Acute Pelvic Inflammatory Disease
Authors
INTRODUCTION
The true incidence of PID is unknown because of inaccuracy of diagnosis. The frequency of recognized PID increased dramatically from 1960 to 1975, with a peak in Sweden in 1977 and in the United States in 1993, after which the frequency began to decrease.1, 2, 3 This increase was particularly dramatic for women younger than 25 years. Among teenagers, the incidence of PID is estimated to be 1.5% annually; PID is the most frequent serious infection that teenagers acquire.
The cause of the rise in the PID rate is not well documented, but, in part, the rise was related to an increase in sexually transmitted agents that cause PID, to changes in sexual behavior, and to changes in contraceptive methods. For example, a parallel rise was observed between the rates of PID and both gonorrhea and chlamydia.4 However, in European countries, the proportion of gonorrhea-associated PID has decreased while there was a dramatic increase of total PID due to diseases other than gonorrhea.5 The influence of changes in sexual behavior among women has been less well documented, but an increase in the proportion of young sexually active women may increase the PID rates. The influence of contraceptive changes is discussed later. In the 1980s, up to 15% of women of reproductive age had prior PID, and many were left with the sequelae of the infection. In 1980 an estimated 3.5% of women 30 years of age were sterile or had an ectopic pregnancy as a result of PID.5
Control of gonorrhea and of chlamydia are one reason that PID rates decreased since the 1980s.4 Chlamydial control reduces PID by 50% or more.6, 7 However, more effort is needed to control these microbes because among the group at highest PID risk, 15–25--olds, only 22% of symptomatic women and 5% of women undergoing a pelvic examination are tested for chlamydia in the United States.8 Recent models suggest that such testing of 30–50% of women or men would markedly reduce the prevalence of chlamydia9 and with it, of PID. Screening control programs for young people require continued attention; Finnish 10–19-year-old women and men had a 40–70% increase in the incidence of C. trachomatis between 1995 and 2005.10
SEQUELAE
Recurrent Pelvic Inflammatory Disease
A subsequent episode occurs in up to 25% of women after an episode of PID.11 The frequency of infertility and other sequelae markedly increases after multiple infections, so attempts should be made to reduce subsequent PID through education, treatment of male sexual partners, and close follow-up. Women with sexually transmitted organisms tend to reacquire these organisms, which further contribute to recurrent PID. The high subsequent tubal infection rate may be related to the susceptibility of damaged fallopian tube mucosa to recurrent infection and perhaps to unrecognized persistent infection.
Infertility
Infertility is one of the most common and dreaded consequences of PID. PID is a major cause of infertility. Tubal disease from prior salpingitis is present in 15–40% of infertile women in developed countries12 and up to 65% of infertile women in developing countries.13 Infertility resulting from tubal occlusion occurs in 8% of women with one episode, 23% of women with two episodes, and 43% of women with three episodes of PID.14, 15, 16, 17 The frequency of tubal occlusion also is related to the type and severity of PID and to age. Infertility is more common when PID is caused by organisms other than gonococci.15, 16 Infertility is more common after laparoscopically documented severe tubal infection (21%) than after moderate (16%) or mild (6%) tubal infection.15, 16 Older women are more likely than young women to develop tubal occlusion after PID.5 Tubal infertility also is increased among women with >3 days of pain before treatment.17 The cost of infertility resulting from PID in the United States is estimated to be $60 billion annually, ten times the cost of treating acute PID.14
Ectopic Pregnancy
The number of ectopic pregnancies reported in the United States doubled in the 1980s but has decreased since 1990.18 Between 1930 and 1980, the frequency of ectopic pregnancy after PID increased,5 perhaps related to antibiotic therapy. Antibiotics reduce the total tubal occlusion rates but they do not reverse all tubal damage sustained before therapy. Tubal damage causes the majority of tubal pregnancies. Tubal damage from prior PID is observed in about 50% of women with an ectopic pregnancy.5 Tubal entrapment of the ovum or slowed tubal transit time from damaged cilia presumably accounts for the tubal rather than intrauterine implantation of the pregnancy.
Ectopic pregnancy occurs in 7% of women with their first pregnancy after PID. 5, 19 The risk of an ectopic pregnancy is about 7 times greater in women after PID than in women without prior PID. Ectopic pregnancy rates increase with the severity of the tubal damage and with the number of episodes of PID.20 The impact of ectopic pregnancy on subsequent infertility is considerable; only about one half of women with an ectopic pregnancy ever again conceive. If a pregnancy occurs following an ectopic pregnancy, the rate of another ectopic pregnancy is about 20%. 20
Chronic Abdominal Pain
Chronic abdominal pain lasting longer than 6 months occurred in 17–18% of women after one or more episodes of PID, compared with 2–5% of women who have never had PID.3, 21 Chronic pain is present in 12% after one episode, 30% after two, and more than 60% after three or more PID episodes.21 The pain is often constant, interferes with work, and results in increased physician visits, hospitalizations, and abdominal operations.22 Increased pain with menses and with intercourse also is common.22 The concept that the pain is related to residual pelvic pathology is strengthened by an increased rate of infertility and dyspareunia among women with pain after PID. Many of the hysterectomies and salpingo-oophorectomies after PID are performed because of chronic pain, although extensive surgery should diminish because pain can often be controlled by ovarian hormonal suppression or by laparoscopic lysis of pelvic adhesions.
PATHOGENESIS AND PATHOLOGY
The vagina normally contains a predominance of facultative lactobacilli and numerous but low concentrations of other facultative and anaerobic bacteria.23 It is unclear whether the high endocervix normally supports a flora24 or if the recovery of bacteria from the cervix represents vaginal contamination. However, bacteria are not recovered by culture from normal endometrium and fallopian tubes, although it is possible that bacteria may transiently colonize the upper genital tract during menses. Factors important for the dissemination of organisms from the cervicovaginal flora to the uterus and fallopian tubes are not well understood, but data collected in several studies suggest that cervicitis, vaginitis, and endometritis are related to PID (Table 1).
Table 1. Factors that enhance or inhibit the development of acute salpingitis
Enhancing factors | Inhibitory factors |
Cervicitis | Cervical mucus |
N. gonorrhoeae | Bactericidal antibodies |
C. trachomatis | Oral contraceptives |
Endometritis | |
Bacterial vaginosis | |
Douching | |
Non-medicated intrauterine device |
Cervicitis is a common infection that is often the source of microbes that cause PID. A reduction in the prevalence of cervicitis probably reduces the rate of PID, and cervicitis should be actively diagnosed and treated.
Cervicitis can be diagnosed by finding mucopus (i.e. yellow or opaque appearance of endocervical mucus on a swab or in situ) or by identifying ten or more white blood cells per 400× power on a Gram stain of cervical mucus.25 The cervical surface has to be wiped free of vaginal material to properly visualize for mucopus. Infection within columnar cervical epithelial cells also often produces erythema, edema, and bleeding of the cervix. Chlamydia trachomatis and Neisseria gonorrhoeae are the most common organisms causing cervicitis,25 and together they can be isolated in more than one half of the women with the infection. The cause of infection in the remaining women is unknown, although Mycoplasma genitalium also causes a proportion of cervicitis.26
Spontaneous PID is limited to menstruating women. Women who develop PID with N. gonorrhoeae or C. trachomatis usually experience the onset of pain within 7 days of their menstrual period.27 This observation suggests that PID caused by these bacteria is influenced by menses. The growth of N. gonorrhoeae, particularly the virulent transparent gonococcal phenotypes, may be stimulated during the secretory phase of the cycle just before menstruation.28 The bacteriostatic effect of the cervical mucus on bacteria, which is greatest during the ovulatory phase of the cycle, is at its minimum before the onset of menses.29 Cervical mucus also probably acts as a mechanical barrier, and the loss of cervical mucus during menses potentially allows bacteria to penetrate into the endometrial cavity at this time. Gonococci,30 chlamydiae,31 and other bacteria appear to attach to sperm, and it is even possible that sperm can act as vectors to carry these bacteria into the uterus and fallopian tubes.
Acute infection also is enhanced by the presence of virulent bacteria and diminished by the presence of specific antibody. Some bacteria are more virulent than others. N. gonorrhoeae and C. trachomatis consistently cause tubal infection in experimental animals and in 10–20% of women with these bacteria in the cervix. In contrast, genital mycoplasmas (except M. genitalium) and bacteria of the normal cervicovaginal flora are less virulent than gonococci or chlamydiae, and they cause PID in only a small proportion of those with these microorganisms in the cervix. Most women with cervical gonorrhea for longer than 1 month have a gonococcal bactericidal antibody that may protect against the development of PID.32 Women without the bactericidal antibody are susceptible to the development of PID.32 It is likely that most women destined to develop salpingitis do so during the first few menses after the acquisition of gonorrhea and before the development of immunity conferred by gonococcal antibodies. C. trachomatis infection may be governed by similar humoral immune mechanisms.
Non-medicated intrauterine devices (IUDs) annually cause acute salpingitis among 1–2% of IUD users. An IUD's tail, particularly a multifilament tail, is capable of assisting movement from the cervicovaginal area to the uterus.33 The tail acts as a reservoir for bacteria through colonization on the surface.34 IUDs may also increase infection rate by promoting anaerobic bacterial growth, by producing microulcers, or by producing a chronic inflammatory reaction of the endometrium35 or the fallopian tube.36
The usual infection ascends along the mucosa from the cervix into the endometrium and to the fallopian tubes. After bacteria reach the uterus, they may be transported to the fallopian tubes by contiguous spread or carried by ciliary motion. Endometritis appears to be an intermediate level of infection between cervicitis and salpingitis. Endometritis diagnosed by plasma cells within the endometrium37 is present in approximately 50% of women with cervicitis.38 Women with endometritis have an increased rate of abnormal vaginal bleeding and uterine tenderness. It is possible for women to have symptoms of endometritis for a considerable time before the development of overt salpingitis, and women with these findings should be scrutinized for cervicitis and endometritis.
Once in the fallopian tube, organisms attach to the mucosa and initiate endosalpingitis. Experimental gonococcal infection has provided a model for study of salpingitis. Gonococci attach to nonciliated mucosal epithelial cells and are phagocytized into the cell. Mucosal cells become markedly distorted. A reaction between IgG antibody and gonococci fixes complement and generates an intense inflammatory reaction that includes white blood cells, edema, vasodilatation, and loss of cilia. Liberation of a gonococcal endotoxin causes tubal cilial destruction on the adjacent uninfected ciliated cells.39N. gonorrhoeae lipopolysaccharide also affects ciliary activity. The intense inflammatory reaction causes the short duration and marked symptoms characteristic of gonococcal PID.
C. trachomatis is engulfed within epithelial cells, where it replicates. The takeover of the epithelial cell metabolism and the rupture of mature chlamydial particles back into the tubal lumen causes death of the epithelial cell during chlamydial infection. The intracellular location of C. trachomatis offers limited protection of the bacteria to immune recognition. In human fallopian tube culture, C. trachomatis invaded ciliated and nonciliated tubal cells,40 and in experimental monkey infection, both cell types were lost. Chlamydial infection also induced an inflammatory response characterized by cytokines, including interferon-γ (INF-γ).41 Expression of major histocompatibility complex class II molecule-bound exogenous antigens against epithelial cells activates an immune response directed against the tubal cell itself, resulting in tubal destruction. Cell-mediated mechanisms play the dominant role in the resolution of chlamydial infection. C. trachomatis DNA in the tube has been identified by immunofluorescence among infertile women, even after antibiotic therapy,42 raising the possibility that chlamydiae can remain in tubal mucosa even after treatment.
