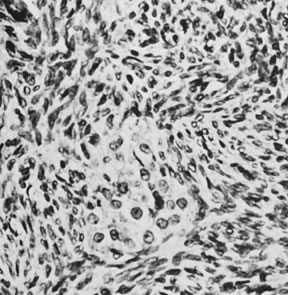 Luteinized thecoma (magnification, ×430). A nest of lutein cells
is surrounded by fibroblasts.
Luteinized thecoma (magnification, ×430). A nest of lutein cells
is surrounded by fibroblasts.
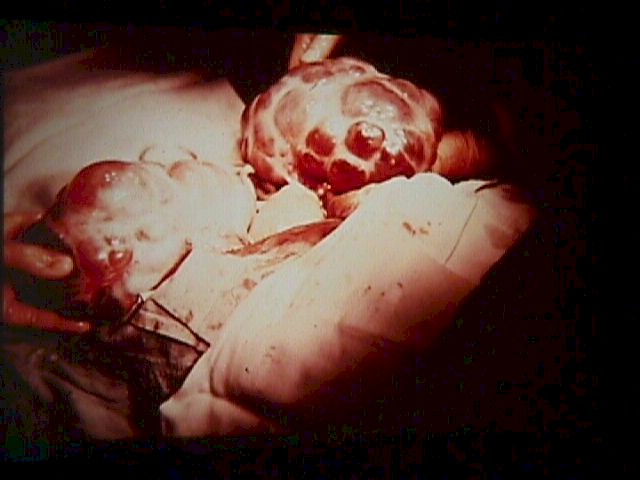 Theca Lutein Cyst (From
Operational Obstetrics & Gynecology - 2nd Edition, The Health Care of Women in
Military Settings, CAPT Michael John Hughey, MC, USNR, NAVMEDPUB 6300-2C,
Bureau of Medicine and Surgery, Department of the Navy, 2300 E Street NW,
Washington, D.C. 20372-5300, January 1, 2000. Original image courtesy Armed
Forces Institute of Pathology)
Theca Lutein Cyst (From
Operational Obstetrics & Gynecology - 2nd Edition, The Health Care of Women in
Military Settings, CAPT Michael John Hughey, MC, USNR, NAVMEDPUB 6300-2C,
Bureau of Medicine and Surgery, Department of the Navy, 2300 E Street NW,
Washington, D.C. 20372-5300, January 1, 2000. Original image courtesy Armed
Forces Institute of Pathology)
Back to Top
Sertoli-Stromal Cell Tumors (Androblastomas)
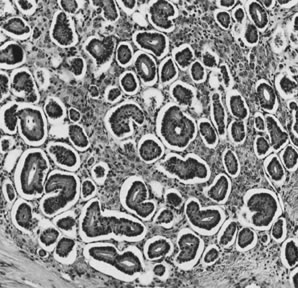 Sertoli-Leydig cell tumor, well differentiated (magnification, ×100). (Morris JM, Scully RE: Endocrine Pathology of the Ovary. St. Louis, CV
Mosby, 1958.)
Sertoli-Leydig cell tumor, well differentiated (magnification, ×100). (Morris JM, Scully RE: Endocrine Pathology of the Ovary. St. Louis, CV
Mosby, 1958.)
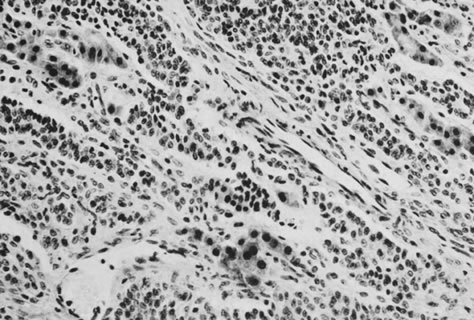 Sertoli-Leydig cell tumor of intermediate differentiation (magnification, ×240).(Morris JM, Scully RE: Endocrine Pathology of the Ovary. St. Louis, CV
Mosby, 1958.)
Sertoli-Leydig cell tumor of intermediate differentiation (magnification, ×240).(Morris JM, Scully RE: Endocrine Pathology of the Ovary. St. Louis, CV
Mosby, 1958.)
Back to Top
Gonadoblastoma
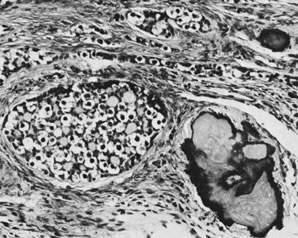 Gonadoblastoma with calcification (magnification, ×130).(Serov SF, Scully RE, Sobin LH: Histological Typing of Ovarian Tumours. Geneva, World
Health Organization, 1973.)
Gonadoblastoma with calcification (magnification, ×130).(Serov SF, Scully RE, Sobin LH: Histological Typing of Ovarian Tumours. Geneva, World
Health Organization, 1973.)
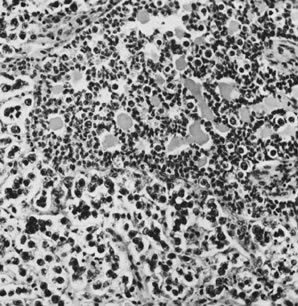 Gonadoblastoma with dysgerminoma (magnification, ×160).(Serov SF, Scully RE, Sobin LH: Histological Typing of Ovarian Tumours. Geneva, World
Health Organization, 1973.)
Gonadoblastoma with dysgerminoma (magnification, ×160).(Serov SF, Scully RE, Sobin LH: Histological Typing of Ovarian Tumours. Geneva, World
Health Organization, 1973.)
Back to Top
Granulosa Cell Tumor
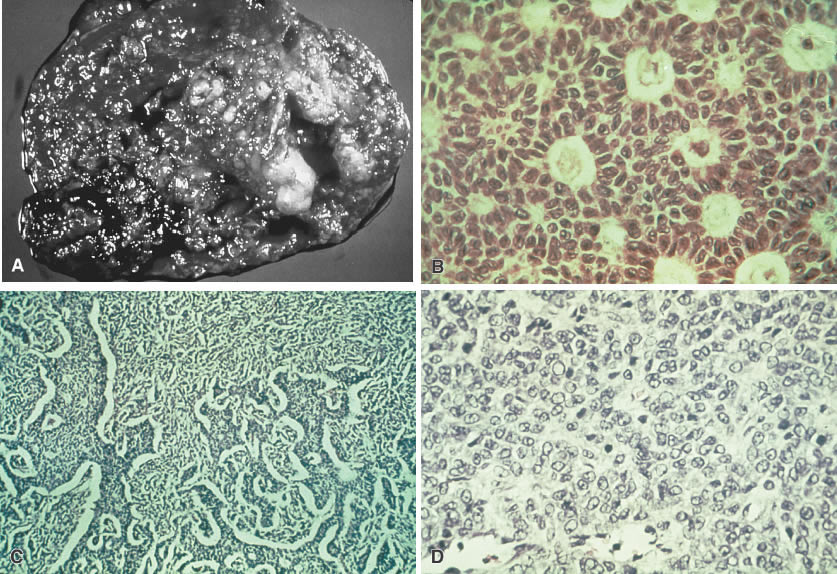 Granulosa cell tumor. A. Tumor is usually solid but may be cystic. Primarily areas of a solid tumor
are shown with areas of hemorrhage. B. Large, round and ovoid nuclei with a longitudinal groove arranged in patterns
are referred to as Call-Exner bodies (microglandular pattern). C. Similar cells in a stromal matrix are arranged in cords and ribbons (trabecular
pattern). D. A variation of cells shows some cellular atypia (sarcomatoid pattern).
Granulosa cell tumor. A. Tumor is usually solid but may be cystic. Primarily areas of a solid tumor
are shown with areas of hemorrhage. B. Large, round and ovoid nuclei with a longitudinal groove arranged in patterns
are referred to as Call-Exner bodies (microglandular pattern). C. Similar cells in a stromal matrix are arranged in cords and ribbons (trabecular
pattern). D. A variation of cells shows some cellular atypia (sarcomatoid pattern).
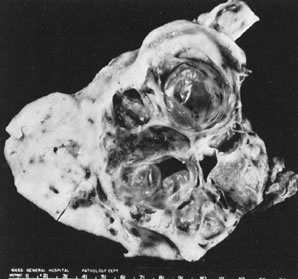 Sectioned surface of granulosa cell tumor with
solid and cystic components.(Scully RE: Ovarian tumors with estrogenic
manifestations. Contemp Ob Gyn 10:83, 1977.)
Sectioned surface of granulosa cell tumor with
solid and cystic components.(Scully RE: Ovarian tumors with estrogenic
manifestations. Contemp Ob Gyn 10:83, 1977.)
