Age and Reproduction
Authors
INTRODUCTION
Human reproduction is profoundly influenced by age. The study of the effect of age on human reproduction is complicated by the numerous physiologic changes occurring in men and women as they age, coupled with powerful environmental and socioeconomic conditions exerting external influences on them. Through the advances of science and modern technology, the average life expectancy of men and women continually increases. Despite this, modern technology has been unable to extend the reproductive potential in women, and the age of onset of menopause remains remarkably constant. Although many factors contribute to the age-related decline in human fertility, the data suggest that maternal aging renders the most significant impact. More specifically, the ovary contains the pacemaker of a woman's 'biologic clock'. Although the focus of this chapter is on maternal aging, the effect of paternal aging on human reproduction is discussed briefly.
BACKGROUND
Part of the reason we are gaining new insights into the effect of age on human reproduction is the work of Steptoe and Edwards, who in 1978, oversaw the birth of the first in vitro fertilization (IVF) baby. Since then, significant strides have been made in the diagnosis and treatment of impaired fertility, and thousands of couples undergo treatment each year, thereby allowing investigators to study the intricate details of human reproduction.
Although it does not seem that the incidence of impaired fertility is increasing, the proportion of women of advancing maternal age who are childless is increasing.24 This increase results from social changes in many populations and reflects the fact that the “baby boomers” are coming of age. An increasing number of individuals born in the postwar baby boom are now 35 to 44 years of age.25 Women are delaying childbearing for a number of reasons, such as changing emphasis on career pursuits, economic forces keeping women in the work force longer, and increasing divorce rates resulting in an increased number of women entering new relationships later in life.26 Taken together, many women are now delaying childbearing until their late thirties and early forties. This has led to an accumulation of data regarding the treatment of women of advancing maternal age. However, assessing age effects on human reproduction is biased if only an infertile population is studied. Fortunately, in the past, various populations have been studied to assess age effects on natural reproduction. These populations include fertile populations not using contraception, fertile populations using contraception, and infertile populations. Before studying human reproduction within various populations, several terms should be clearly defined.
DEFINITIONS AND POPULATIONS
Infertility is defined as 1 year of unprotected intercourse without conception. A fertile couple has achieved a pregnancy resulting in a live birth. A fertility rate is defined by dividing the number of live births in a given time divided by the total population of women between the ages of 15 and 44. Fecundability refers to the ability of a woman to achieve a pregnancy within one menstrual cycle. Fecundity refers to the ability of a woman to achieve a live birth within one menstrual cycle. Since the advent of IVF, a clinical pregnancy has been defined as a pregnancy associated with rising serum β-human chorionic gonadotropin levels in conjunction with the presence of a gestational sac detected by ultrasonography. A clinical pregnancy rate is calculated by dividing the total number of clinical pregnancies divided by the total number of menstrual cycles. Natural populations refer to fertile populations who do not practice birth control.