In experimental infection in monkeys, repeated C. trachomatis inoculation results in an accelerated tubal inflammatory reaction and increased tubal scarring.43 The first exposure of the tube to C. trachomatis produced a self-limited infection and no tubal scarring, but repeated inoculation produced tubal occlusion and peritubal adhesions.43, 44 A cytokine response of INF-γ and various interleukins occur with experimental infection.41 With acute infection, 60% of the lymphocytes were cytotoxic lymphocytes, which together with finding porforin indicates activation of cytotoxic lymphocytes.41 Repetitive infection may produce the severe permanent tubal damage by the immunopathogenic lymphocyte response. The importance of cytotoxic lymphocytes is furthered by the observation that chlamydial salpingitis occurs more frequently among humans with human leukocyte antigen-31 (HLA-31).45 HLA-31 is an MHC class I molecule that presents cytoplasmically processed antigen to cytotoxic lymphocytes. The impact of genetics on infection is complex. This class II molecule HLA DRA *0301 was associated with both chlamydial and gonococcal cervicitis and infertility, suggesting genetics can increase both susceptability and disease severity.46
Helper T lymphocytes may also play a role in the immunopathology of chlamydial salpingitis. Helper T lymphocytes participate in a delayed-type hypersensitivity (DTH) response. DTH is produced by placing chlamydial heat shock protein-60 (CHSP-60) in the conjunctiva of animals previously infected with Chlamydia.47 Antibody to CHSP-60 increases the risk of developing chlamydial PID fivefold after chlamydial cervical infection in humans45 and is associated with severe tubal disease observed with acute infection48 and with tubal infertility49, 50 and ectopic pregnancy.51
Local and serum IgA, IgM, and IgG class antibodies are made in response to chlamydial infection. Partial protection against reinfection has been observed, but this protection appears short and serotype specific.52 Antibody appears to play a small role compared with cell-mediated immune responses in the resolution of infection. While C. trachomatis screening control programs drive the prevalence and rate of PID down, a dichotomy may exist where such control programs might not allow the development of protective antibody to C. trachomatis, which might limit tubal damage.53
Tubo-ovarian Abscess
N. gonorrhoeae and C. trachomatis do not produce abscesses in animals or humans.54 Abscesses form from a combination of facultative and anaerobic bacteria. Anaerobes dominate the local environment of low oxygen tension.55
Gonococcal infection is usually limited to the mucosa of the tube. In contrast, aerobic and anaerobic bacteria and perhaps chlamydiae often cause inflammation below the basement membrane,33 which can result in fibrosis and permanent tubal damage. Inflammation can also spread to the muscularis and serosal layers. The tubal lumen remains open early in the infection, and infected material may be exuded through the fimbriated end, producing a localized or generalized peritonitis and contamination of ovarian, bowel, and peritoneal surfaces. Later in infection, the fimbriae can be obstructed. Intraabdominal abscess formation occurs in 5% to 15% of patients with acute salpingitis as a result of a mixed aerobic and anaerobic infection. Abscess can occur within the tube, within the ovary, or between genital and gastrointestinal structures.
Classic genital Mycoplasma may not cause primary tubal infection. Little is known about Mycoplasma infection in humans, but in studies of grivet monkeys, Mycoplasma hominis cause inflammation along the vascular and lymphatic channels of the broad ligament.56M. genitalium pathology requires study. Tubal serosal swelling occurs, but tubal mucosa is neither inflamed nor infected. Lymphatic or hematogenous spread of organisms other than Mycoplasma from the uterus to the broad ligament and ovaries is uncommon among nonpregnant women because of the endometrial-myometrial barrier.
A popular but unproved hypothesis suggests that aerobic and anaerobic bacteria frequently invade the fallopian tubes after an initial gonococcal or chlamydial infection. The common finding of concomitant tubal infection with these bacteria supports this hypothesis. Virulent bacteria are present in the vagina. Aerobic and anaerobic bacteria are also capable of producing primary salpingitis after uterine instrumentation, in the presence of an IUD, and occasionally without any known risk factor. The presence of particularly virulent organisms such as streptococci or Haemophilus or the presence of high concentrations of organisms, as in the case of bacterial vaginosis, may be prerequisites for a primary infection with those organisms. Difficult-to-culture microbes in the vagina are highly associated with bacterial vaginosis.57 The presence of these microbes can be detected by DNA identification techniques. Some of these difficult-to-culture microbes associated with bacterial vaginosois were identified in tubal specimens of women with PID.58
After bacteria are eliminated by antibiotics, residual inflammatory cells remain in the tubal submucosa for prolonged periods. However, ordinary bacteria were uncommonly recovered from the tube, ovaries, or adjacent structures after antibiotic therapy.59 An exception may occur with C. trachomatis, which has been recovered from the fallopian tubes and peritubal tissue of women undergoing tuboplasty months or years after an episode of PID.42C. trachomatis has been identified in 35% of women with gross tubal inflammation and in 20% without gross tubal inflammation,60 which raises the possibility that a prolonged infection and an inflammatory response can result from chlamydiae.
Age
The highest rate of PID occurs among the youngest sexually active women. The PID rate of 15- to 19-year-old women is approximately three times that of 20- to 24-year-old women and is approximately twice the PID rate of 25- to 29-year old women.5 PID is clearly the most common serious infectious disease of young women. However, sexually transmitted disease (STD) rates are highest in young women and age is no longer an important risk factor for PID when N. gonorrhoeae and C. trachomatis are controlled.61 Thus, age is a risk factor for the acquisition of these two microbes, but is only a risk marker for PID.
Intrauterine Devices
Women with a non-medicated IUD are two to three times more likely to develop PID than nonusers.62, 63 IUD use was a risk factor for PID even in later reports.63 A foreign body increases infection rates, and human64 and primate65 IUD users frequently have intrauterine bacterial colonization, while nonusers remain without bacteria.66 Despite controversy about the selection of control groups and the criteria used to diagnose PID in some studies, the combined weight of epidemiologic and in vitro and in vivo data represents evidence that non-medicated IUDs can cause PID. Progesterone dampens an immunology response; and in preliminary studies, progesterone IUDs appear to have a low PID rate. However, large well controlled studies are needed of progesterone users.
A small 1–2% of women will develop signs of PID within 3 weeks of the IUD insertion and is probably related to microbes pushed up the uterus during insertion. The severity of PID among IUD users is similar to nonusers. The impact of IUD-caused salpingitis is further emphasized by reports of threefold increased rates of primary infertility among copper IUD users67 and ectopic pregnancy among IUD users compared with women who never used an IUD.
Multiple mechanisms are probably responsible for the increased rate of PID among IUD users. Early studies suggested that bacteria that were introduced into the uterus during the insertion of an IUD were cleared within 48 hours68 and that the endometrial cavity of an IUD user was sterile. However, later data indicate endometrial bacteria in all women with tailed IUDs months after insertion, although not in women who used tailless IUDs or no IUD.64
Anaerobic vaginitis is more common among IUD users than IUD nonusers, and anaerobic Actinomyces species were identified in large concentrations more frequently among IUD users than nonusers.69 These observations raise the possibility that an IUD promotes the growth of anaerobic bacteria, perhaps to a critical level, which contributes to infection. Although Actinomyces israelii has been isolated from only a few IUD-associated PID infections, until the mechanism and PID attack rate are better described, IUD-using women with Actinomyces identified on Papanicolaou smear should be told of the organism's presence and asked to immediately report abnormal discharge, bleeding, or pain. Symptomatic women with Actinomyces should have the IUD removed and receive antibiotic treatment. However, asymptomatic women with Actinomyces should not be treated or necessarily have the IUD removed, particularly if plans include reinsertion of another IUD. It is estimated that the net rate of PID would be higher if the IUD was removed and reinserted than if it were left in situ because of the low risk of PID from Actinomyces.
Douching
Douching has been related to salpingitis and ectopic pregnancy.70 Douching three or more times a month with each commercial product was associated with a threefold increased rate of PID when demographic variables, gonorrhea, and Chlamydia infection were controlled in a multivariate analysis.71 However, a subsequent prospective study of over 1100 women found no increase in incidence of PID among women who douch.72 Douching was usually undertaken as a habit and not in response to symptoms of infection. Because there is no known benefit to douching, in any case, patients should be discouraged from the practice.
Contraceptives
Barrier contraception reduces the acquisition of STDs and appears to confer a modest reduction in the rate of PID. However, barrier methods need to be used consistently to protect against STDs, and male condom use is usually inconsistent.
Oral contraceptive (OC) use, perhaps because of the progesterone content, appears to reduce the rate of PID.5, 73 OCs may markedly reduce the rate of PID (up to 80%) among patients with C. trachomatis but have little effect among patients with N. gonorrhoeae or neither organism.73 OCs may downregulate the immune response to C. trachomatis salpingitis. Mild salpingitis is significantly more likely to result when a woman is on OCs than if she uses an IUD or other contraceptives.74
Previous Pelvic Inflammatory Disease
Women with prior PID are twice as likely to develop the infection as those who have never had PID. From 20% to 25% of patients with one bout of PID suffer subsequent PID episodes.75, 14 Although these subsequent episodes of PID could represent recrudescences of a chronic latent bacterial infection, many appear to represent reinfection. Fortunately, recurrent bouts of PID are not as common when adequate antibiotic therapy is administered. Untreated male sexual patterns remain an important cause of reinfection. It is also likely, although unproved, that many recurrences result because tubal epithelium, previously damaged by infection, is susceptible to bacterial colonization because of ciliary destruction or depressed local defense mechanisms.
Untreated Male Sexual Contacts
Untreated males with urethral N. gonorrhoeae and C. trachomatis infection are an important source of the initial and subsequent episodes of PID. More than 80% of male sexual partners have not been treated for gonorrhea when the female presents with PID.76 About one-half of the male contacts with gonorrhea were asymptomatic, and 40% of asymptomatic men had urethral N. gonorrhoeae. The males remained asymptomatic for as long as 180 days after the females were treated. Symptomatic nongonococcal urethritis and asymptomatic chlamydial urethritis are also common among male contacts of patients with PID. Examination and treatment of male contacts are crucial to decrease the recurrence of PID.
Surgical Procedures
Cervical vaginal microorganisms are pushed into the endometrium by surgical procedures, including IUD insertion, dilation and curettage, hysterosalpingography, and induced abortion. Such procedures occurred before the onset of PID in 12% of cases.77 PID has been reported in 1.5% of women within the first 3 months of IUD insertion.78 Induced abortion led to PID in up to 20% of women with untreated C. trachomatis79 and bacterial vaginosis.80 Treatment of these infections before induced abortions reduces the rate of subsequent PID to baseline.81
Human Immunodeficiency Virus Infection
Women with human immunodeficiency virus (HIV) infection may have increased rates of PID. The seroprevalence of HIV was higher in US studies82, 83 and in one Kenyan study84 among those with PID compared with controls without PID. Patients with PID appear to have more abscesses84, 85 and more frequently require surgery86 than PID patients without HIV. Early hospitalization and intravenous treatment is recommended for HIV-positive women with PID,87 but in general HIV-infected women respond appropriately to antobiotics.84
MICROBIAL ETIOLOGY
Sexually transmitted bacteria, most notably N. gonorrhoeae and C. trachomatis, are recovered from a large number of women with spontaneous PID. A small number of women have genital Mycoplasma recovered from the peritoneum. The rate with which STD microorganisms are isolated from patients with PID differs widely in various studies, probably because of different baseline rates of these STD bacteria between the populations and the severity of infection.