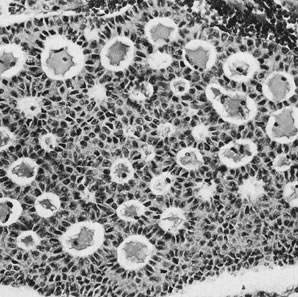 Granulosa cell tumor, microfollicular pattern with Call-Exner bodies (magnification, ×290).(Scully RE, Morris JM: Functioning ovarian tumors. In Meigs JV, Sturgis
SH [eds]: Progress in Gynecology, Vol 3. New York, Grune & Siralton, 1957, by
permission.)
Granulosa cell tumor, microfollicular pattern with Call-Exner bodies (magnification, ×290).(Scully RE, Morris JM: Functioning ovarian tumors. In Meigs JV, Sturgis
SH [eds]: Progress in Gynecology, Vol 3. New York, Grune & Siralton, 1957, by
permission.)
 Granulosa cell tumor, trabecular pattern (magnification, ×200).(Serov SF, Scully RE, Sobin LH: Histological Typing of Ovarian Tumours. Geneva, World
Health Organization, 1973.)
Granulosa cell tumor, trabecular pattern (magnification, ×200).(Serov SF, Scully RE, Sobin LH: Histological Typing of Ovarian Tumours. Geneva, World
Health Organization, 1973.)
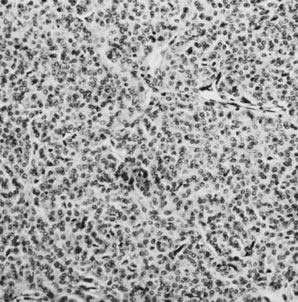 Granulosa cell tumor, diffuse pattern (magnification, ×200).(Scully RE: Ovarian tumors with estrogenic manifestations. Contemp Ob Gyn 10:83, 1977.)
Granulosa cell tumor, diffuse pattern (magnification, ×200).(Scully RE: Ovarian tumors with estrogenic manifestations. Contemp Ob Gyn 10:83, 1977.)
Back to Top
Hilus Cell Tumor
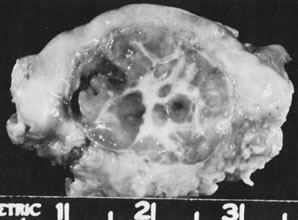 Sectioned surface of hilus cell tumor.(Scully RE: Sex cord-stromal tumors. In Blaustein A [ed]: Pathology
of the Female Genital Tract. New York, Springer-Verlag, 1977.)
Sectioned surface of hilus cell tumor.(Scully RE: Sex cord-stromal tumors. In Blaustein A [ed]: Pathology
of the Female Genital Tract. New York, Springer-Verlag, 1977.)
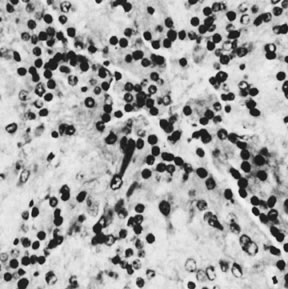 Hilus cell tumor (magnification, ×320). Note large crystal of Reinke
near center.(Scully RE: Androgenic lesions of the ovary. In Grady HG, Smith DE [eds]: The
Ovary, International Academy of Pathology, Monograph
no. 3. Baltimore, Williams & Wilkins, 1962.)
Hilus cell tumor (magnification, ×320). Note large crystal of Reinke
near center.(Scully RE: Androgenic lesions of the ovary. In Grady HG, Smith DE [eds]: The
Ovary, International Academy of Pathology, Monograph
no. 3. Baltimore, Williams & Wilkins, 1962.)
Back to Top
Dysgerminoma
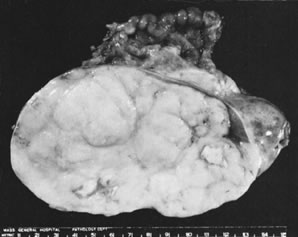 Sectioned surface of dysgerminoma. Note lobulated appearance and foci of
caseation-like necrosis.(Scully RE: Recent progress in ovarian cancer. Hum Pathol 1:73, 1970.)
Sectioned surface of dysgerminoma. Note lobulated appearance and foci of
caseation-like necrosis.(Scully RE: Recent progress in ovarian cancer. Hum Pathol 1:73, 1970.)
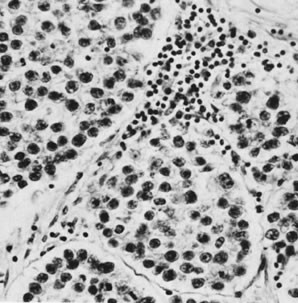 Dysgerminoma with lymphocytes in stroma (magnification, ×300).(Scully RE: Germ cell tumors of the ovary and fallopian tube. In Meigs
JV, Sturgis SH [eds]: Progress in Gynecology, Vol 4. New York, Grune & Stratton, 1963, by permission.)
Dysgerminoma with lymphocytes in stroma (magnification, ×300).(Scully RE: Germ cell tumors of the ovary and fallopian tube. In Meigs
JV, Sturgis SH [eds]: Progress in Gynecology, Vol 4. New York, Grune & Stratton, 1963, by permission.)
 Dysgerminoma. A. In situ picture of a unilateral dysgerminoma shows it is bosselated and surrounded
by a dense capsule. B. Grossly, a cut surface of this dysgerminoma reveals a yellow-tan, soft, homogeneous, and
brainlike consistency. C. Histologic picture of a dysgerminoma shows cells are analogous to the
undifferentiated germ cells of an embryonal gonad (i.e., well-defined
clusters of cells separated by a fibrovascular system, infiltration [lymphocytes]).
Dysgerminoma. A. In situ picture of a unilateral dysgerminoma shows it is bosselated and surrounded
by a dense capsule. B. Grossly, a cut surface of this dysgerminoma reveals a yellow-tan, soft, homogeneous, and
brainlike consistency. C. Histologic picture of a dysgerminoma shows cells are analogous to the
undifferentiated germ cells of an embryonal gonad (i.e., well-defined
clusters of cells separated by a fibrovascular system, infiltration [lymphocytes]).
Back to Top
Endodermal Sinus Tumor
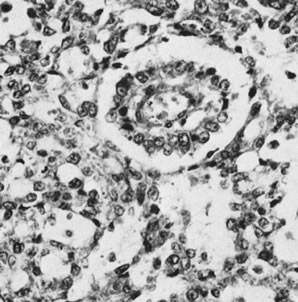 Endodermal sinus tumor, reticular pattern with Schiller-Duval body (magnification, ×300).(Scully RE: Germ cell tumors of the ovary and fallopian tube. In Meigs
JV, Sturgis SH [eds]: Progress in Gynecology, Vol 4. New York, Grune & Stratton, 1963, by permission.)
Endodermal sinus tumor, reticular pattern with Schiller-Duval body (magnification, ×300).(Scully RE: Germ cell tumors of the ovary and fallopian tube. In Meigs
JV, Sturgis SH [eds]: Progress in Gynecology, Vol 4. New York, Grune & Stratton, 1963, by permission.)
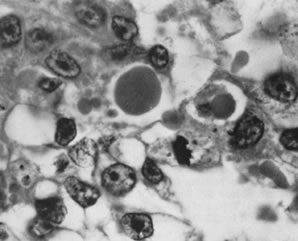 Endodermal sinus tumor with hyaline bodies (magnification, ×750).(Serov SF, Scully RE, Sobin LH: Histological Typing of Ovarian Tumours. Geneva, World
Health Organization, 1973.)
Endodermal sinus tumor with hyaline bodies (magnification, ×750).(Serov SF, Scully RE, Sobin LH: Histological Typing of Ovarian Tumours. Geneva, World
Health Organization, 1973.)
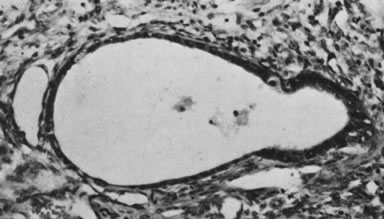 Endodermal sinus tumor, polyvesicular vitelline pattern (magnification, ×190). Secondary
yolk sac at right is budding from larger primary
yolk sac.(Serov SF, Scully RE, Sobin LH: Histological Typing of Ovarian Tumours. Geneva, World
Health Organization, 1973.)
Endodermal sinus tumor, polyvesicular vitelline pattern (magnification, ×190). Secondary
yolk sac at right is budding from larger primary
yolk sac.(Serov SF, Scully RE, Sobin LH: Histological Typing of Ovarian Tumours. Geneva, World
Health Organization, 1973.)
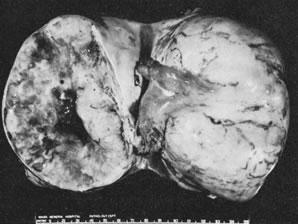 Sectioned surface of yolk sac tumor.(Scully RE: Recent Progress in Ovarian Cancer. Hum Pathol 1:73, 1970.)
Sectioned surface of yolk sac tumor.(Scully RE: Recent Progress in Ovarian Cancer. Hum Pathol 1:73, 1970.)