When examining natural populations, the Hutterites deserve mention. A Protestant sect of Swiss immigrants living in the northern United States and Southern Canada, the Hutterites represent one of the most fertile populations on record. Even this highly fertile population demonstrated significant impairment of fertility with increasing age. Infertility occurred in 10% of women 35 or younger, 33% in women between the ages of 36 and 40, and 87.5% of women 41 to 45 years old.27 Not only did infertility rise with increasing maternal age, but the interval of time between conceptions rose with increasing maternal age. Hendershot has confirmed that older couples require an increased length of time to achieve a pregnancy.28
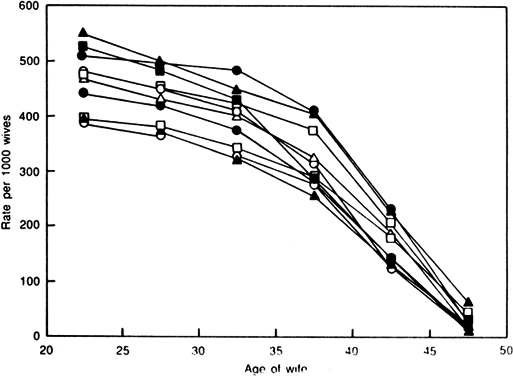
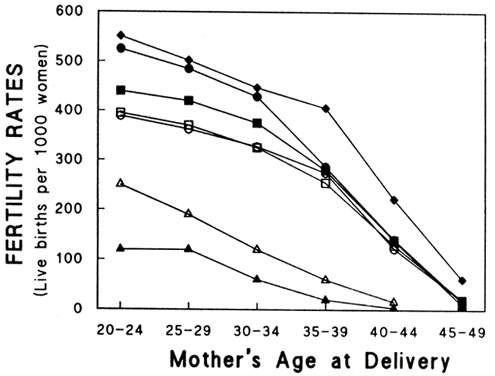
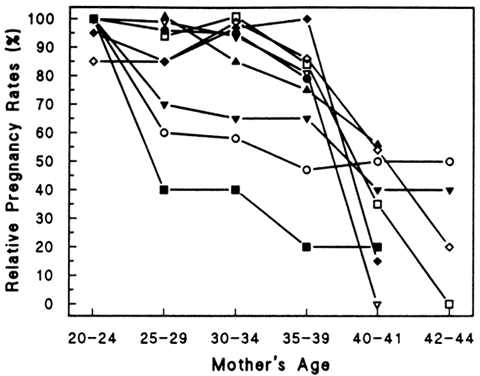
Many other natural populations have been studied as well.29 Menken and colleagues have examined several natural populations and graphically depicted their results.30 Figure 1 demonstrates an impressive effect of age on fertility in several populations in which family size was not limited by social norms. However, many social factors, such as the general decrease in coital frequency among aging couples, must be considered when studying natural populations.6,31,32 Their data demonstrate a relatively slow decline in fertility rates among women over age 25, which accelerates in the mid-thirties and plummets by the early forties. In an attempt to control for some of the social influences that likely interfere with the study of age and declining fertility, Menken examined several population where birth control was not practiced and late marriages were common among nulliparous women. Figure 2 shows profound age-related changes in fertility. Maroulis also examined age-related changes in natural populations but included United States populations who practiced birth control.33
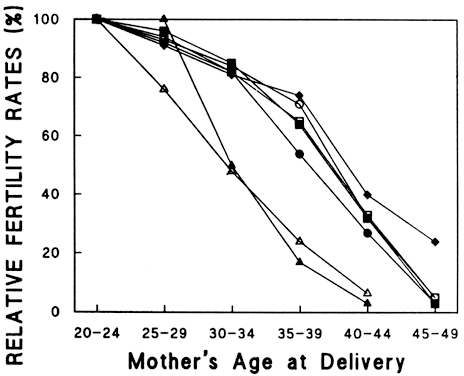
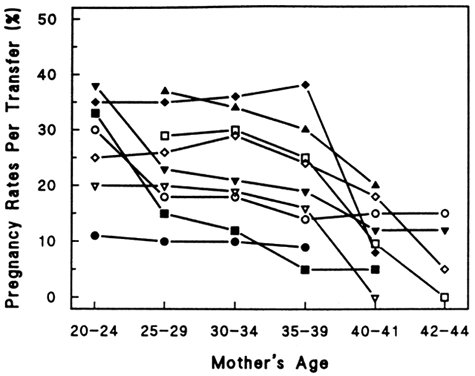
Figure 3 demonstrates a decrease in fertility rates among all groups with increasing age. Figure 4 demonstrates a steeper relative rate of decline in fertility rates among U.S. populations using contraception compared with natural populations. This interesting finding may result from significant use of contraception and avoidance of pregnancy in aging couples, as well as other social and economic influences of modern industrialized nations. In a study evaluating spontaneous deliveries in the United States, Hansen found that less than 0.7% of live births occur in women older than 40 years of age.34 Maroulis went on to evaluate age-related decreases in fertility rates in women undergoing assisted reproductive technologies (ART) at different ART centers. He demonstrated similar age-related decreases (Figs. 5 and 6). Hopefully, a general model to explain the age-related decline in human fertility will emerge as we explore the physiologic processes involved with aging.
PHYSIOLOGIC ASPECTS OF AGING
Aging affects all organs of the human body, but each organ is affected to different degrees. Some organ systems, although adversely affected by age, may be functionally restored through medical intervention. Multiple theories explaining the mechanisms of aging have been described over the years. No single theory provides an inclusive explanation. Although a thorough discussion of each of the theories is beyond the scope of this chapter, some include age-related changes in chromosome exchange and gene expression, programmed cell death and apoptosis, reactive oxygen species and mitochondrial DNA damage, and telomere shortening. These theories and their application to general physiologic aging and ovarian aging continue to receive considerable attention within the research community. In the near future, one or more of these theories may enhance our understanding of female reproductive aging.
In the following sections, we consider the role of genetics in female reproductive aging. We then examine various general pathologic conditions and environmental exposures that occur with increasing frequency as women age that may influence reproductive aging. We evaluate the age effect of the organs that are directly related to the reproductive system, such as the adrenal glands, hypothalamus, pituitary gland, fallopian tubes, uterus, and ovaries.