Cervical isolation is not a reliable indicator that it is the only bacteria in the tube or even that it is in the tube. A sizable proportion of patients with PID have N. gonorrhoeae and C. trachomatis cervical infections. However, a wide variety of bacteria can be recovered from the fallopian tubes in the presence of all types of cervical infection. Women thought to have gonococcal PID have been found to have fallopian tube infection caused by a combination of N. gonorrhoeae and C. trachomatis, by N. gonorrhoeae and endogenous aerobic and anaerobic bacteria, by endogenous bacteria alone, and by mycoplasmas. Similarly, women without gonorrhea in the cervix can have tubal infection caused by the same variety of microorganisms. Although a large number of possible combinations exist, it is useful to discuss each group separately.
Gonorrhea
Between 10% and 19% of women with N. gonorrhoeae in the cervix have signs of PID.88, 89, 90 Gonococcal virulence factors may explain why only some women develop PID. Transparent colonies of N. gonorrhoeae attach more readily to epithelial cells than opaque-appearing colonies, and transparent but not opaque colony-forming gonococci have been recovered from the abdomen of patients with gonococcal salpingitis.21 Bactericidal antibody develops in women with cervical gonorrhea present for more than 1 month, and specific gonococcal antibody appears to protect against PID.25
A wide range of N. gonorrhoeae recovery is present in PID. Cervical N. gonorrhoeae has been recovered from 80% of some urban American populations with PID88 and from less than 10% in European studies (75–85) (Table 2). In the 17 studies cited in Table 2, the mean N. gonorrhoeae recovery rate was 26%. These studies were selected because C. trachomatis cultures were also performed. Among studies that found high N. gonorrhoeae rates, there tended to be a lower C. trachomatis rate.
Table 2. Comparison of Chlamydia trachomatis and Neisseria gonororhoeae cervical isolation and N. gonorrhoeae tubal isolation among women with acute pelvic inflammatory disease
Cervical infection | Tubal/ | |||
Study | No. of patients | C. trachomatis | N. gonorrhoeae | N. gonorrhoeae |
Henry-Suchet et al.60 | 17 | (38%) | 0/4 | 1/4 (25%) |
Møller et al.90 | 166 | (22%) | 9 (5%) | |
Mårdh et al.91 | 60 | (38%) | 4 (7%) | |
Gjønnaess et al.92 | 65 | (46%) | 5 (8%) | 0/65 |
Mårdh et al.93 | 63 | (36%) | 11 (17%) | 1/14 (7%) |
Adler et al.22 | 78 | (5%) | 14 (18%) | |
Ripa et al.94 | 206 | (33%) | 39 (19%) | |
Osser and Persson95 | 209 | (47%) | 41 (20%) | |
Paavonen96 | 106 | (25%) | 27 (25%) | |
Paavonen et al.97 | 101 | (32%) | 25 (25%) | |
Paavonen et al.98 | 228 | (30%) | 60 (26%) | |
| Cohen et al.84 | 133 | (11%) | (30%) | |
| Ness99 | 831 | (21%) | 170 (20%) | |
Eilard et al.100 | 22 | (27%) | 7 (32%) | 1/22 (5%) |
Bowie and Jones101 | 43 | (51%) | 15 (35%) | |
Eschenbach et al.76 | 204 | (20%) | 90 (44%) | 7/54 (13%) |
Sweet et al89 | 39 | (5%) | 18 (46%) | 8/35 (23%) |
Cunningham et al.102 | 104 |
| 56 (54%) | 30/104 (29%) |
Thompson et al.88 | 30 | (10%) | 24 (80%) | 10/30 (33%) |
Totals | 2705 | 591/2329 (25%) | 640/2092 (24%) | 58/328 (18%) |
*Isolation of N. gonorrhoeae from the peritoneum of the total number of women studied.
N. gonorrhoeae is the most common tubal isolate from women with acute PID and the most common recovered from the cervix. N. gonorrhoeae is usually recovered from the fallopian tube or abdomen of 20–30% of women with the bacteria in the cervix and from 5% to 30% of all women with PID. Because of the exceptional virulence of N. gonorrhoeae, it is easy to assume that gonococci initiate or play a major part in the infection when it is present in the cervix. However, a significant proportion of women with gonococcal PID have no bacteria or bacteria other than gonococci isolated from the fallopian tube. A decrease in the proportion of patients from whom gonococci are recovered is noted when symptoms are present more than 7 days.89 Gonococcal tubal infection could have an initial stage when the gonococci produce the infection and a later stage of infections from other organisms. The decreased rate of gonococcal recovery in the later stage may be a consequence of inhibition by leukocytes, or it may reflect their intracellular location. Women in whom N. gonorrhoeae is the sole bacteria isolated from the tubes tend to respond to therapy more rapidly than do women from whom other bacteria are cultured, suggesting that polymicrobial infection reflects a distinct stage of infection.
Chlamydia
The proportion of PID attributed to Chlamydia is higher than that attributed to the gonococcus. C. trachomatis has been isolated from the cervix of 5–56% of women with PID. The mean recovery rate of C. trachomatis is 30%, with widely variable rates84, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 100, 101, 102, 103, 104 (Table 3). Most of these studies used culture to identify C. trachomatis, and because DNA detection of Chlamydia by ligase or polymerase chain reaction (PCR) is more sensitive than culture,105 additional women with PID were probably infected with Chlamydia. Two recent studies used DNA detection of C. trachomatis and N. gonorrhoeae.84, 104 C. trachomatis was isolated from the fallopian tubes of about one-third of all women with the organism in the cervix and from 10% of all women with PID. A complicating finding is that chlamydiae, in contrast to gonococci, can remain in the fallopian tube area for months and years after an initial infection. Women with chlamydial salpingitis may develop progressive tubal damage caused by persistent infection if it is not effectively treated.
Table 3. Chlamydia trachomatis infection among women with pelvic inflammatory disease
Chlamydia trachomatis | ||||
Study | No. of patients | Present in cervix | Present in tubes/ | Antibody titer |
Adler et al.22 | 78 | 4 (5%) |
| 24/78 (31%)† |
Sweet et al.89 | 39 | 2 (5%) | 0/35 | 5/22 (23%) |
Thompson et al.88 | 30 | 3 (10%) | 3/30 (10%) |
|
Eschenbach et al.76 | 100 | 20 (22%) | 1/54 (2%) | 15/74 (20%) |
Møller et al.90 | 166 | 37 (22%) |
| 34 (20%) |
Paavonen96 | 106 | 27 (25%) |
| 19/72 (26%) |
Eilard et al.100 | 22 | 6 (27%) | 2 (9%) |
|
Paavonen et al.98 | 228 | 69 (30%) |
| 32/167 (19%) |
Paavonen et al.97 | 101 | 32 (32%) |
| 18 (18%) |
Mårdh et al.93 | 63 | 19/53 (36%) | 6/20 (30%) |
|
Ripa et al.94 | 156 | 52 (33%) |
| 37/80 (46%) |
Henry-Suchet et al.60 | 16 | 6 (38%) | 4/17 (24%) |
|
Mårdh et al.91 | 60 | 23 (38%) |
| 22/60 (37%) |
Gjønnaess et al.92 | 65 | 26/56 (46%) | 5/31 (16%)‡ | 24/60 (40%) |
Osser and Persson95 | 111 | 52 (47%) |
| 37/72 (51%) |
| Ness et al.104 | ||||
| Cohen84 | ||||
Bowie and Jones101 | 43 | 22 (51%) |
|
|
Skaug et al.103 | 34 | 1956 (%) |
| 16 (47%) |
Totals | 1418 | 419/1399 (30%) | 21/209 (10%) | 281/986 (28%) |
*Antibody change.
†Criteria of antibody response not given.
‡Additional women had C. trachomatis in the peritoneum but not in the cervix.
No characteristic gross appearance of the fallopian tubes distinguishes chlamydial from gonococcal infection. C. trachomatis has been recovered in the presence of mild and severe tubal inflammation. However, women with C. trachomatis often have milder clinical manifestations than women with other forms of tubal infection.106 Despite the more benign clinical findings, women with C. trachomatis have tubal damage at least as severe as that of women with N. gonorrhoeae and more severe than that of women with neither. Between 20% and 50% of women with PID have serologic evidence of chlamydial infection that approximates the cervical isolation rate (Table 3). However, between 10% and 30% of culture-negative women have antibody evidence of chlamydial infection.
The presence of C. trachomatis does not exclude a concomitant N. gonorrhoeae infection. A sizable proportion of women with C. trachomatis in the cervix also have N. gonorrhoeae isolated. N. gonorrhoeae has been recovered from the same proportion of women with (23%) as without (22%) C. trachomatis. Similarly, C. trachomatis was isolated from the cervix of 33% of women with N. gonorrhoeae and from 31% of women without N. gonorrhoeae (Table 4). Concomitant infection with both organisms is common. These data underscore the rationale of treating women with PID for both organisms.
Table 4. Concomitant Neisseria gonorrhoeae and Chlamydia trachomatis infection among women with acute pelvic inflammatory disease
| Study | N. gonorrhoeae-positive cultures among women with and without C. trachomatis | C. trachomatis-positive cultures among women with and without N. gonorrhoeae | ||
With | Without | With | Without | |
Eschenbach et al.76 | 10/20 (50%) | 41/80 (51%) | 10/51 (20%) | 10/49 (20%) |
Møller et al.90 | 1/37 (3%) | 8/129 (6%) | 1/9 (11%) | 36/157 (23%) |
Paavonen96 | 8/27 (30%) | 19/79 (24%) | 8/27 (30%) | 19/79 (24%) |
Paavonen et al.98] | 17/69 (25%) | 43/159 (27%) | 17/60 (28%) | 52/168 (31%) |
Paavonen et al.97 | 6/32 (19%) | 20/69 (29%) | 6/26 (23%) | 26/75 (35%) |
Mårch et al.93 | 5/19 (26%) | 6/34 (18%) | 5/11 (45%) | 14/52 (27%) |
Gjønnaess et al.92 | 2/26 (8%) | 3/39 (8%) | 2/5 (40%) | 24/60 (40%) |
Osser and Persson95 | 12/52 (23%) | 3/59 (5%) | 12/14 (86%) | 39/90 (43%) |
Bowie and Jones101 | 10/22 (45%) | 5/21 (24%) | 10/15 (67%) | 12/28 (43%) |
Totals | 71/304 (23%) | 148/669 (22%) | 71/218 (33%) | 232/758 (31%) |
The importance of C. trachomatis is further emphasized in the study of infertile women and ectopic pregnancy. Although N. gonorrhoeae tends to produce mucosal infection, C. trachomatis and other aerobic and anaerobic bacteria may produce infection below the basement membrane, resulting in permanent tubal damage. Chlamydia antibody was present in 35–90% of infertile women with tubal abnormalities detected by hysterosalpingography or direct tubal visualization or both, compared with 0% to 50% of infertile women with no tubal abnormality107, 108, 109, 110, 111, 112, 113, 114, 115 (Table 5). These data suggest that a significant amount of tubal infertility is caused by chlamydial salpingitis. The relative importance of N. gonorrhoeae in these women usually has not been simultaneously determined, but in one report infertility rates after chlamydial salpingitis (23%), gonococcal salpingitis (24%), and nonchlamydial nongonococcal salpingitis (22%) were similar.116 Higher Chlamydia antibody rates have also been detected among patients with ectopic pregnancy than controls.117C. trachomatis was isolated from the tubes of some patients with an ectopic pregnancy, suggesting that an ongoing infection may further contribute to tubal pregnancy.