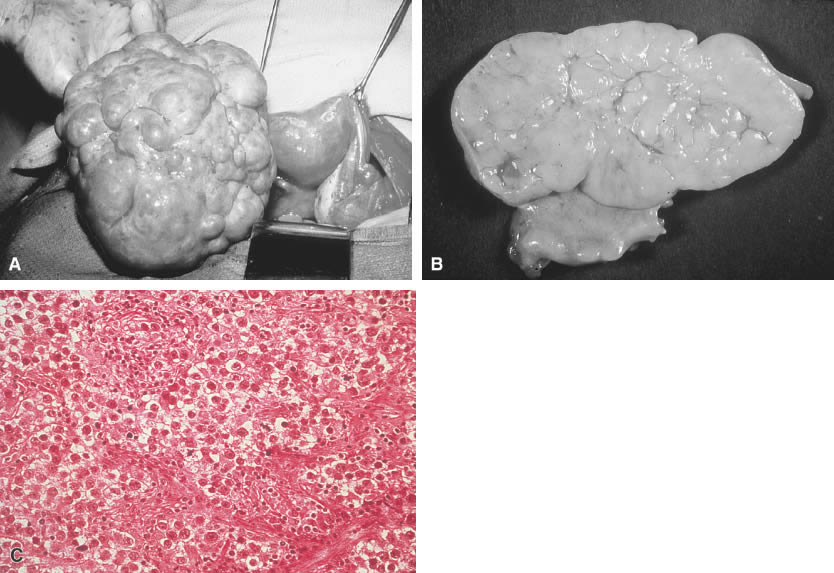
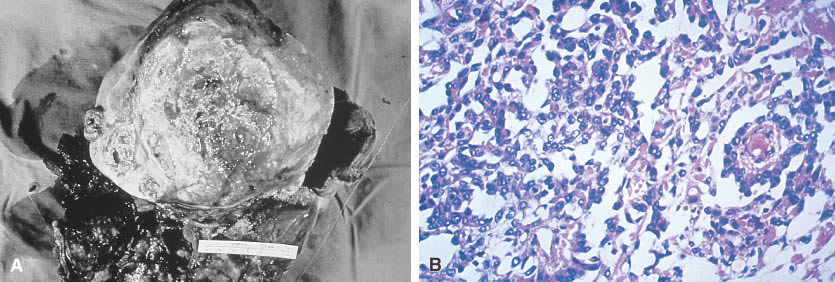 Yolk sac (endodermal sinus) tumor. A. Yolk sac tumor is classically yellow-red-gray, soft, and friable with
multiple cystic areas representing areas of hemorrhage and necrosis. B. A pattern is shown of pleomorphic, poorly differentiated cells with papillary
clusters and a pseudopapillary projection with a vascular component
within the center (Schiller-Duvall body).
Yolk sac (endodermal sinus) tumor. A. Yolk sac tumor is classically yellow-red-gray, soft, and friable with
multiple cystic areas representing areas of hemorrhage and necrosis. B. A pattern is shown of pleomorphic, poorly differentiated cells with papillary
clusters and a pseudopapillary projection with a vascular component
within the center (Schiller-Duvall body).
Back to Top
Serous Cystadenoma of
Low Malignant Potential
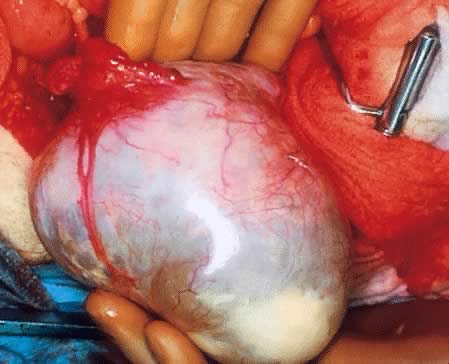 External surface of a serous cystadenoma of low malignant potential showing
a smooth, tense capsule with prominent veins and thickened fibrous
areas.(Courtesy of Dr. Richard Stock, Naval Hospital, Portsmouth, VA.)
External surface of a serous cystadenoma of low malignant potential showing
a smooth, tense capsule with prominent veins and thickened fibrous
areas.(Courtesy of Dr. Richard Stock, Naval Hospital, Portsmouth, VA.)
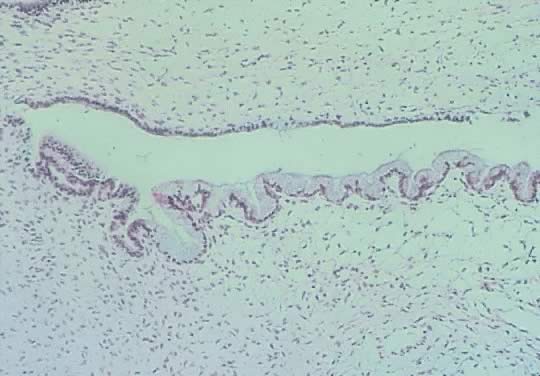 Cystadenoma exhibiting lining consisting of cuboidal epithelium serous
type ( top) and mucus-producing tall, columnar, endocervical-type epithelium with
basally arranged nuclei ( bottom ). The mixture of epithelia frequently is found in serous or mucinous
cystadenomas of the ovary (hematoxylin and eosin,
Cystadenoma exhibiting lining consisting of cuboidal epithelium serous
type ( top) and mucus-producing tall, columnar, endocervical-type epithelium with
basally arranged nuclei ( bottom ). The mixture of epithelia frequently is found in serous or mucinous
cystadenomas of the ovary (hematoxylin and eosin,
 520). 520).
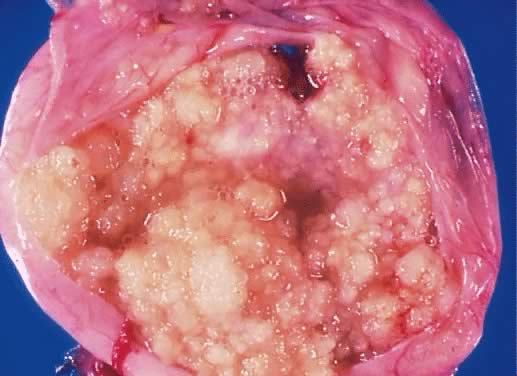 Serous cystadenoma of low malignant potential demonstrating an exuberant
growth of papillary projections arising from the internal surface of
the tumor. Bubbly serous fluid is present in the lumen.(Courtesy of Dr. Richard Stock.)
Serous cystadenoma of low malignant potential demonstrating an exuberant
growth of papillary projections arising from the internal surface of
the tumor. Bubbly serous fluid is present in the lumen.(Courtesy of Dr. Richard Stock.)
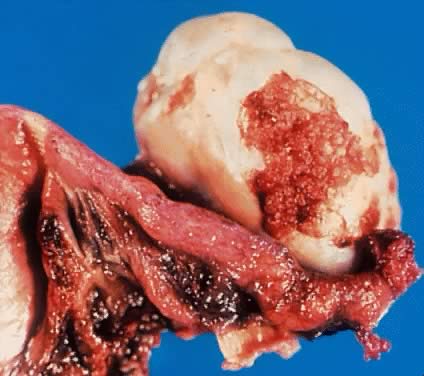 External surface of an ovary showing a serous cystadenoma of low malignant
potential exhibiting papillary growth. The lateral aspect of the uterus
and the entire fallopian tube are seen clearly. The papillary growth
does not represent invasion.(Courtesy of Dr. Richard Stock.)
External surface of an ovary showing a serous cystadenoma of low malignant
potential exhibiting papillary growth. The lateral aspect of the uterus
and the entire fallopian tube are seen clearly. The papillary growth
does not represent invasion.(Courtesy of Dr. Richard Stock.)
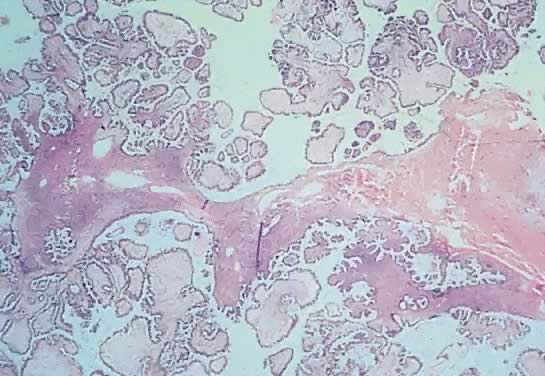 Photomicrograph of a serous cystadenoma of low malignant potential. The
tumor is composed mainly of papillary structures of different sizes resting
on stroma made up of fibrous tissue. Tufting is observed in the
interpapillary space (
Photomicrograph of a serous cystadenoma of low malignant potential. The
tumor is composed mainly of papillary structures of different sizes resting
on stroma made up of fibrous tissue. Tufting is observed in the
interpapillary space ( 100). 100).