Genetics
So complex are the determinants of female fecundity that even the intrauterine environment in which a female fetus develops may profoundly affect her reproductive potential and the timing of her onset of menopause.35,36 In a comprehensive update on premature ovarian failure (POF), Anasti summarized the relation of genetic disorders and future fecundity.37 He emphasized that many of the causes of POF are related to chromosomal abnormalities that may exert their effect in utero on germ cell migration, oocyte proliferation, oocyte depletion, and other complex mechanisms. For instance, the POF associated with Turner's syndrome is caused by accelerated follicular atresia during late fetal development. Other chromosomal abnormalities may lead to follicle dysfunction, enzyme deficiencies, or signal defects. Numerous other inherited genetic abnormalities have long been known to cause infertility and POF. Moreover, investigators have shown a correlation between the age of onset of menopause and more subtle genetic factors through family studies. For instance, Torgerson and colleagues showed a correlation between mothers and daughters and the age of onset of menopause.38 Likewise, Cramer and coworkers demonstrated an association between sisters with POF and the age of onset at menopause.39 The cumulative data rather convincingly suggest that a woman's endowed follicular pool, which provides the foundation for her reproductive potential, is established by events occurring at the time of fetal formation. From then on, her reproductive potential is modified continuously throughout her life.
Pathologic Conditions
As women age, they experience an increase in exposure to an assortment of pathologic processes. For example, the number of women who develop uterine fibroids increases with age. As many as 20% to 50% of women may have uterine fibroids, and the incidence increases with age.40 Uterine fibroids have been implicated in infertility.41 As increasing numbers of women delay childbearing until later years, fibroids are becoming an increasingly important problem. Fertility increases after surgical removal of uterine fibroids in natural populations.42,43,44 Women who have submucosal or intramural fibroids demonstrate a significant reduction in implantation rate and pregnancy rate when undergoing ART.45 By working through the logic of such arguments, it becomes clear that these pathologic processes are likely to adversely influence a woman's ability to conceive. Similarly, endometriosis represents another pathologic process related to aging and impaired fertility.
Endometriosis is a common gynecologic condition that is found in 3% to 10% of the general population in the reproductive-age group and 25% to 35% of an infertile population.46,47 The relation between endometriosis and infertility is well supported by the literature.48 The incidence of endometriosis increases with increasing age.49,50 Physicians can expect to see an increase in endometriosis-associated infertility as women delay childbearing. Westrom identified another interesting finding associated with impaired fertility and aging through his work on pelvic inflammatory disease.51 He found that the incidence of postinfection infertility after the first exposure was significantly higher in women 25 to 34 years of age compared with women between the ages of 15 and 24. This age difference was eliminated, however, when women had multiple episodes of infection. Still another interesting age-related problem is demonstrated through the increased incidence of ectopic gestation found in women of advancing maternal age. There is a threefold increase in ectopic pregnancies in women between the ages of 35 and 44 compared with women aged between 15 and 24 years old.52,53 Over time, cumulative exposure to these pathologic conditions and more likely contribute to the age-related decline in female reproductive capacity.
In addition to specific pathologic processes directly related to the reproductive tract that lead to impaired fertility, a host of medical conditions caused by the effects of aging on other endocrine organ systems may indirectly lead to impaired fertility. An example is provided by diabetes. The incidence of type II diabetes increases with advancing age.54,55 We know that even impaired glucose tolerance, a condition often present before the diagnosis of overt diabetes is made, has a negative impact on ovarian function and ovulation.56,57 Another example is thyroid disease. The incidence of thyroid disease in women increases significantly with age.58,59 And thyroid disease is known to be associated with oligomenorrhea, amenorrhea, menorrhagia, and anovulation, leading to impaired fertility.60,61 A theme is established that demonstrates the negative influence of aging on reproduction. This theme continues as we examine the impact of continually increasing exposure to various external environmental factors as women age.
Effects of Environmental Exposures
With increasing age, women are exposed to potential environmental toxins that are likely to impair fertility. An increasing number of women are exposed to toxins in the workplace and the environment.62 More than 50 synthetic chemicals that are ubiquitous in the environment have been implicated as reproductive toxins.63 Sharara and colleagues describe the biologic basis of adverse effects of environmental toxins on female reproduction and discuss the mechanisms of action of environmental toxins. Although they acknowledge that some environmental chemicals are present in low concentrations or have low affinities to induce biologic responses, they highlight the fact that many environmental toxins are lipid soluble, and over decades, exposure may be significant because of bioaccumulation in the food chain. Chronic exposure as women age may result in adverse reproductive outcomes. They also discuss endocrine disruptors, heavy metals, solvents, industrial chemical, and pesticides. Perhaps most importantly, they discuss cigarette smoking and the effects of active and passive smoking on female reproduction.