Table 5. Presence of chlamydial antibody among infertile women with and without tubal abnormality
Infertile women with chlamydia antibody | ||||
Study | Method to | Women with | Women without | P* |
Punonen et al.107 | H | 21/23 (91%) | 52/105 (49%) | <0.001 |
Cevenini et al.108 | H | 36/40 (90%) | 8/30 (27%) | <0.001 |
Moore et al.109 | L | 24/33 (73%) | 0/35 (0%) | <0.001 |
Conway et al.110 | H/L | 36/48 (75%) | 23/75 (31%) | <0.001 |
Gump et al.111 | H/L | 34/53 (64%) | 38/134 (28%) | <0.001 |
Jones et al.112 | H/L | 46/77 (60%) | 13/77 (17%) | <0.001 |
Gibson et al.113 | H/L | 33/58 (57%) |
| <0.001 |
Henry-Suchet et al.114 | L/L | 32/64 (50%) | 8/40 (20%) | <0.005 |
Kane et al.115 | L | 25/70 (35%) | 6/52 (12%) | <0.005 |
H, hysterosalpingography; L, Laparoscopy; H/L, laparoscopy or hysterosalpingography; L/L, laparoscopy or laparotomy.
*Calculated p value using Yates correction of χ2.
Genital Mycoplasmas
Culture, serologic, and experimental lines of evidence suggest some role for genital Mycoplasma. Patients with PID frequently have M. hominis (55% to 70%) isolated from the cervix. However, M. hominis was recovered from the tubal area in only 4% to 17% of women with PID (Table 6).
Table 6. Evidence of genital Mycoplasma infection among women with acute salpingitis
Study | Cervical isolation (%) | Tubal isolation (%) | Antibody change (%) |
Mycoplasma hominis | |||
Sweet et al.118 | 73 | 4 |
|
Eschenbach et al.76 | 72 | 4 | 20 |
Mårdh and Westrõm119 | 62 | 8 |
|
Thompson et al.88 | 60 | 17 |
|
Møller et al.90 | 55 |
| 30 |
Mårdh et al.91 |
|
| 12 |
Ureaplasma urealyticum | |||
Eschenbach et al.76 | 81 | 2 | 18 |
Mårdh and Westrom119 | 56 | 4 |
|
Sweet et al.118 | 54 | 15 |
|
Thompson et al.88 | 33 | 20 |
|
Henry-Suchet et al.60 | 24 | 17 |
|
Sweet et al.89 |
| 9 |
|
Serologic evidence of acute mycoplasmal infection is more common than recovery of the organism from the tubal area. Increases in IgG antibody to M. hominis have been identified in 10–30% of patients with PID; most of these women also had significant serum elevations of M. hominis IgM antibody, which is further evidence of acute infection. The fact that more patients have serologic evidence than fallopian tube culture evidence of acute mycoplasmal infection may be attributable to the propensity of mycoplasmas to cause parametritis rather than salpingitis, as discussed in the Pathogenesis and Pathology section.
U. urealyticum is commonly isolated from the cervix and can be isolated from the tubes. Although U. urealyticum serum antibody titers changed significantly in up to 18% of PID cases, its isolation into primate and human tubes fails to produce evidence of infection. There appears to be a minimal or no role for U. urealyticum in PID.
Mycoplasma genitalium has produced tubal infection in monkeys.120M. genitalium is present by PCR in up to 25% of women attending STD clinics121 and serum antibody to M. genitalium is present in one-third of women with acute PID, but no gonorrhea or chlamydia.122M. genitalium is difficult to culture but DNA detection found M. genitalium in 16% or 58 women with symptoms of PID and endometritis.123
Aerobic and Anaerobic Bacteria
Aerobic and anaerobic bacteria are isolated from the cul-de-sac and tube of up to 60% of women with acute salpingitis.102 The frequency of isolation from direct tubal cultures obtained through the laparoscope has been lower in Sweden. Although the frequency of these bacteria may be overestimated as a result of contamination of the culdocentesis samples by normal vaginal flora, aerobic and anaerobic bacteria are clearly important pathogens in acute salpingitis. The disparity between the findings of investigators in the United States and Sweden may be partially explained by population differences; women with PID in US studies tend to report for care later in the course of infection than do Swedish women. Finding other aerobic and anaerobic bacteria among women with gonococcal PID is probably attributable to the ability of nongonococcal bacteria to invade areas of fallopian tubes with inflammation and reduced redox potential after an initial gonococcal infection. An analogous situation probably occurs after primary C. trachomatis infection. Aerobes and anaerobes are often primary pathogens among women with instrumentations, and IUDs, and perhaps those with recurrent salpingitis and bacterial vaginosis.
The most common aerobic isolates are streptococci, Escherichia coli, and Haemophilus influenzae. The most common anaerobic organisms consist of peptostreptococci and Bacteroides species. As mentioned before, difficult-to-culture microbes, such as Leptotrichia and Atopo, associated with bacterial vaginosis were present in tubal specimens using DNA detection; and this method may continue to identify new pathogens.58 Anaerobic bacteria, alone or more commonly as part of a polymicrobial infection, are present in 15–60% of women with PID. Patients with the most severe clinical findings, such as peritonitis, often have aerobic and anaerobic bacterial infections. Anaerobic bacteria are virtually always present in intraabdominal abscesses caused by PID. The important role of anaerobes is further illustrated by the finding that patients with anaerobic pelvic infection respond poorly to therapy and tend to develop sequelae. Fallopian tube occlusion was more frequent among patients with C. trachomatis or anaerobic salpingitis than those with N. gonorrhoeae salpingitis.124
CLINICAL MANIFESTATIONS
Abdominal pain is the most common symptom of PID, although the pain may be mild or, absent in at least 5% of patients with laparoscopically verified PID.125 From 30% to 75% of women with occluded tubes have never had recognized PID or abdominal pain severe enough to receive antibiotic therapy.109 Physicians must be aware that mild or atypical symptoms may represent PID. When present, pain usually was present for less than 10 days.106 About 75% of patients with gonococcal or chlamydial PID complain of vaginal discharge of recent onset. Intermenstrual or excessive menstrual bleeding, probably because of endometritis, occurs in 40% of patients.125 Dysuria or urinary frequency occurs in 15% of women. Fever is present in only about 40% of patients,125 most commonly in those with severe clinical disease. Nausea and vomiting occur later in the course of PID than in appendicitis and are assumed to result from contiguous bowel inflammation. In patients with gonococci or chlamydiae, the onset of pain tends to occur with menses. In general, gonococcal PID has a rapid onset; the pain is usually of short duration, the temperature is often higher than 100.4°F (38°C), and patients have a cervical exudate. Gonococcal PID is not usually associated with a history of previous PID or with IUD use. In contrast, chlamydial PID is characterized by a significantly longer duration of usually less severe pain, a higher sedimentation rate, and less frequently a fever than gonococcal PID.106 Women with chlamydial PID more often have undergone recent genital tract instrumentation than patients with gonococcal PID.
A syndrome of perihepatitis associated with gonococcal salpingitis was described in the 1920s. Chlamydial salpingitis also causes perihepatitis. The pleuritic pain is caused in the early stage by inflammation of the liver capsule. During the later stage, "violin string" adhesions form between the liver and the anterior abdominal wall. Perihepatitis is usually diagnosed by severe, pleuritic, most frequently right, upper quadrant pain. The onset of pleuritic pain is most likely to occur after or simultaneously with the onset of lower abdominal pain, although it can occur occasionally without any recognized lower abdominal pain. Symptomatic perihepatitis occurs in 5% to 10% of women with salpingitis, but it is frequently not considered in the differential diagnosis of right upper quadrant pain, which can be misdiagnosed as cholecystitis or pneumonia. To limit diagnostic errors, a careful pelvic examination should be performed in any sexually active woman who presents with pleuritic or right upper quadrant abdominal pain.
The most widely accepted mechanism of infection has been the spread of intraabdominal bacteria from the fallopian tubes to the liver surface. However, clinical and experimental evidence suggests that lymphatic or hematogenous bacterial spread from the pelvis to the liver may be a more likely route than direct intraabdominal extension. An immune component is also possible, with increased levels of CHSP-60 found in cases of perihepatitis.126
The physical findings consist of moderate-to-severe liver tenderness without hepatomegaly. A transient friction rub is occasionally present. Signs of salpingitis are usually present on a pelvic examination. Results of serum bilirubin and liver function tests are normal or only mildly elevated. The gallbladder may not be visualized with an oral cholecystogram during the acute phase. Laparoscopy provides the most precise diagnosis; a purulent or fibrinous exudate over the liver capsule is visualized early in the infection, followed by adhesions later in the course. The pain usually resolves rapidly after antibiotic therapy is administered.
DIAGNOSIS
Salpingitis has a broad spectrum of clinical manifestations, varying from mild to severe. Insistence on rigid diagnostic criteria such as fever, abdominal tenderness, leukocytosis, and an elevated sedimentation rate can uncover only the most overt cases of PID while excluding most cases. In patients with severe to moderate, clinically overt PID, it is possible to establish the diagnosis with reasonable certainty by a combination of history, physical examination, Gram stain of cervical secretions, and examination of the male sexual partner. Laparoscopy is of limited benefit for the 4% of women with clinically severe salpingitis.