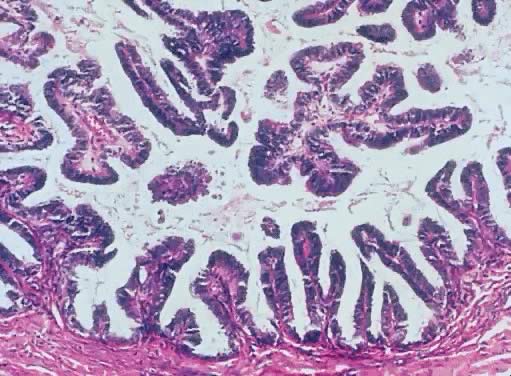 Inner lining of a serous cystadenoma of low malignant potential showing
papillations with epithelial pluristratification, hyperchromasia, and
tufting; there is some degree of nuclear atypia (
Inner lining of a serous cystadenoma of low malignant potential showing
papillations with epithelial pluristratification, hyperchromasia, and
tufting; there is some degree of nuclear atypia ( 420). 420).
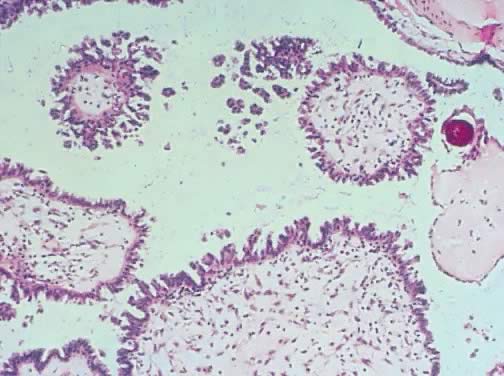 Serous cystadenoma of low malignant potential demonstrating papillae, stratification
of epithelial lining, tufting, stromal edema with hyalinization, and
focal acute inflammatory cell infiltrate. On the upper right-hand
corner, a psammoma body is clearly seen (hematoxylin and eosin,
Serous cystadenoma of low malignant potential demonstrating papillae, stratification
of epithelial lining, tufting, stromal edema with hyalinization, and
focal acute inflammatory cell infiltrate. On the upper right-hand
corner, a psammoma body is clearly seen (hematoxylin and eosin,
 420). 420).
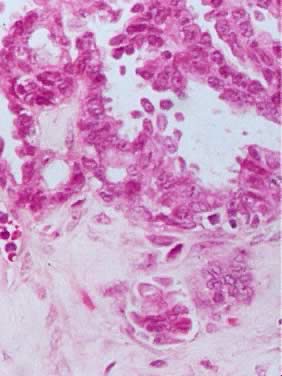 Photomicrograph of a serous tumor of low malignant potential showing a
focus of microinvasion represented by small nests of tumor cells penetrating
the tumor stroma near the epithelial-stromal interphase. The nests
are surrounded by a small clear space that should be filled with serous
fluid. There is no stromal necrosis or inflammation around the tumor
nests (
Photomicrograph of a serous tumor of low malignant potential showing a
focus of microinvasion represented by small nests of tumor cells penetrating
the tumor stroma near the epithelial-stromal interphase. The nests
are surrounded by a small clear space that should be filled with serous
fluid. There is no stromal necrosis or inflammation around the tumor
nests ( 400). 400).
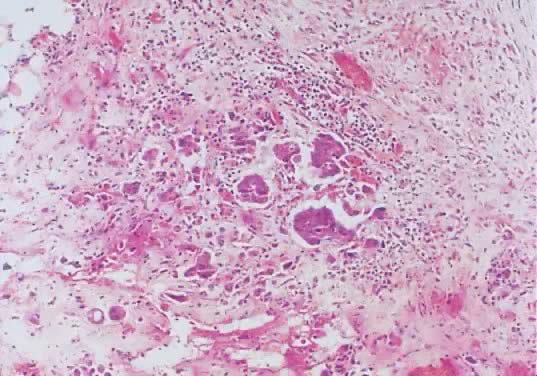 Omental invasive implants associated with a serous ovarian tumor of low
malignant potential. The malignant histologic characteristics of the
epithelium, its stromal invasion by single and papillary nests of cells, the
inflammatory response, and the fibroblastic proliferation are evident (hematoxylin
and eosin,
Omental invasive implants associated with a serous ovarian tumor of low
malignant potential. The malignant histologic characteristics of the
epithelium, its stromal invasion by single and papillary nests of cells, the
inflammatory response, and the fibroblastic proliferation are evident (hematoxylin
and eosin,  420). 420).
Back to Top
Mucinous Cystadenoma
of Low Malignant Potential
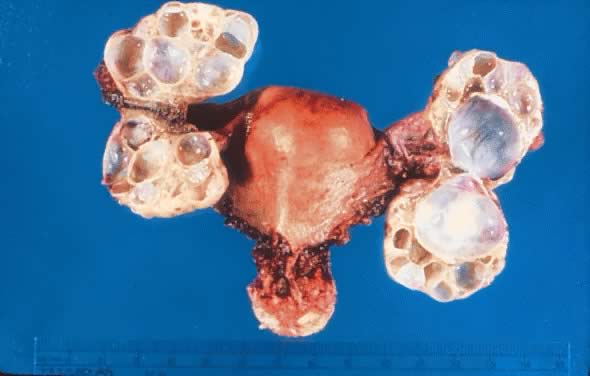 Uterus with bilateral ovarian mucinous cystadenomas of low malignant potential
showing multioculations and thick cyst walls.(Courtesy of Dr. Richard Stock.)
Uterus with bilateral ovarian mucinous cystadenomas of low malignant potential
showing multioculations and thick cyst walls.(Courtesy of Dr. Richard Stock.)
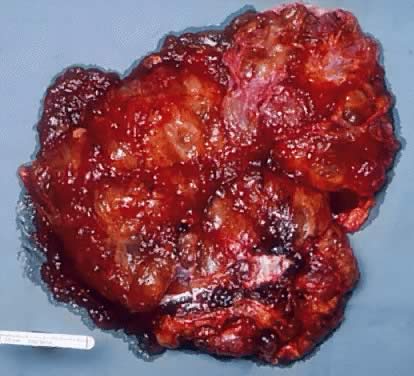 Gross appearance of a mucinous cystadenoma of low malignant potential. The
external surface of the tumor is irregular and multilobulated. The
lobules are of different size and shape. The tumor is reddish brown. The
lobules were fluctuant on palpation. Some areas of the tumor are firm. On
cut section, multiple mucus-filled cysts are seen. Numerous small
tan papillary structures are present in the inner surface of some
of the cysts.
Gross appearance of a mucinous cystadenoma of low malignant potential. The
external surface of the tumor is irregular and multilobulated. The
lobules are of different size and shape. The tumor is reddish brown. The
lobules were fluctuant on palpation. Some areas of the tumor are firm. On
cut section, multiple mucus-filled cysts are seen. Numerous small
tan papillary structures are present in the inner surface of some
of the cysts.
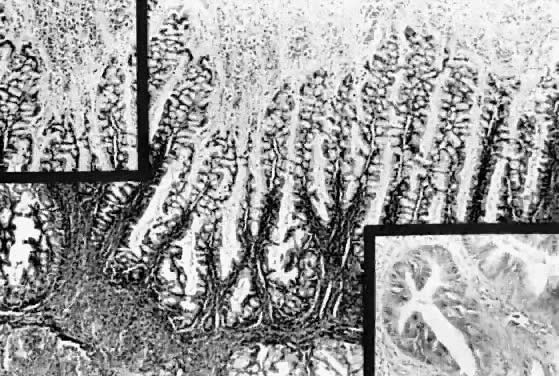 Mucinous cystadenoma of low malignant potential, endocervical or müllerian
type. Observe the marked papillation similar to that seen in
serous tumors. Tufting and inflammatory exudate in the cyst lumen are
seen (hematoxylin and eosin,
Mucinous cystadenoma of low malignant potential, endocervical or müllerian
type. Observe the marked papillation similar to that seen in
serous tumors. Tufting and inflammatory exudate in the cyst lumen are
seen (hematoxylin and eosin,  212). Inset, upper left. Papillation and inflammatory exudate. Inset, lower right. Epithelial atypia of gland-like spaces ( 212). Inset, upper left. Papillation and inflammatory exudate. Inset, lower right. Epithelial atypia of gland-like spaces ( 420). 420).