Active and passive exposure to cigarette smoke increases with age, and to quote Sharara, “Cigarettes represent the ultimate legal delivery system of hundreds of reproductive toxicants and carcinogens. Cigarette smoke contains more than 4000 chemical compounds, including 43 carcinogens or poisons and more than 300 polycyclic aromatic hydrocarbons.” Smoking among women is beginning at an earlier age, and they are smoking more cigarettes on average.64 The effect of cigarette smoking on female reproduction has consistently demonstrated impairment of fertility and fecundity in natural populations and populations undergoing ART. For instance, Van Voorhis and coworkers reported, for women undergoing ART, a 50% reduction in implantation rate and ongoing pregnancy rate in active smokers compared with nonsmokers.65 In natural populations, fecundity decreases, and the incidence of spontaneous abortion increases.66,67 Ovarian reserve appears to be adversely affected by cigarette smoking.68 Ovarian reserve in smokers between the ages of 35 and 39 is decreased compared with that of nonsmokers between the ages of 35 and 39.69 Women who smoke reach menopause 1 to 4 years earlier than their nonsmoking age-matched counterparts.70 These last two factors imply that smoking accelerates follicular loss and results in earlier entry into the perimenopause, accelerating the age effect on fertility and fecundity.
Additional adverse exposures include radiation and chemotherapeutic agents, which are known to have deleterious effects on female reproduction, and the insult is more profound with advancing age.71 Independent of any of the aforementioned exposures, all human organ systems undergo adverse age-related changes. We focus on the age-related changes of specific organ systems.
Adrenal Gland Changes
The adrenal gland is a vital organ to the endocrine system and the adrenal gland synthesizes and secretes several hormones that are related to the reproductive system. The adrenal glands produce and secrete many of the same hormones that are concurrently produced and secreted by the ovaries. Various hyperandrogenic adrenal disorders such as congenital adrenal hyperplasia and late-onset adrenal hyperplasia have a detrimental effect on female reproduction. However, little is known about the age-related decrease in adrenal androgen production and its effect, if any, on the age-related decline in female reproduction. Because adrenal steroidogenesis is markedly different between species, it is difficult to find an animal model to study that resembles human adrenal physiology. The cause of the changes in adrenal activity that occur with age are not clearly understood, but what little is known about the age-related changes in adrenal function does not implicate the adrenal gland as having a major role in the age-related decline in reproduction.
Early in life, adrenal androgen production is quite low. Adrenarche marks the onset of a significant increase in the production of adrenal steroids and is independent of gonadarche.72 Once adrenarche occurs, adrenal androgen production rises steadily until around the age of 25, at which time an irreversible decline ensues.73,74,75 The primary circulating androgens include androstenedione, testosterone, dehydrotestosterone (DHT), dehydroepiandrosterone (DHEA), and dehydroepiandrosterone sulfate (DHEAS). The adrenal glands and the ovaries in equal amounts produce androstenedione. Approximately 25% of testosterone is produced by the adrenal glands, 25% is produced by the ovaries, and the remaining 50% is derived from the peripheral conversion of androstenedione to testosterone. Androstenedione and testosterone are converted to DHT in the liver and the skin. About 90% of DHEA is produced in the adrenal glands, and 10% is produced by the ovaries, whereas DHEAS is produced almost exclusively in the adrenal glands. With the adrenal glands contributing a significant amount of these steroid hormones to the circulating pool of androgen hormones in the endocrine environment, age-related changes in the adrenal glands may be expected to contribute to the age-related changes in reproductive system.