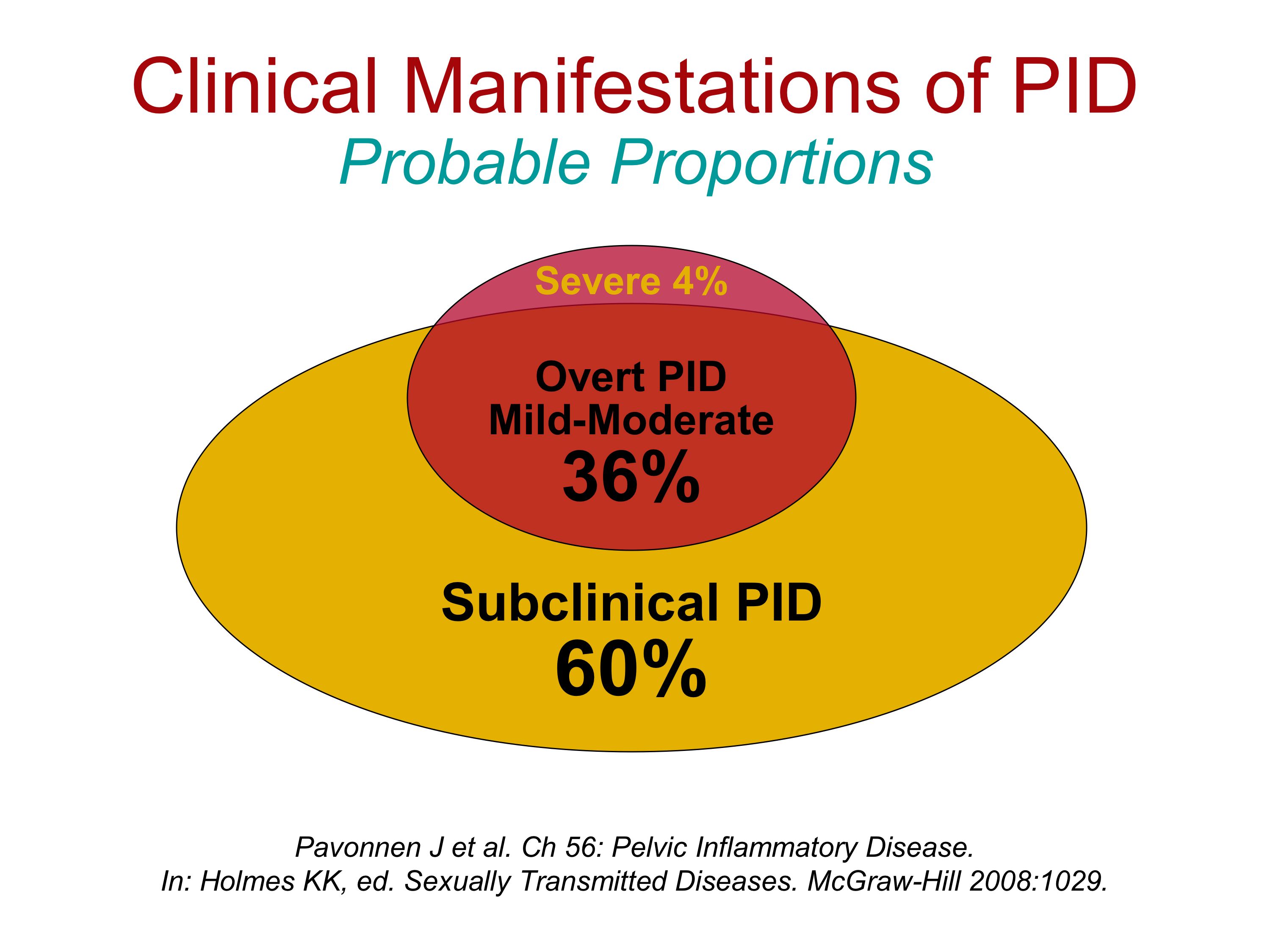
Several studies have addressed the difficulties of accurately establishing a clinical diagnosis of PID (107–112) (Table 7). Among patients with a clinical diagnosis of acute PID, salpingitis could be confirmed by laparoscopy in only about 63%, whereas 17% of patients had other diseases, most commonly benign bleeding ovarian cysts, ectopic pregnancy, appendicitis, and endometriosis. Of the women clinically diagnosed as having PID, 3% had had appendicitis, and 4% have an ectopic pregnancy (Table 7). About 20% of patients had no pathologic pelvic findings visualized by laparoscopy.
Table 7. Visually confirmed salpingitis and of other pathology among women with suspected acute salpingitis
Pathology | Jacobson and Westrõm107 | Chaparro et al.109 | Kolmorgen et al.110 | Cohen84 | Wølner-Hanssen et al.111 | Allen and Schoon112 | Total |
Acute salpingitis | 532 | 103 | 92 | 133 | 76 | 63 | 999 (63%) |
Normal findings | 184 | 51 | 33 | 2 | 22 | 13 | 305 (19%) |
Other | 98 | 69 | 57 | 15 | 6 | 27 | 272 (17%) |
Bleeding ovarian cyst | 12 | 39 | 15 | 3 | 1 | 1 | 71 |
Ectopic pregnancy | 11 | 27 | 14 | 5 |
| 8 | 65 |
Appendicitis | 24 | 2 | 18 |
|
|
| 44 |
Endometriosis | 16 |
| 2 |
| 3 |
| 21 |
Up to 60% of salpingitis is not diagnosed or treated (Figure 1).109 The aim should be to diagnose all cases of PID by increasing the sensitivity while sacrificing specificity. To increase the number of patients treated for PID, those with mild or atypical signs and symptoms need to be detected. Patients with mucopurulent cervicitis and mild pelvic pain or tenderness and patients with abnormal bleeding with menses, mild pelvic pain, and uterine tenderness (representing endometritis) need to be treated for PID. However, the already low 60% specificity of PID is even lower in this group with more mild clinical findings, but the advantage of this approach is that treatment of a larger number of patients for PID may lower the rate of sequelae (with treatment of a higher number of those without PID).
Although it is not practical to perform laparoscopy on every woman suspected to have salpingitis, laparoscopy should be used in certain situations. Laparoscopy remains the most accurate method to diagnose salpingitis and it is relatively safe. It should be used for women with severe abdominal rigidity to exclude appendicitis, intraperitoneal bleeding, or a ruptured abscess. Laparoscopy is cost effective for women with persistent pain after one or more courses of antibiotics for presumptive PID. Such women rarely have active infection and instead have adhesions from prior salpingitis, other diseases (most commonly endometriosis), or normal pelvic structures in those with chronic pelvic pain from undetermined sources. Laparoscopy is also useful among women with an unclear cause of mild abdominal pain who would otherwise not be treated with antibiotics because of a clinical diagnosis other than salpingitis.
History
Clinical symptoms do not accurately predict salpingitis observed at laparoscopy. Lower abdominal pain is the most frequent symptom among patients with acute salpingitis visualized at laparoscopy, although the pain may be mild or, as mentioned, even absent. The abdominal pain is usually continuous, bilateral, and most severe in the lower quadrants. It frequently increases with movement, a Valsalva maneuver, and intercourse. Intermittent sharp pain that lasts for a few seconds or minutes is unlikely to be caused by PID. Pain resulting from salpingitis had been present for less than 15 days in 85% of patients with visually confirmed salpingitis.127 Chronic pain, particularly intermittent pain of more than 3 weeks' duration, also is unlikely to be caused by PID. Women with chronic pain should usually undergo laparoscopy rather than antibiotic treatment for PID.
The presence or absence of other symptoms is of limited use to establish the diagnosis of PID. Women with visually confirmed salpingitis had the same rate of increased vaginal discharge, irregular vaginal bleeding, urinary symptoms, and vomiting as women without salpingitis.125 A history of fever or chills may be helpful to distinguish patients with salpingitis from patients without infection, but most women do not have these symptoms. Salpingitis is particularly common among women with a recent episode of PID and among sexual contacts of men with gonococcal or chlamydial urethritis.
Physical Examination
Patients with salpingitis commonly have tenderness of the lower abdomen, cervix, uterus, and both adnexa. Cervical motion tenderness is particularly common in PID. Unilateral salpingitis occurs in fewer than 10% of women with PID; most women with unilateral adnexal tenderness on physical examination were found to have bilateral salpingitis on laparoscopy.125 Other diagnoses should be considered when unilateral adnexal tenderness is present. Few physical findings are specific for salpingitis, and patients with other disease or no disease have physical findings similar to those of patients with PID. Grossly abnormal cervical discharge is found is less than one-half of these patients, although cervical and vaginal leukocytosis is common.128 A temperature over 100.4°F (38°C) is detected in only about one-third of patients with visually confirmed salpingitis.125
Despite our almost universal belief that women with the greatest abdominal and adnexal tenderness have the greatest amount of tubal damage, clinical signs do not predict the severity of tubal disease observed at laparoscopy.129 However, women with mild clinical manifestations require the same treatment as women with more severe findings.
Laboratory Tests
While history and physical examination signs have a low sensitivity with moderate specificity, several laboratory tests are both sensitive and specific (Kahn). The hematocrit and white blood cell count rate are of benefit when abnormal. However, only 45% of patients with laparoscopically confirmed salpingitis had a white blood cell count of 7900 or greater, and only 75% had a sedimentation rate greater than 15 mm/hour.125 An elevated C-reactive protein value may be more predictive of PID than these traditional tests.130
A Gram stain of cervical secretions is not specific but most women with PID have an increased number of polymorphonuclear leukocytes (PMNs) in the Gram-stained cervical discharge or cervicitis.The findings of ten or more PMNs establishes a diagnosis of cervicitis.25 To exclude a possible ectopic pregnancy, serum pregnancy tests should be performed for any woman who may be pregnant.
Endometrial Biopsy and Culdocentesis
Endometrial biopsy has been used to study the pathogenesis of PID.37, 38 However, the presence of plasma cells within endometrium,37 indicative of endometritis, may also be used to diagnose PID. Endometritis has a sensitivity of 70–90% and specificity of 70–90% for laparoscopically diagnosed PID 37, 38, 131, 132. Finding plasma cells in the endometrium can help to increase the number of patients identified with PID. Aspiration of purulent culdocentesis fluid can be useful to establish the diagnosis of salpingitis in those with signs of peritonitis. Culdocentesis is of benefit when the abdominal fluid contains white blood cells or nonclotting blood. Appendicitis or other causes of peritonitis that could also be associated with a purulent exudate should be excluded. Abdominal exploration is necessary when nonclotting blood is present to diagnose ectopic pregnancy or a bleeding ruptured ovarian cyst. Culdocentesis is of no benefit for diagnosis when no fluid or fluid devoid of while blood cells is obtained.
Examination of Male Sexual Partners
An opportunity to help establish a diagnosis of gonorrhea or chlamydia is provided by an examination of male sexual partners of patients with suspected PID. Even male partners without an overt discharge should have urethral material obtained. The sample should be tested for N. gonorrhoeae and C. trachomatis. Finding gonococci within the urethral by Gram stain is strong evidence that a symptomatic female sexual contact has salpingitis. Chlamydial PID should be suspected when the male sexual contact has a urethral discharge or urethral white blood cells in the absence of gonococci.
Ultrasonography
Ultrasound is a specific noninvasive test to help diagnose PID and follow the course of severe PID.133 An adnexal mass that represents an abscess appears as a cystic mass containing variable amounts of debris. An abscess can often be differentiated from an inflammatory pelvic mass, which consists of swollen tubes, ovary, and bowel. However, the ultrasonographic appearance of an inflammatory mass is extremely variable, and abscesses may be cystic or solid or have a fluid level. Ultrasonography should be performed to determine whether a large adnexal mass is an inflammatory mass or an abscess. Ultrasonography has been used to guide needles to drain abscesses when they are close to the vaginal or abdominal surface.134 However, ultrasound is less useful to diagnose PID when a mass is not present among women with minimal distortion of the tube from infection. Ultrasound correctly diagnosed PID confirmed by laparoscopy in 94% of patients with severe PID but in only 80% with moderate and 64% with mild PID.133
Gonorrhea and Chlamydia Testing
Both N. gonorrhoeae and C. trachomatis should also be sought by DNA testing. However, with rare exception, the recovery of other bacteria from the cervix is not useful to diagnose PID.
During culdocentesis laparoscopy or laparotomy, purulent material can be obtained from the pelvic cavity. Culture material also can be obtained by cannulating the tube with a small catheter, bronchoscopy brush, or a calcium alginate swab. If an abscess is present, pus can be aspirated with a syringe and needle. If the abscess is removed, a section of the abscess wall should be sent for culture. N. gonorrhoeae, anaerobic bacteria, and C. trachomatis are more likely to be recovered from infected tissue or epithelial cells brushed from the tubes than from pus.