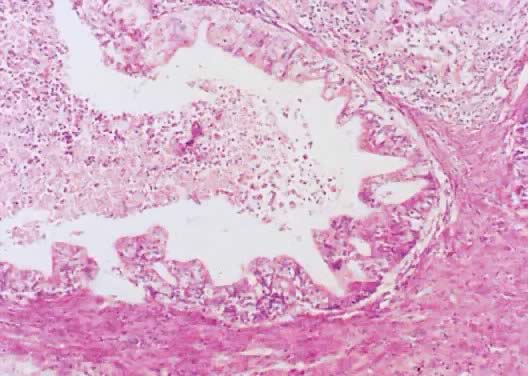 Mucinous cystadenoma of low malignant potential, endocervical type, exhibiting
a filigree pattern, pluristratification with atypia, stroma with
mucinous material and inflammatory cell infiltration at the upper right-hand
corner, and cellular debris with inflammatory cell exudate in
the lumen (hematoxylin and eosin,
Mucinous cystadenoma of low malignant potential, endocervical type, exhibiting
a filigree pattern, pluristratification with atypia, stroma with
mucinous material and inflammatory cell infiltration at the upper right-hand
corner, and cellular debris with inflammatory cell exudate in
the lumen (hematoxylin and eosin,  520). 520).
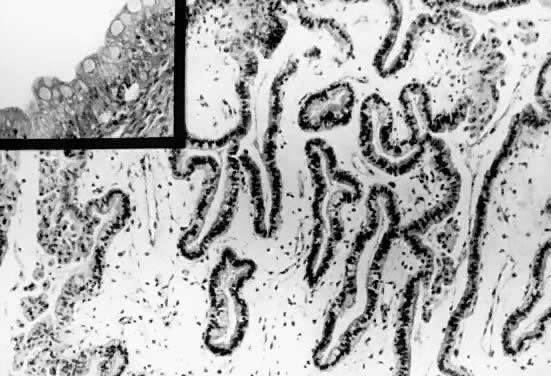 Papillations and tufting in a mucinous cystadenoma of low malignant potential, intestinal
type. Spaces are lined with a mixture of goblet cells
and tall, columnar intestinal-type epithelium. The stroma is edematous
and exhibits fine capillaries. Areas with metaplastic changes are
present on the left side and upper right-hand corner (hematoxylin and
eosin,
Papillations and tufting in a mucinous cystadenoma of low malignant potential, intestinal
type. Spaces are lined with a mixture of goblet cells
and tall, columnar intestinal-type epithelium. The stroma is edematous
and exhibits fine capillaries. Areas with metaplastic changes are
present on the left side and upper right-hand corner (hematoxylin and
eosin,  212). Inset. Higher magnification illustrating goblet cells (hematoxylin and eosin, 212). Inset. Higher magnification illustrating goblet cells (hematoxylin and eosin,
 420). 420).
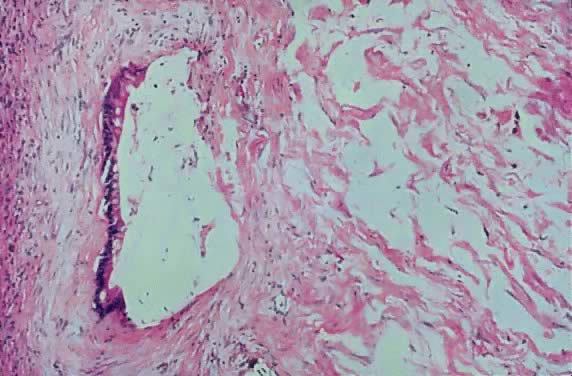 Stroma of an ovarian mucinous cystadenoma of low malignant potential showing
pools of mucus and atypical mucus-producing columnar epithelial
cells of intestinal type in the stroma. Goblet cells are seen easily. The
mucus pools are mostly acellular, although three hyperchromatic atypical
nuclei are seen at the upper right-hand corner (hematoxylin and
eosin,
Stroma of an ovarian mucinous cystadenoma of low malignant potential showing
pools of mucus and atypical mucus-producing columnar epithelial
cells of intestinal type in the stroma. Goblet cells are seen easily. The
mucus pools are mostly acellular, although three hyperchromatic atypical
nuclei are seen at the upper right-hand corner (hematoxylin and
eosin,  420). 420).
Back to Top
Endometrioid Tumor of Low Malignant Potential
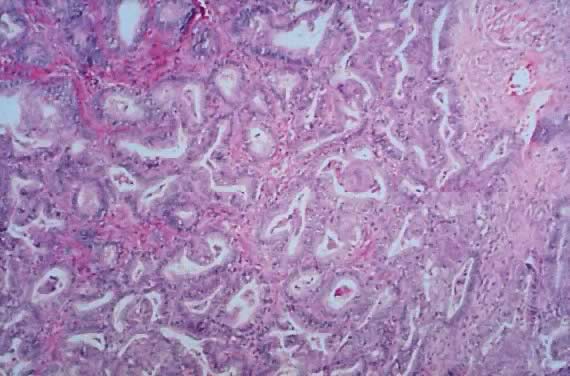 Photomicrograph of an endometrioid tumor of low malignant potential. The
tumor is composed of endometrial-like glands simulating atypical complex
hyperplasia. The glands are arranged in groups separated by dense
fibrous stroma (
Photomicrograph of an endometrioid tumor of low malignant potential. The
tumor is composed of endometrial-like glands simulating atypical complex
hyperplasia. The glands are arranged in groups separated by dense
fibrous stroma ( 100). 100).
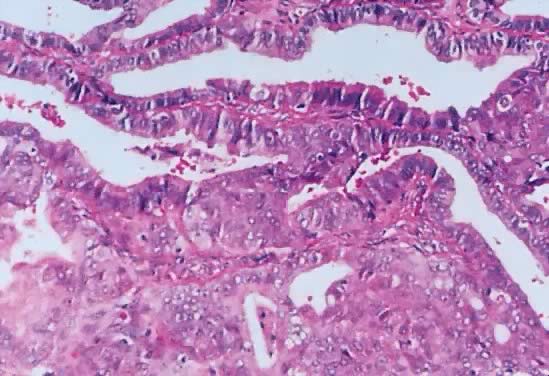 Photomicrograph of an endometrioid tumor of low malignant potential. Glands
similar to those observed in atypical complex, and atypical simple
hyperplasia are observed. The glands are irregular. The epithelium form, in
some areas, solid masses of atypical cells showing glands within
glands. The stroma is formed by dense fibrous tissue. No stromal necrosis
is observed (
Photomicrograph of an endometrioid tumor of low malignant potential. Glands
similar to those observed in atypical complex, and atypical simple
hyperplasia are observed. The glands are irregular. The epithelium form, in
some areas, solid masses of atypical cells showing glands within
glands. The stroma is formed by dense fibrous tissue. No stromal necrosis
is observed ( 200). 200).
Back to Top
Clear Cell Tumor of Low
Malignant Potential
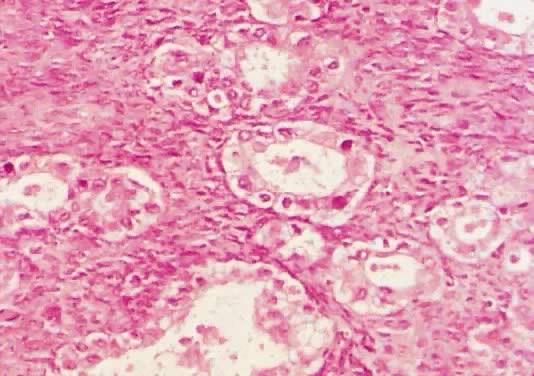 Photomicrograph of a clear cell tumor of LMP showing tubular structures
lined by low columnar epithelium. The cytoplasm in most epithelial cells
is clear. Some nuclei are hyperchromatic. The intertubular stroma
is dense (
Photomicrograph of a clear cell tumor of LMP showing tubular structures
lined by low columnar epithelium. The cytoplasm in most epithelial cells
is clear. Some nuclei are hyperchromatic. The intertubular stroma
is dense ( 200). 200).
Back to Top
Brenner Tumor of Low Malignant Potential
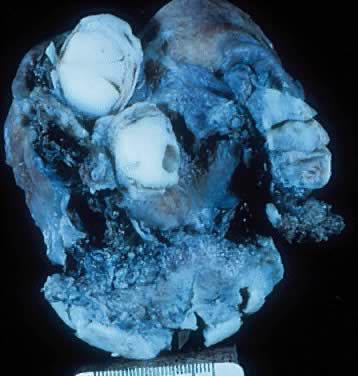 Gross appearance an ovarian transitional cell tumor of low malignant potential (Brenner
tumor of LMP). Notice irregular cystic spaces and two
round-oval masses approximately 1.5 cm in diameter. These masses are
solid. In the lower mass, a small cyst space is noted. The inner lining
of the cystic spaces is shaggy.
Gross appearance an ovarian transitional cell tumor of low malignant potential (Brenner
tumor of LMP). Notice irregular cystic spaces and two
round-oval masses approximately 1.5 cm in diameter. These masses are
solid. In the lower mass, a small cyst space is noted. The inner lining
of the cystic spaces is shaggy.
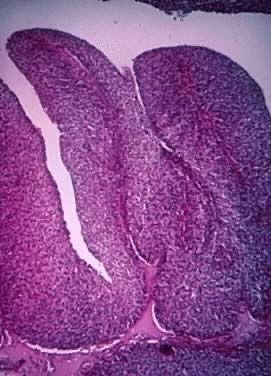 Papillations showing a thin, central fibrovascular core present in a proliferating
Brenner tumor. The epithelial lining consists of 10 to 20 layers
of transitional cell-type epithelium. The cellular atypia and pattern
resemble those of transitional cell carcinoma (grades 1 to 2) of
the urinary tract (hematoxylin and eosin,
Papillations showing a thin, central fibrovascular core present in a proliferating
Brenner tumor. The epithelial lining consists of 10 to 20 layers
of transitional cell-type epithelium. The cellular atypia and pattern
resemble those of transitional cell carcinoma (grades 1 to 2) of
the urinary tract (hematoxylin and eosin,
 400).(Courtesy of Dr. Laurence Roth.) 400).(Courtesy of Dr. Laurence Roth.)
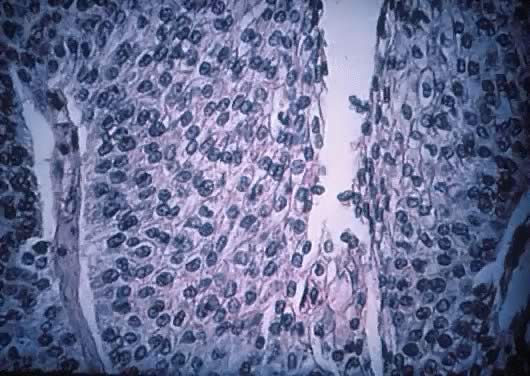 Transitional cell-like epithelium of proliferating Brenner tumor showing
atypical nuclear changes similar to those of transitional cell carcinoma
of the urinary bladder (grades 1 to 2) (hematoxylin and eosin,
Transitional cell-like epithelium of proliferating Brenner tumor showing
atypical nuclear changes similar to those of transitional cell carcinoma
of the urinary bladder (grades 1 to 2) (hematoxylin and eosin,
 700).(Courtesy of Dr. Laurence Roth.) 700).(Courtesy of Dr. Laurence Roth.)
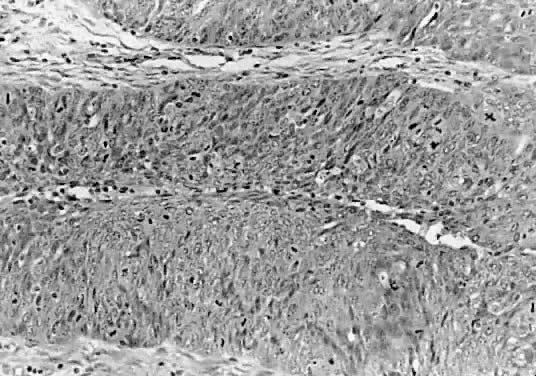 Section illustrating Brenner tumor of low malignant potential with histologic
characteristics simulating a transitional carcinoma grade 3. Observe
the number of layers, loss of polarity of nuclei, hyperchromasia, and
abundant and abnormal mitosis (hematoxylin and eosin,
Section illustrating Brenner tumor of low malignant potential with histologic
characteristics simulating a transitional carcinoma grade 3. Observe
the number of layers, loss of polarity of nuclei, hyperchromasia, and
abundant and abnormal mitosis (hematoxylin and eosin,
 420). 420).
Back to Top
Cystadenofibroma with Atypia
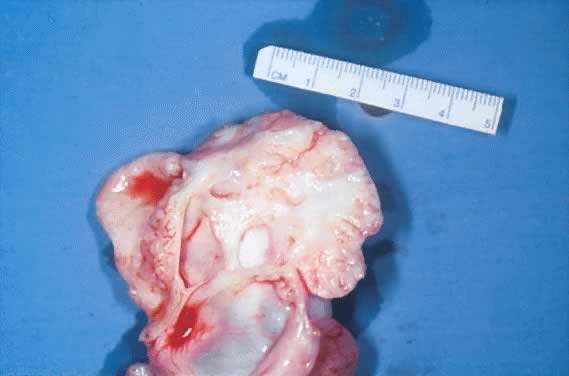 Gross appearance of a cystic adenofibroma of LMP. The tumor involves part
of the ovary. It is slightly lobulated and made up of cystic and solid
structures. The tumor is firm and hard on cut section. It is yellowish
gray. The cystic spaces show a smooth inner lining.
Gross appearance of a cystic adenofibroma of LMP. The tumor involves part
of the ovary. It is slightly lobulated and made up of cystic and solid
structures. The tumor is firm and hard on cut section. It is yellowish
gray. The cystic spaces show a smooth inner lining.
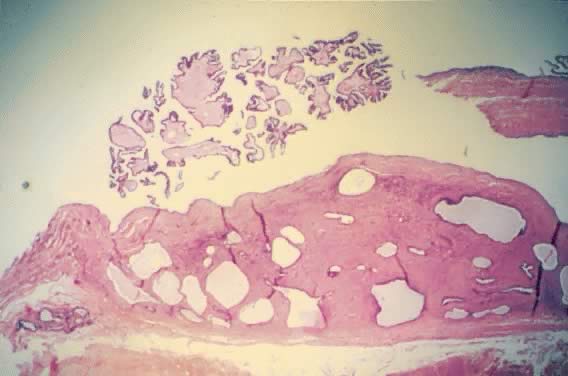 Cystadenofibroma with atypia. The stroma is composed of dense, fibrous
tissue and dilated cyst-like glands lined with cuboidal epithelial cells. Papillary
areas at the top demonstrate dense, fibrous stroma lined
with atypical epithelial cells (hematoxylin and eosin,
Cystadenofibroma with atypia. The stroma is composed of dense, fibrous
tissue and dilated cyst-like glands lined with cuboidal epithelial cells. Papillary
areas at the top demonstrate dense, fibrous stroma lined
with atypical epithelial cells (hematoxylin and eosin,
 170). 170).
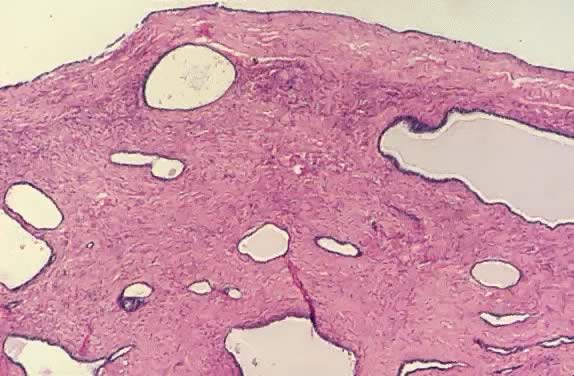 Close-up of cystadenofibroma with atypia showing dense, fibrous stroma
and dilated cystic glands lined with cuboidal epithelial cells showing
nuclear hyperchromasia (hematoxylin and eosin,
Close-up of cystadenofibroma with atypia showing dense, fibrous stroma
and dilated cystic glands lined with cuboidal epithelial cells showing
nuclear hyperchromasia (hematoxylin and eosin,
 420). 420).
Back to Top
Borderline Serous Tumor
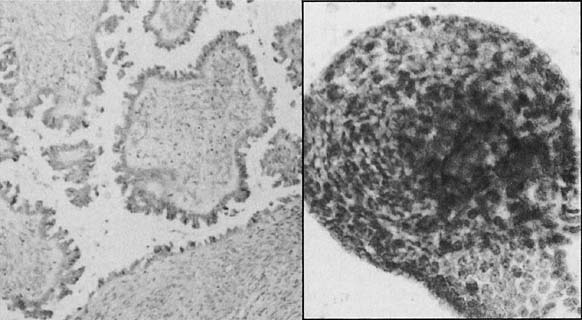 Left. Histologic section of a borderline serous tumor. (Hematoxylin and
eosin, × 520). Right. A cluster of small monomorphic cells from
the neoplasm.(Papanicolaou, × 900).
Left. Histologic section of a borderline serous tumor. (Hematoxylin and
eosin, × 520). Right. A cluster of small monomorphic cells from
the neoplasm.(Papanicolaou, × 900).
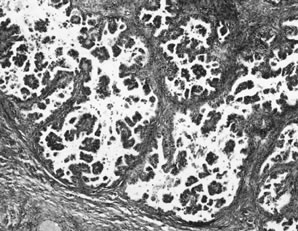 Atypical proliferative serous tumor. Hierarchical branching pattern with
detachment of cell clusters and without invasion characterizes this
tumor, which is also referred to as serous borderline tumor.
Atypical proliferative serous tumor. Hierarchical branching pattern with
detachment of cell clusters and without invasion characterizes this
tumor, which is also referred to as serous borderline tumor.
Back to Top
Pseudomyxoma Peritonei
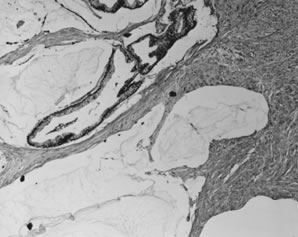 Disseminated peritoneal adenomucinosis. In this form of pseudomyxoma peritonei, large pools of acellular mucin dissect through tissues. Occasional mucinous
glands, seen at upper left, display minimal cytologic atypia.