TREATMENT
Adequate treatment of salpingitis includes an assessment of the severity of the infection, administration of antibiotics to eradicate a wide variety of possible organisms, use of other health measures, close patient follow-up, and treatment of male sexual partners. Treatment within 3 days of the onset of symptoms reduces infertility.17
Because of costs, most patients with salpingitis are not hospitalized in the United States. Mild-to-moderate PID responds as readily to outpatient as to inpatient regimens.104, 132 However, noncompliant patients may need hospitalization because of a high failure rate among noncompliant women who receive oral antibiotics on an outpatient basis.135 Patients with the following features should be hospitalized: severe peritonitis (severe nausea or a temperature 38°C or higher), suspected pelvic abscess, pregnancy, failure to respond to outpatient antibiotics, an unclear diagnosis of salpingitis, and perhaps concominant HIV infection.
Antibiotic therapy should ideally be directed toward the microbes recovered from the fallopian tube. However, fallopian tube cultures are not easily obtained and in most cases antibiotics are administered empirically. There has understandably been much interest in the appropriate antibiotic treatment of women with PID and probably an unrealistic hope that antibiotic therapy can markedly reduce sequelae. This hope was based on the belief that the newer broad-spectrum regimens that are effective against chlamydiae, anaerobes, and gonorrhea would improve the outcome. However, data from Sweden indicate identical infertility rates among women who received antibiotics with widely different antimicrobial spectrums.16 The data suggest that most permanent tubal damage has occurred before antibiotic therapy is begun. This possibility is reinforced by the finding that the most predictive parameter of subsequent infertility was the degree of tubal damage observed laparoscopically before therapy.4, 16 Thus, a cornerstone of treatment should be to prevent salpingitis by the diagnosis and treatment of cervicitis and endometritis.
Women with mild manifestations are just as likely as women with severe clinical manifestations to have severe tubal damage;20 thus, all women with suspected PID should receive a recommended regimen.136 Antibiotic regimens recommended by the Centers for Disease Control are designed to be effective against N. gonorrhoeae, C. trachomatis, and the most commonly isolated aerobic and anaerobic bacteria.87 Each regimen has its strong and weak points (Table 8). Alternative parenteral regimens include ofloxacin plus metronidazole, ampicillin/sulbactam plus doxycycline or ciprofloxacin, doxycycline, and metronidazole. Three reviews were published about the treatment of PID.137, 138, 139 The clinical response to the administration of each of the various antibiotic regimens was usually favorable, and no particular regimen has been shown to provide a superior clinical response over another. However, the combination of doxycycline and metronidazole consistently performs poorly.137, 139 Subsequent fertility would be expected to provide a more sensitive index of therapeutic success than the initial clinical response, but subsequent fertility among women receiving several different antibiotics either was similar16, 104 or not reported.140
Table 8. Guidelines for the treatment of acute pelvic inflammatory disease
Treatment | Dose | Duration (days) |
Hospitalized patients | ||
Regimen A | ||
Hospital regimen | ||
Doxycycline plus | 100 mg IV q 12 hr |
|
Cefoxitin or | 2 g IV q 6 hr |
|
Cefotetan | 2g IV q 12 hr |
|
Discharge regimen doxycycline | 100 mg PO bid | Total 14 days |
Regimen B | 900 mg IV q 8 hr |
|
Hospital regimen | 2 mg/kg loading, then 1.5 mg/kg | |
Clindamycin plus gentamicin | IV q 8 hr | |
Discharge regimen (choice of one) | ||
Clindamycin or | 450 mg PO qid | Total 14 days |
Doxycycline | 100 mg PO bid | Total 14 days |
Nonhospitalized patients | ||
Regimen A | ||
Ofloxacin | 400 mg PO bid | Total 14 days |
Metronidazole | 500 mg PO bid | Total 14 days |
Regimen B | ||
Loading dose (choice of one) | ||
Cefoxitin or | 2.0 g IM | |
Ceftriaxone or equivalent | 250 mg IM | |
Followed with doxycycline | 100 mg PO q 12 hr | 14 days |
Compiled from 2006 Guidelines for treatment of sexually transmitted disease. MMWR Morb Mortal Wkly Rep, 55:1, 2006.
Each antibiotic regimen should be selected for the situation in which it is most potentially useful. For example, the cefoxitin or cefotetan-doxycycline combination is an effective overall regimen that can inhibit N. gonorrhoeae (including penicillinase-producing strains), C. trachomatis, most aerobic, and more than 90% of (but not all) anaerobic bacteria. Clindamycin and an aminoglycoside can inhibit virtually all anaerobes and most aerobic bacteria, but clindamycin is less active than tetracycline or against Chlamydia and high intravenous clindamycin doses (2400 mg daily) are required to inhibit Chlamydia. This regimen, however, is well tested for the treatment of abscesses. Cephalosporins should not be given as a single agent, because although patients usually respond clinically, C. trachomatis persists despite therapy,141 and persistent Chlamydia may increase tubal damage. Thus, an anti-chlamydia agent must be added to the Lep ?
Women with an IUD should have it removed, usually after some interval of antibiotic therapy. All patients should be closely followed and reexamined at one and three weeks after beginning therapy to document a satisfactory clinical response. Patients with a slow response should be examined more frequently. All recent male sexual contacts of women with acute salpingitis, including those who are asymptomatic, should be examined, sampled, and treated, if necessary.
Abscess has decreased with the use of antibiotics that inhibit anaerobic bacteria, particularly Bacteroides fragilis, during the acute PID phase. However, immediate surgery is necessary to prevent septic shock in patients with a ruptured pelvic abscess. Early operative transvaginal drainage is the preferred method to treat a fluctuant pelvic abscess that becomes attached to the vagina in the midline of the cul-de-sac. More difficulty arises in the treatment of an inflammatory mass that is not attached to the vagina but requires transperitoneal drainage. Because even large masses do not always represent abscess formation, the current recommendation is that antibiotic therapy aimed particularly at anaerobes should be started and continued if the mass size declines. Surgery can then be delayed unless a definite abscess is identified by the clinical course (i.e. the patient fails to improve clinically or the mass remains unchanged in size). However, an abscess greater than 6 cm in diameter demonstrated by scans is likely to resolve slowly, recurs within 3 months, or not respond to antibiotics alone. Thus, large abscesses are best treated with both antibiotics and drainage. With this approach, few patients require transabdominal drainage.
Open laparoscopy can be used to guide percutaneous placement of catheters into abscesses.142 The catheter is used to drain pus and irrigate the abscess cavity. The catheter is left to drain for 24 to 48 hours or until drainage decreases and the patient's condition improves. Care must be taken to avoid bowel injury and to identify multiloculated abscesses. About 90% of large abscesses are cured with percutaneous drainage,134 which has the advantage of obviating extensive surgery. If abdominal exploration is necessary, the least extensive amount of surgery likely to be effective should be performed, particularly among women who wish to remain fertile. For women with a unilateral adnexal abscess or an abscess within loops of bowel, total abdominal hysterectomy and bilateral salpingooophorectomy represent surgical overkill. Simple drainage or even removal of a unilateral abscess without removal of the opposite tube and ovary and the uterus is usually successful for a patient on an adequate antibiotic regimen, which includes antibiotics that inhibits B. fragilis. This conservative approach is mandatory for women who still desire children to allow for possible future in-vitro fertilization.
Despite prompt diagnosis and treatment, sequelae are common. About 20% of patients with salpingitis suffer persistent abdominal pain without evidence of an abscess or adnexal pathology except for mild tenderness. If the pain continues for several weeks, laparoscopy should be performed to exclude other pelvic pathology or pelvic adhesions, which can then be lysed. In many instances of chronic pain, there is no discernible pelvic pathology. Laparoscopy should be performed before repeated antibiotic courses are given to patients with persistent pelvic pain and no objective evidence of salpingitis.
REFERENCES
Washington AE, Cates W, Zardi AA. Hospitalization for pelvic inflammatory disease: epidemiology and trends in the United States 1975 to 1981. JAMA 251:2529, 1984 |
|
Weström L. Decrease in the incidence of women treated in hospital for acute salpingitis in Sweden. Genitourin Med 64: 59, 1988 |
|
Expert Committee on Pelvic Inflammatory Disease. Pelvic inflammatory disease: research directions in the 1990s. Sex Transm Dis 18: 46, 1991 |
|
Adler MQ. Trends for gonorrhea and pelvic inflammatory disease in England and Wales and for gonorrhea in a defined population. Am J Obstet Gynecol 138: 901, 1980 |
|
Westrom L. Incidence, prevalence, and trends of acute pelvic inflammatory disease and its consequences in industrialized countries. Am J Obstet Gynecol 138: 880, 1980 |
|
Scholes D, et al. Prevention of pelvic inflammatory disease by screening for cervical chlamydial infection. N Engl J Med 334: 1399, 1996 |
|
Østergaard L, et al. Home sampling versus conventional swab sampling for screening of Chlamydia trachomatis in women: A cluster-randomized 1-year follow-up study. Clin Infect Dis 31: 951-957, 2000 |
|
Hoover K, et al. Low rates of both asymptomatic chlamydia screening and diagnostic testing of women in U.S. outpatient clinics. Obstet Gynecol 112: 891-898, 2008 |
|
Regan DG, Wilson DP, Hocking JS. Coverage is the key for effective screening of Chlamydia trachomatis in Australia. J Infect Dis 198: 349-358, 2008 |
|
Hiltunen-Back E, Haikala O, Kautiainen H, et al. Sex Transm Dis 30: 737-41, 2003 |
|
Falk V. Treatment of acute non-tuberculous salpingitis with antibiotics alone and in combination with glucocorticoids. A prospective double blind controlled study of the clinical course and prognosis. Acta Obstet Gynecol Scand 44 (Suppl 6), 3-118, 1965 |