Disseminated peritoneal adenomucinosis. In this form of pseudomyxoma peritonei, large pools of acellular mucin dissect through tissues. Occasional mucinous
glands, seen at upper left, display minimal cytologic atypia.
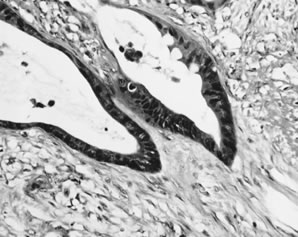 Peritoneal mucinous carcinomatosis. In this form of pseudomyxoma peritonei, angulated glands with moderate-to-severe nuclear atypia invade tissues.
Peritoneal mucinous carcinomatosis. In this form of pseudomyxoma peritonei, angulated glands with moderate-to-severe nuclear atypia invade tissues.
Back to Top
Serous Adenocarcinoma
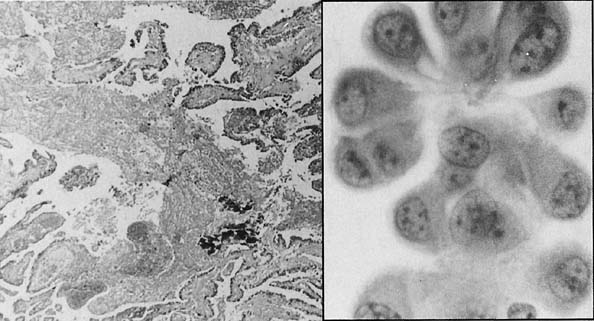 Left. Histologic section of an invasive serous adenocarcinoma. (Hematoxylin
and eosin, × 260). Right. A cluster of cells from the tumor, showing marked pleomorphism and nuclear
atypia.(Papanicolaou, × 1200).
Left. Histologic section of an invasive serous adenocarcinoma. (Hematoxylin
and eosin, × 260). Right. A cluster of cells from the tumor, showing marked pleomorphism and nuclear
atypia.(Papanicolaou, × 1200).
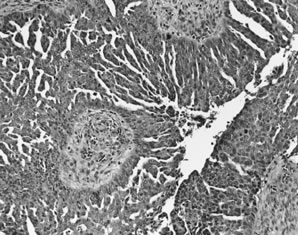 Serous carcinoma. Markedly complex and stratified papillary proliferation
with moderate-to-severe nuclear atypia.
Serous carcinoma. Markedly complex and stratified papillary proliferation
with moderate-to-severe nuclear atypia.
Back to Top
Serous Psammocarcinoma
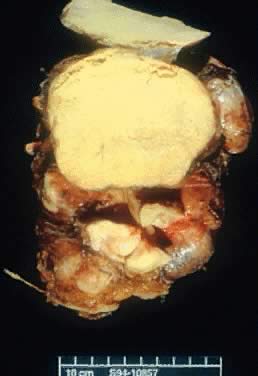 Gross view of an ovarian psammocarcinoma. Multiple tumor nodules are noted. The
largest one is yellowish. The cut surface has a gritty and solid
consistency.
Gross view of an ovarian psammocarcinoma. Multiple tumor nodules are noted. The
largest one is yellowish. The cut surface has a gritty and solid
consistency.
 Serous psammocarcinoma of the ovary. The section shows numerous psammoma
bodies in the stroma, cyst lumen, and papillae (hematoxylin and eosin,
Serous psammocarcinoma of the ovary. The section shows numerous psammoma
bodies in the stroma, cyst lumen, and papillae (hematoxylin and eosin,
 85). 85).
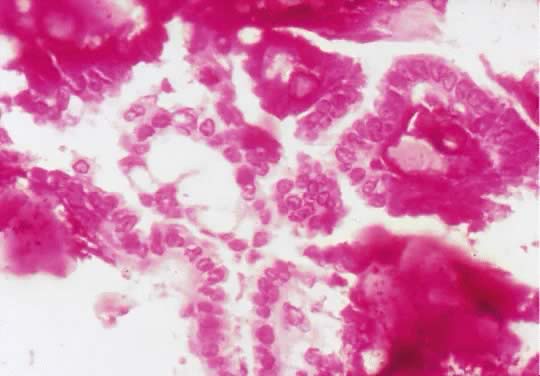 Photomicrograph of a psammocarcinoma to illustrate psammoma bodies in the
papillary component of the tumor. Atypical cuboidal epithelial cells
with prominent nucleoli line the papillae (
Photomicrograph of a psammocarcinoma to illustrate psammoma bodies in the
papillary component of the tumor. Atypical cuboidal epithelial cells
with prominent nucleoli line the papillae ( 200). 200).
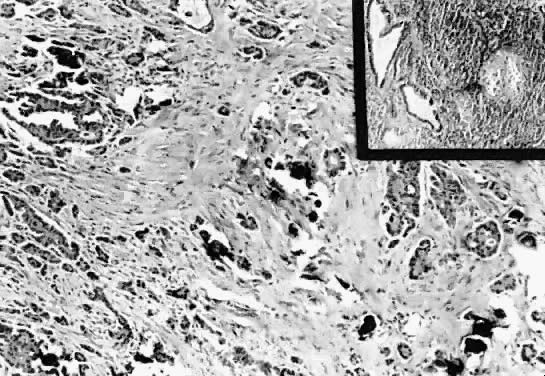 Serous psammocarcinoma. The epithelial component diffusely infiltrates
the desmoplastic stroma. Few psammoma bodies are present (hematoxylin
and eosin,
Serous psammocarcinoma. The epithelial component diffusely infiltrates
the desmoplastic stroma. Few psammoma bodies are present (hematoxylin
and eosin,  212). Inset. Section of a periaortic lymph node involved by tumor. Observe the atypical
glandular epithelium. The glands in the center exhibit atypical papillary
structures (hematoxylin and eosin, 212). Inset. Section of a periaortic lymph node involved by tumor. Observe the atypical
glandular epithelium. The glands in the center exhibit atypical papillary
structures (hematoxylin and eosin,  212). 212).
Back to Top
Endometrioid Carcinoma
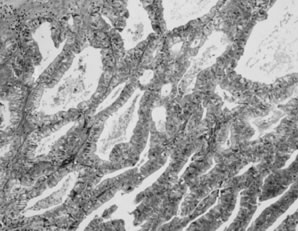 Endometrioid carcinoma. Crowded, back-to-back glands lined by tall columnar
epithelium resembling endometrial epithelium invade the stroma.
Endometrioid carcinoma. Crowded, back-to-back glands lined by tall columnar
epithelium resembling endometrial epithelium invade the stroma.
Back to Top
Clear Cell Carcinoma
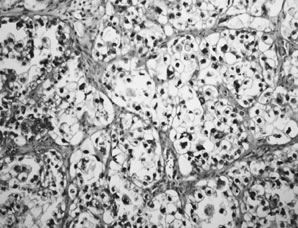 Clear cell carcinoma. Sheets of cells with clear cytoplasm and mild-to-moderate
nuclear atypia are characteristic.
Clear cell carcinoma. Sheets of cells with clear cytoplasm and mild-to-moderate
nuclear atypia are characteristic.
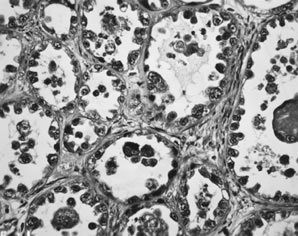 Clear cell carcinoma. Glands lined by epithelium displaying severe nuclear
atypia and a hobnail pattern characterized by nuclei protruding into
the lumena of the glands are present. In this pattern, clear cytoplasm
is less conspicuous.
Clear cell carcinoma. Glands lined by epithelium displaying severe nuclear
atypia and a hobnail pattern characterized by nuclei protruding into
the lumena of the glands are present. In this pattern, clear cytoplasm
is less conspicuous.
Back to Top
Embryonal Carcinoma
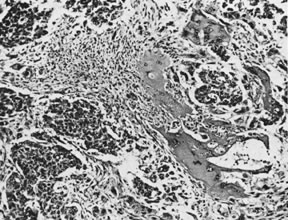 Embryonal carcinoma with syncytiotrophoblast cells (magnification, ×34). AFIP
Neg. 75-14208.(Kurman RJ, Norris HJ: Embryonal carcinoma of the ovary. A clinicopathologic
entity distinct from endodermal sinus tumor resembling embryonal
carcinoma of the adult testis. Cancer 38:2420, 1976.)
Embryonal carcinoma with syncytiotrophoblast cells (magnification, ×34). AFIP
Neg. 75-14208.(Kurman RJ, Norris HJ: Embryonal carcinoma of the ovary. A clinicopathologic
entity distinct from endodermal sinus tumor resembling embryonal
carcinoma of the adult testis. Cancer 38:2420, 1976.)
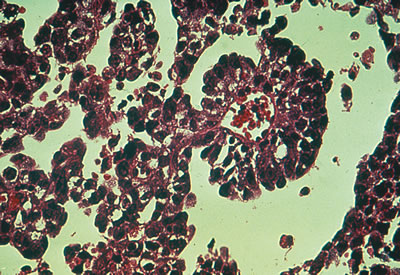 Embryonal carcinoma. Histologic pattern resembles glomeruli, vacuolated
cytoplasm, and glandlike spaces.
Embryonal carcinoma. Histologic pattern resembles glomeruli, vacuolated
cytoplasm, and glandlike spaces.
Back to Top
Choriocarcinoma
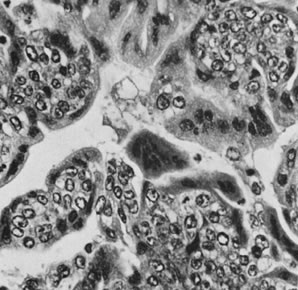 Choriocarcinoma (magnification, ×350).(Serov SF, Scully RE, Sobin LH: Histological Typing of Ovarian Tumours. Geneva, World
Health Organization, 1973.)
Choriocarcinoma (magnification, ×350).(Serov SF, Scully RE, Sobin LH: Histological Typing of Ovarian Tumours. Geneva, World
Health Organization, 1973.)
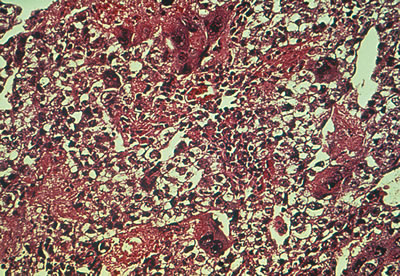 Choriocarcinoma. Microscopic view of nongestational choriocarcinoma reveals
cells resembling syncytiotrophoblasts and cytotrophoblasts. There
is usually extensive hemorrhage and necrosis.
Choriocarcinoma. Microscopic view of nongestational choriocarcinoma reveals
cells resembling syncytiotrophoblasts and cytotrophoblasts. There
is usually extensive hemorrhage and necrosis.
Back to Top
Mixed Germ Cell Tumor
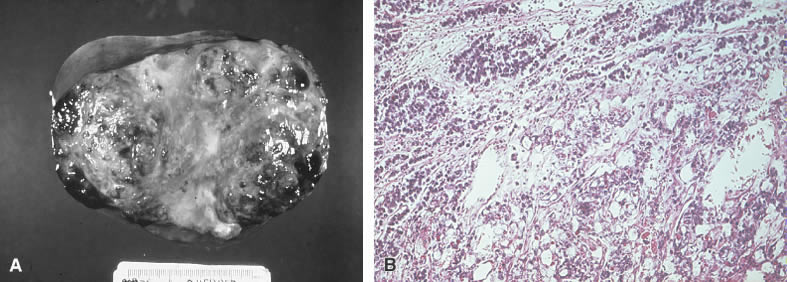 Mixed germ cell tumor. A. A nondescript solid tumor has no defining characteristics. B. Dysgerminoma elements are associated with a yolk sac carcinoma.
Mixed germ cell tumor. A. A nondescript solid tumor has no defining characteristics. B. Dysgerminoma elements are associated with a yolk sac carcinoma.
Back to Top
Krukenberg Tumor
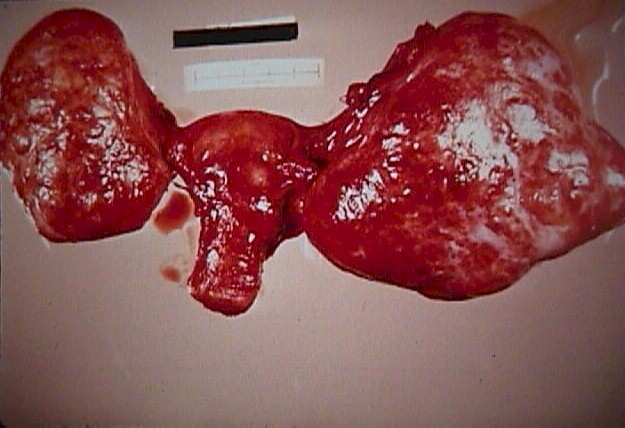 Krukenberg
Tumor (From Operational Obstetrics & Gynecology
- 2nd Edition, The Health Care of Women in Military Settings, CAPT Michael
John Hughey, MC, USNR, NAVMEDPUB 6300-2C, Bureau of Medicine and Surgery,
Department of the Navy, 2300 E Street NW, Washington, D.C. 20372-5300,
January 1, 2000. Original image courtesy Armed Forces Institute of Pathology) Krukenberg
Tumor (From Operational Obstetrics & Gynecology
- 2nd Edition, The Health Care of Women in Military Settings, CAPT Michael
John Hughey, MC, USNR, NAVMEDPUB 6300-2C, Bureau of Medicine and Surgery,
Department of the Navy, 2300 E Street NW, Washington, D.C. 20372-5300,
January 1, 2000. Original image courtesy Armed Forces Institute of Pathology)
| 












 Infarction
of a Dermoid Cyst (From Operational Obstetrics &
Gynecology - 2nd Edition, The Health Care of Women in Military Settings,
CAPT Michael John Hughey, MC, USNR, NAVMEDPUB 6300-2C, Bureau of Medicine
and Surgery, Department of the Navy, 2300 E Street NW, Washington, D.C.
20372-5300, January 1, 2000. Original image courtesy Armed Forces Institute
of Pathology)
Infarction
of a Dermoid Cyst (From Operational Obstetrics &
Gynecology - 2nd Edition, The Health Care of Women in Military Settings,
CAPT Michael John Hughey, MC, USNR, NAVMEDPUB 6300-2C, Bureau of Medicine
and Surgery, Department of the Navy, 2300 E Street NW, Washington, D.C.
20372-5300, January 1, 2000. Original image courtesy Armed Forces Institute
of Pathology)









 70
pound mucinous cystadenoma (From Operational Obstetrics
& Gynecology - 2nd Edition, The Health Care of Women in Military Settings,
CAPT Michael John Hughey, MC, USNR, NAVMEDPUB 6300-2C, Bureau of Medicine
and Surgery, Department of the Navy, 2300 E Street NW, Washington, D.C.
20372-5300, January 1, 2000. Original image courtesy Michael John Hughey,
MD. All rights reserved.)
70
pound mucinous cystadenoma (From Operational Obstetrics
& Gynecology - 2nd Edition, The Health Care of Women in Military Settings,
CAPT Michael John Hughey, MC, USNR, NAVMEDPUB 6300-2C, Bureau of Medicine
and Surgery, Department of the Navy, 2300 E Street NW, Washington, D.C.
20372-5300, January 1, 2000. Original image courtesy Michael John Hughey,
MD. All rights reserved.)










 Theca Lutein Cyst (From
Operational Obstetrics & Gynecology - 2nd Edition, The Health Care of Women in
Military Settings, CAPT Michael John Hughey, MC, USNR, NAVMEDPUB 6300-2C,
Bureau of Medicine and Surgery, Department of the Navy, 2300 E Street NW,
Washington, D.C. 20372-5300, January 1, 2000. Original image courtesy Armed
Forces Institute of Pathology)
Theca Lutein Cyst (From
Operational Obstetrics & Gynecology - 2nd Edition, The Health Care of Women in
Military Settings, CAPT Michael John Hughey, MC, USNR, NAVMEDPUB 6300-2C,
Bureau of Medicine and Surgery, Department of the Navy, 2300 E Street NW,
Washington, D.C. 20372-5300, January 1, 2000. Original image courtesy Armed
Forces Institute of Pathology)





























































 Krukenberg
Tumor (From Operational Obstetrics & Gynecology
- 2nd Edition, The Health Care of Women in Military Settings, CAPT Michael
John Hughey, MC, USNR, NAVMEDPUB 6300-2C, Bureau of Medicine and Surgery,
Department of the Navy, 2300 E Street NW, Washington, D.C. 20372-5300,
January 1, 2000. Original image courtesy Armed Forces Institute of Pathology)
Krukenberg
Tumor (From Operational Obstetrics & Gynecology
- 2nd Edition, The Health Care of Women in Military Settings, CAPT Michael
John Hughey, MC, USNR, NAVMEDPUB 6300-2C, Bureau of Medicine and Surgery,
Department of the Navy, 2300 E Street NW, Washington, D.C. 20372-5300,
January 1, 2000. Original image courtesy Armed Forces Institute of Pathology)