|
Hull MG, et al. Population study of causes, treatment, and outcome of infertility. Br Med J 291: 1693, 1985 |
|
Cates W, et al. Sexually transmitted diseases, pelvic inflammatory disease, and infertility: an epidemiologic update. Epidemiol Rev 12: 199, 1990 |
|
Khatamee MA. Infertility: a preventable epidemic? Int J Fertil 34: 246, 1988 |
|
Weström L. Effect of acute pelvic inflammatory disease on fertility. Am J Obstet Gynecol 121: 707, 1975 |
|
Weström L, Losif S, Svensson L, et al. Infertility after acute salpingitis: results of treatment with different antibiotics. Curr Ther Res 26: 752, 1979 |
|
Hillis SD et al. Delayed care of pelvic inflammatory disease as a risk factor for impaired fertility. Am J Obstet Gynecol 168: 1503, 1993 |
|
Centers for Disease Control. Ectopic pregnancy—United States, 1990-1992. MMWR Morb Mortal Wkly Rep 44: 46, 1995 |
|
Westrom L, Bengtsson LPH, Mardh P-A. Incidence, trends and risks of ectopic pregnancy in a population of women. BMJ 282: 15, 1981 |
|
Joesoef R et al. Recurrence of ectopic pregnancy: the role of salpingitis. Am J Obstet Gynecol 165: 46, 1991 |
|
Weström L, Svensson L. Chronic pain after acute pelvic inflammatory disease. In Belfort P et al. (eds): Advances in Gynecology and Obstetrics. Proceedings of the XIIth World Congress of Gynecology and Obstetrics, Rio de Janeiro 6: 265, 1988 |
|
Adler MW, Belsey EH, O'Connor BH. Morbidity associated with pelvic inflammatory disease. Br J Vener Dis 58: 151, 1982 |
|
Hillier SL, Krohn MA, Rabe LK et al. The normal vaginal flora, H2O2-producing lactobacilli and bacterial vaginosis in pregnant women. Clin Infect Dis 16(Suppl 4): S273, 1993 |
|
Gorbach SC, Menda KB, Thadepalli H, et al. Anaerobic microflora of the cervix in healthy women. Am J Obstet Gynecol 117: 1053, 1973 |
|
Brunham RC, Paavonen J, Stevens CE et al. Mucopurulent cervicitis: the ignored counterpart in women of urethritis in men. N Engl J Med 311: 1, 1984 |
|
Manhart LE, Critchlow CW, Holmes KK, et al. Mucopurulent cervicitis and Mycoplasma genitalium. J Infect Dis 187:650, 2003 |
|
Falk V, Krook G. Do results of culture for gonococci vary with sampling phase of menstrual cycle? Acta Derm Venereol (Stockh) 47: 190, 1967 |
|
Draper DL, James JF, Brooks GF et al. Comparison of virulence markers of peritoneal and fallopian tube isolates with endocervical N. gonorrhoeae isolates from women with acute salpingitis. Infect Immun 27: 882, 1980 |
|
Zucherman H, Kahane A, Carmel S. Antibacterial activity of human cervical mucus. Gynecol Invest 6: 265, 1975 |
|
James AN, Knox JM, Williams PR. Attachment of gonococci to sperm: influence of physical and chemical factors. Br J Vener Dis 52: 128, 1976 |
|
Wolner-Hanssen P, Mardh P-A: In vitro tests of adherence of Chlamydia trachomatis to human spermatozoa. Fertil Steril 42: 102, 1984 |
|
Buchanan TM, Eschenbach DA, Knapp JS et al. Gonococcal salpingitis is less likely to recur with Neisseria gonorrhoeae of the same principal outer membrane protein antigenic type. Am J Obstet Gynecol 138: 978, 1980 |
|
Tatum HJ, Schmidt FA, Phillips D, et al. The Dalkon Shield controversy: structural and bacteriological studies of IUD tails. JAMA 231: 711, 1975 |
|
Banks HL, Williamson HO. Scanning electron microscopy of Dalkon Shield tails. Fertil Steril 40: 334, 1983 |
|
Moyer DL, Mishell DR. Reaction of human endometrium to the uterine foreign body. Am J Obstet Gynecol 111: 66, 1971 |
|
Smith MR, Soderstrom R. Salpingitis: a frequent response to intrauterine contraception. J Reprod Med 16: 159, 1976 |
|
Kiviat NB, Wolner-Hanssen P, Eschenbach DA, et al.: Endometrial histopathology in patients with culture-proved upper genital tract infections and laparoscopically diagnosed acute salpingitis. Am J Surg Pathol 14: 167, 1990 |
|
Paavonen J, Kiviat N, Brunham RC, et al: Prevalence and manifestation of endometritis among women with cervicitis. Am J Obstet Gynecol 152: 280, 1985 |
|
McGee ZA, Melly MA, Gregg CR, et al. Virulence factors of gonococci: Studies using human fallopian tube organ culture. In Brooks GF, Gotschlich EL, Holmes KK et al. (eds): Immunobiology of N. gonorrhoeae, p 258. Washington, DC: American Society for Microbiology, 1978 |
|
Cooper MD, et al. Chlamydia trachomatis infection of human fallopian tube organ cultures. J Gen Microbiol 136: 1109, 1990 |
|
Van Voorhis WC, Barrett LK, Cosgrove Sweeney C, et al.: Analysis of lymphocyte phenotype and cytokine activity in the inflammatory infiltrates of the upper genital tract of female macaques infected with Chlamydia trachomatis. J Infect Dis 174: 647, 1996 |
|
Patton DL, et al. Detection of Chlamydia trachomatis in fallopian tube tissue in women with postinfectious tubal infertility. Am J Obstet Gynecol 171: 95, 1994 |
|
Patton DL. Immunopathology and histopathology of experimental chlamydial salpingitis. Rev Infect Dis 7: 746, 1982 |
|
Patton DL, et al. The effects of Chlamydia trachomatis on the female reproductive tract of Macaca nemestrina after a single challenge following repeated cervical inoculations. Obstet Gynecol 76: 1271, 1990 |
|
Kimani J, et al. Risk factors for Chlamydia trachomatis pelvic inflammatory disease among sex workers in Nairobi, Kenya. J Infect Dis 173: 1437, 1996 |
|
Ness RB, Brunham RC, Shen C, Bass DC; PID Evaluation Clinical Health (PEACH) study investigators. Associations among human leukocyte antigen (HLA) class II DQ variants, bacterial sexually transmitted diseases, endometritis, and fertility among women with clinical pelvic inflammatory disease. Sex Transm Dis. 31: 301-4, 2004 |
|
Taylor HR, et al Chlamydial heat shock proteins and trachoma. Infect Immun 58: 3061, 1990 |
|
Eckert LO, et al. Prevalence and correlates of antibody to chlamydial heat shock protein in women STD clinic attendees and women with confirmed PID. J Infect Dis 175: 1453, 1997 |
|
Toye B, et al. Association between antibody to the chlamydial heat shock protein and tubal infertility. J Infect Dis 168: 1236, 1993 |
|
Tiitinen A, Surcel HM, Halttunen M, et al. Chlamydia trachomatis and chlamydial heat shock protein 60-specific antibody and cell-mediated responses predict tubal factor infertility. Hum Reprod 21: 1533-8, 2006 |
|
Brunham RC, et al. Chlamydia trachomatis-associated ectopic pregnancy: serologic and histologic correlates. J Infect Dis 165: 1076, 1992 |
|
Grayston JT, et al. Trachoma vaccine studies in volunteer students of the National Defense Medical Care Center II. Response to challenge eye inoculation of egg grown trachoma virus. Clin Med J 8: 312, 1961 |
|
Brunham RC, Pourbohloul B, Mak S, et al. The unexpected impact of a Chlamydia trachomatis infection control program on susceptibility to reinfection. J Infect Dis 192: 1836-1844, 2005 |
|
Cox SM, et al. Role of Neisseria gonorrhoeae and Chlamydia trachomatis in intraabdominal abscess formation in the rat. J Reprod Med 36: 202, 1991 |
|
Bieluch VM, Tally FP. Pathophysiology of abscess formation. Clin Obstet Gynecol 10:93, 1983 |
|
Moller BR, Freundt EA, Block FT, et al. Experimental infection of the genital tract of female grivet monkeys by Mycoplasma hominis. Infect Immun 20: 258, 1979 |
|
Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med 353; 1899, 2005 |
|
Hebb JK, Cohen CR, Astete SG, et al. Detection of novel organisms associated with salpingitis, by use of 16S rDNA polymerase chain reaction. J Infect Dis 190: 2109-20, 2004 |
|
Lukasik J. A comparative evaluation of the bacteriological flora of the uterine cervix and fallopian tubes in cases of salpingitis. Am J Obstet Gynecol 87: 1028, 1963 |
|
Henry-Suchet J, Catalan F, Leffredo V, et al. Microbiology of specimens obtained by laparoscopy from controls and from patients with pelvic inflammatory disease or infertility with tubal obstruction: Chlamydia trachomatis and Ureaplasma urealyticum. Am J Obstet Gynecol 138: 1022, 1980 |
|
Eschenbach D, et al. Epidemiology of acute pelvic inflammatory disease. Abstract C03-088, International Society for STD Research, Banff, Canada, October 6-9, 1991 |
|
Senanayake P, Kramer DG: Contraception and the etiology of pelvic inflammatory disease: new perspectives. Am J Obstet Gynecol 138: 852, 1980 |
|
Lee ND, Rubin GL, Ory HW, et al. Type of intrauterine device and the risk of pelvic inflammatory disease. Obstet Gynecol 62: 1, 1983 |
|
Sparks RA, Purrier BAG, Watt PJ, et al. Bacteriological colonization of uterine cavity: role of tailed intrauterine contraceptive device. Br J Med 282: 1189, 1981 |
|
Skangalis M, Mahoney CJ, O'Leary WM. Microbial presence in the uterine cavity as affected by varieties of intrauterine contraceptive devices. Fertil Steril 37: 263, 1982 |
|
Sparks H, et al. Bacteriological colonization of uterine cavity: Role of tailed intrauterine contraceptive device. Br Med J 282:1189, 1981 |
|
Daling JR, et al. The intrauterine device and primary tubal infertility. N Engl J Med 326: 203, 1992 |
|
Mishell DR, Moyer DE. Association of pelvic inflammatory disease with the intrauterine device. Clin Obstet Gynecol 12: 179, 1969 |
|
Hagar WD, Douglas B, Majmudar B, et al. Pelvic colonization with Actinomyces in women using intrauterine contraceptive devices. Am J Obstet Gynecol 135: 680, 1979 |
|
Daling JR, et al. Vaginal douching and the risk of tubal pregnancy. Epidemiology 2: 40, 1991 |
|
Wolner-Hanssen P, Eschenbach DA, Paavonen J, et al. Association between douching and acute pelvic inflammatory disease. JAMA 263: 1936, 1990 |
|
Ness RB, Hillier SL, Kip KE, et al. Douching, pelvic inflammatory disease, and incident gonococcal and chlamydial genital infection in a cohort of high-risk women. Am J Epidemiol, 161:186-95, 2005 |
|
Wolner-Hanssen P, Eschenbach DA, Paavonen J, et al. Decreased risk of symptomatic chlamydial pelvic inflammatory disease associated with oral contraceptive use. JAMA 263: 54, 1990 |
|
Wolner-Hanssen P, et al. Laparoscopic findings and contraceptive use in women with signs and symptoms suggestive of acute salpingitis. Obstet Gynecol 66: 233, 1985 |
|
Falk V. Treatment of acute non-tuberculous salpingitis with antibiotics alone and in combination with glucocorticoids. A prospective double blind controlled study of the clinical course and prognosis. Acta Obstet Gynecol Scand 44 (Suppl 6), 3-118, 1965 |
|
Eschenbach DA, Buchanan TM, Pollock HM, et al. Polymicrobial etiology of acute pelvic inflammatory disease. N Engl J Med 293: 166, 1975 |
|
Weström L, Mardh PA. Chlamydial salpingitis. Br Med Bull 39:145, 1983 |
|
Tietze C, Lewit S. Evaluation of intrauterine devices: ninth progress report of the cooperative statistical program. Stud Fam Plan 55: 1, 1970 |
|
Giertz G, Kallings I, Nordenvall M, et al. A prospective study of Chlamydia trachomatis infection following legal abortion. Acta Obstet Gynecol Scand 66: 107, 1987 |
|
Larsson PG, Bergman B, Forsum U, et al. Mobiluncus and clue cells as predators of PID after first trimester abortion. Acta Obstet Gynecol Scand 68: 217-20, 1989 |
|
Larsson PG, Platz-Christensen JJ, Thejla H, et al. Incidence of pelvic inflammatory disease after first-trimester legal abortion in women with bacterial vaginosis after treatment with metronidazole: a double-blind, randomized study. Am J Obstet Gynecol 166: 100-3, 1992 |
|
Safrin S, et al. Seroprevalence and epidemiologic correlates of human immunodeficiency virus infections in women with acute pelvic inflammatory disease. Obstet Gynecol 75: 666, 1990 |
|
Sperling RS, et al. Seroprevalence of human immunodeficiency virus in women admitted to the hospital with pelvic inflammatory disease. J Reprod Med 36: 122, 1991 |
|
Cohen C, Sinei S, Reilly M, et al. Effect of human immunodeficiency virus type I infection upon acute salpingitis: a laparoscopic study. J Infect Dis 178: 1352, 1998 |
|
Irwin KI. Potential for bias in studies of the influence of human immunodeficiency virus infection on the recognition, incidence, clinical course, and microbiology of pelvic inflammatory disease. Obstet Gynecol 84: 463, 1994 |
|
Korn AP, et al. Pelvic inflammatory disease in human immunodeficiency virus infected women. Obstet Gynecol 82: 765, 1993 |
|
2006 Guidelines for treatment of sexually transmitted diseases. MMWR Morb Mortal Wkly Rep 55:1, 2006 |
|
Thompson SE, Hagar WD, Wong K-H. The microbiology and therapy of acute pelvic inflammatory disease in hospitalized patients. Am J Obstet Gynecol 136: 179, 1980 |
|
Sweet RL, Draper DL, Hadley WK. Etiology of acute salpingitis: influence of episode number and duration of symptoms. Obstet Gynecol 58: 62, 1981 |
|
Moller BR, Mardh P-A, Akrons S, et al. Infection with Chlamydia trachomatis, Mycoplasma hominis, and Neisseria gonorrhoeae in patients with acute pelvic inflammatory disease. Am Vener Dis Assoc 8: 198, 1981 |
|
Mardh P-A, Ripa T, Svensson L et al: Role of Chlamydia trachomatis infection in acute salpingitis. N Engl J Med 296: 1377, 1977 |
|
Gjonnaess H, Dolaken K, Anestad G, et al. Pelvic inflammatory disease: etiologic studies with emphasis on chlamydial infection. Obstet Gynecol 59: 550, 1982 |
|
Mardh P-A, Lind I, Svensson L, et al. Antibodies to Chlamydia trachomatis, Mycoplasma hominis, and Neisseria gonorrhoeae in sera from patients with acute salpingitis. Br J Vener Dis 57: 125, 1981 |
|
Ripa KT, Svensson L, Treharne JD, et al. Chlamydia trachomatis infection in patients with laparoscopically verified acute salpingitis. Am J Obstet Gynecol 138: 960, 1980 |
|
Osser S, Persson K. Epidemiologic and serodiagnostic aspects of chlamydial salpingitis. Obstet Gynecol 59: 206, 1982 |
|
Paavonen J. Chlamydia trachomatis in acute salpingitis. Am J Obstet Gynecol 138: 957, 1980 |
|
Paavonen J, Saiku P, Vestermen E. Chlamydia trachomatis in acute salpingitis. Br J Vener Dis 55: 203, 1979 |
|
Paavonen J, Valtonen VV, Kasper DC. Serologic evidence for the role of Bacteroides fragilis and Enterobacteriaceae in the pathogenesis of acute pelvic inflammatory disease. Lancet 1: 293, 1981 |
|
Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the Pelvic Inflammatory Disease Evaluation and Clnical Health (PEACH) Randomized Trial. Am J Obstet Gynecol 186(5), 929-937, 2002 |
|
Eilard T, Brorsson J-E, Hamark B, et al. Isolation of chlamydia in acute salpingitis. Scand J Infect Dis 9 (Suppl): 82, 1976 |
|
Bowie WR, Jones H. Acute pelvic inflammatory disease in outpatients and associates with Chlamydia trachomatis and Neisseria gonorrhoeae. Ann Intern Med 95: 685, 1981 |
|
Cunningham FG, Hauth JC, Gilstrap LC: The bacterial pathogenesis of acute pelvic inflammatory disease. Obstet Gynecol 52: 161, 1978 |
|
Skaug K, Vik ISS, Wvigstad E, et al. Chlamydial serum IgG antibodies in patients with acute salpingitis measured by an enzyme-linked immunoabsorbent assay. Acta Pathol Microbiol Immunol Scand 90: 67, 1982 |
|
Ness RB, Trautmann G, Richter HE, et al. Effectiveness of treatment strategies of some women with pelvic inflammatory disease: a randomized trial. Obstet Gynecol. 106: 573-80, 2005 |
|
Pasternack P, Vuorinen P, Kuukankorpi A, et al. Determination of Chlamydia trachomatis infections in women by Amplicor PCR: comparisons of diagnostic performance with urine and cervical specimens. J Clin Microbiol 34: 995, 1996 |
|
Svensson L, Westrom L, Ripa KT, et al. Differences in some clinical and laboratory parameters in acute salpingitis related to culture and serologic finding. Am J Obstet Gynecol 138: 1017, 1980 |
|
Punnonen J, Terho P, Nikkanen V, et al. Chlamydial serology in infertile women by immunofluorescence. Fertil Steril 31: 656, 1979 |
|
Cevenini R, Possati G, LaPlacva M. Chlamydia trachomatis infection in infertile women. In Mardh P-A, Holmes KK, Oriel JD et al. (eds): Chlamydial Infection, pp 189–192. New York: Elsevier Biomed Press, 1982 |
|
Moore DE, Spadoni L, Foy H-M, et al. Increased frequency of serum antibodies to Chlamydia trachomatis in infertility due to tubal disease. Lancet 2: 514, 1982 |
|
Conway D, Glazener CMA, Caul EO, et al. Chlamydia serology in fertile and infertile women. Lancet 1: 191, 1984 |
|
Gump DW, Gibson M, Ashikaga T. Evidence of prior pelvic inflammatory disease and its relationship to C. trachomatis antibody and intrauterine contraceptive device use in infertile women. Am J Obstet Gynecol 146: 143, 1983 |
|
Jones RB, Ardery BR, Hui SC, et al. Correlation between serum antichlamydial antibodies and tubal factor as a cause of infertility. Fertil Steril 38: 553, 1982 |
|
Gibson M, Gump D, Ashikage T, et al. Patterns of adnexal inflammatory damage: Chlamydia, the intrauterine device, and history of pelvic inflammatory disease. Fertil Steril 41: 47, 1984 |
|
Henry-Suchet J, Catalan F, Loffredo V, et al. Chlamydia trachomatis associated with chronic inflammation in abdominal specimens from women selected for tuboplasty. Fertil Steril 36: 599, 1981 |
|
Kane JL, Woodland RN, Forsey T. Chlamydia trachomatis and infertility. Lancet 1: 736, 1984 |
|
Svensson L, Mardh P-A, Westrom L. Infertility after acute salpingitis with special reference to Chlamydia trachomatis. Fertil Steril 40: 322, 1983 |
|
Brunham RC, Binns B, McDowell J, et al. Chlamydia trachomatis infection in women with ectopic pregnancy. Obstet Gynecol 67: 722, 1986 |
|
Sweet RL, Mills J, Hadley KW, et al. Use of laparoscopy to determine the microbiologic etiology of acute salpingitis. Am J Obstet Gynecol 134: 781, 1979 |
|
Mardh P-A, Westrom L. Tubal and cervical cultures in acute salpingitis with special reference to Mycoplasma hominis and T-strain mycoplasmas. Br J Vener Dis 46: 179, 1970 |
|
Taylor-Robinson D. The history of Mycoplasma genitalium in sexually transmitted diseases. Genitourin Med 71: 1, 1995 |
|
Palmer HM, et al. Detection of Mycoplasma genitalium in the genitourinary tract of women by the polymerase chain reaction. Int J STD AIDS 2: 261, 1991 |
|
Moller BR, et al. Serological evidence implicating Mycoplasma genitalium in pelvic inflammatory disease. Lancet 1: 1102, 1984 |
|
Cohen CR, Manhart LE, Bukusi EA, et al. Association between Mycoplasma genitalium and acute endometritis. Lancet 359-765, 2002 |
|
Brunham RC, Binnes B, Guijon F, et al. Etiology and outcome of acute pelvic inflammatory disease. J Infect Dis 158: 510, 1988 |
|
Jacobson L, Westrom L. Objectivized diagnosis of acute pelvic inflammatory disease. Am J Obstet Gynecol 105: 1088, 1969 |
|
Money DM, et al. Antibodies to the chlamydial 60 kDa heat shock protein are associated with laparoscopically confirmed perihepatitis. Am J Obstet Gynecol 176: 870, 1997 |
|
Westrom L, Mardh P-A. Epidemiology, etiology and prognosis of acute salpingitis: a study of 1457 laparoscopically verified cases. In Hobson D, Holmes KK (eds): Nongonococcal Urethritis and Related Diseases. Washington, DC: American Society for Microbiology, 1977 |
|
Westrom L. Clinical manifestations and diagnosis of pelvic inflammatory disease. J Reprod Med 28: 703, 1983 |
|
Eschenbach DA, et al. Acute pelvic inflammatory disease: association of clinical and laboratory findings with laparoscopic findings. Obstet Gynecol 89: 184, 1997 |
|
Kahn JG, Walker CK, Washington AE, et al. Diagnosing pelvic inflammatory disease. A comprehensive analysis and considerations for a new model. JAMA 226:2594, 1991 |
|
Paavonen J, Aine R, Teisala K, et al. Comparison of endometrial biopsy and peritoneal blood cytology with laparoscopy in the diagnoses of acute pelvic inflammatory disease. Am J Obstet Gynecol 151: 645, 1985 |
|
Eckert LO, Thwin SS, Hillier SL, Kiviat NB, Eschenbach DA. The antimicrobial treatment of subacute endometritis: A proof of concept study. Am J Obstet Gynecol 190: 305-313, 2004 |
|
Spirtos NJ, et al. Sonography in acute pelvic inflammatory disease. J Reprod Med 27: 312, 1982 |
|
Nelson AL, et al. Endovaginal ultrasonographically guided transvaginal drainage for treatment of pelvic abscesses. Am J Obstet Gynecol 172: 1926, 1995 |
|
Thompson SE, Brooks C, Eschenbach DA, et al. High failure rates in outpatient treatment of salpingitis with either tetracycline alone or penicillin/ampicillin in combination. Am J Obstet Gynecol 152: 635, 1985 |
|
Eschenbach D. Treatment of pelvic inflammatory disease. Clin Infect Dis 44: 961-963, 2007 |
|
Walker CK , et al. Pelvic inflammatory disease: meta-analysis of antimicrobial regimen efficacy. J Infect Dis 168: 969, 1993 |
|
Dodson MG. Antibiotic regimens for treating acute pelvic inflammatory disease: an evaluation. J Reprod Med 39: 285, 1994 |
|
Haggerty, CL and Ness RB. Newest approaches to treatment of pelvic inflammatory disease: a review of recent randomized clinical trials. Clin Infect Dis 44: 953-960, 2007 |
|
Brunham RC. Therapy for acute pelvic inflammatory disease: a critique of recent treatment trials. Am J Obstet Gynecol 148: 235, 1984 |
|
Sweet RL, Schachter J, Robbie MO. Failure of 6-lactam antibiotics to eradicate Chlamydia trachomatis in the endometrium despite apparent clinical cure of acute salpingitis. JAMA 250: 2641, 1983 |
|
Henry-Suchet J, Soler A, Loffredo V. Laparoscopic treatment of tuboovarian abscesses. J Reprod Med 29: 579, 1984 |