One of the principal secretory hormones of the adrenal glands is cortisol. Circulating levels of cortisol do not change with age.76 The adrenal glands respond normally to corticotropin stimulation with aging.77 On the contrary, the adrenal androgens do demonstrate age-related changes, but it becomes important to try to separate the ovarian and adrenal contribution to this decline before implicating either organ. For instance, beginning in the mid-twenties, androstenedione levels progressively decrease.78 Because the ovarian and adrenal contribution of androstenedione is split, oophorectomized women were studied as well. Although androstenedione levels decreased with advancing age, these levels did not change significantly until women were in their early fifties at the time of the menopausal transition.74 The levels also reach a nadir and plateau in women when they reach their sixties. Androstenedione levels do not decrease significantly when correlated to the time of last menses, thereby suggesting that the ovarian contribution of androstenedione is already minimal by the time menopause is reached.79
Testosterone levels decline with age. Most testosterone is derived from the conversion of DHEA and DHEAS to testosterone, and although testosterone levels change slowly through the perimenopause, the 24-hour testosterone levels do change significantly with age.75 Circulating DHEA and DHEAS peak in the mid-twenties and begin to drop independent of menopause or oophorectomy.80,81,82 The reason these adrenal androgens fall with age has been studied intensively, and it does not appear to be caused by changes in the metabolic clearance rate or the peripheral metabolism of these adrenal androgens.83,84,85,86,87 There is no change in adrenal androgen secretion because of a decrease in the size of the adrenal cortex with age.88 Because oophorectomy does not increase the changes in adrenal androgen secretion, it is unlikely that increasing gonadotropins are responsible for the adrenal changes. Although elevated androgens are known to disrupt the hypothalamic-pituitary-ovarian axis, the decreasing levels of adrenal androgens do not seem to have a significant impact on the hypothalamic-pituitary-ovarian axis or reproductive aging as far as indirect evidence suggests.
Hypothalamic-Pituitary Changes
The menstrual cycle is controlled by the neuroendocrine system. The cyclic nature of the menstrual cycle requires an exquisitely coordinated endocrine system that is intricately regulated through feedback signals. The highest signals are generated through hypothalamic-pituitary interactions. In the past, studies of the hypothalamicpituitary interaction have been performed primarily in rodents, but rodents exhibit regulatory mechanisms that are entirely different from humans. Because of this, extrapolation of biologic mechanisms of hypothalamic-pituitary aging characteristic of rodents to humans is somewhat limited. In humans, the first sign of reproductive aging is a rise in follicle-stimulating hormone (FSH) levels independent of luteinizing hormone (LH) levels (monotropic FSH rise).89,90 Whether this change is caused by intrinsic aging at the level of the hypothalamic-pituitary system or to a change in responsiveness of the hypothalamic-pituitary system to feedback signals remains uncertain.During the past few years, we have started to understand some of the characteristics of hypothalamic-pituitary interactions in humans. In monkeys, the monotropic FSH rise can be induced by altering the gonadotropin-releasing hormone (GnRH) pulse generator.91 It has been postulated that changes in the frequency or amplitude of pulses secreted from the GnRH pulse generator might explain this phenomenon in humans, but Klein and coworkers were unable to demonstrate any differences in LH pulse frequency, LH pulse amplitude, or mean LH levels across menstrual cycle phases when comparing younger and older women.92 It is generally accepted that the LH secretion pattern accurately reflects the secretion pattern of the GnRH pulse generator.93 Alexander and coworkers also examined basal pulsatile LH secretion by monitoring pulse frequency and amplitude and failed to demonstrate a difference between younger and older oophorectomized women.94
Other investigators have studied the stores of GnRH to determine whether differences exist between younger and older women. Parker and coworkers studied the hypothalamic content of GnRH stores in young premenopausal women and postmenopausal women. Postmenopausal women demonstrated significantly less GnRH secretion than the younger premenopausal women. This finding implies a decrease in synthesis or storage of GnRH in older women. However, the hypothalamic content of GnRH was also measured in younger women who had undergone bilateral oophorectomy, and they demonstrated lower GnRH content than that of the normal younger women.95 Other investigators have looked at the bioactivity of the FSH molecule, suggesting that intrinsic changes in the molecule may demonstrate different activity, but the bioactivity does not differ with advancing age.96 The study also found no difference in ovarian steroid secretion of estradiol and progesterone; however, it did show accelerated recruitment and ovulation of a dominant follicle. This observation is consistent with Treolar's findings of a shortening of menstrual cycle length in women of advancing reproductive age.97 This occurs before there is a lengthening of menstrual intervals, followed by the eventual cessation of menstrual flow. Other investigators have also shown shortening of the menstrual cycle.98
The hypothalamic-pituitary axis retains its responsiveness to ovarian steroids and its sensitivity to positive and negative feedback mechanisms.99 The pituitary gland undergoes morphologic changes with aging, but these changes do not alter the functional units of the gland.100,101 Several investigators have convincingly shown that the monotropic FSH rise is associated with decreasing inhibin-B and inhibin-A levels in women, suggesting a decrease in the negative feedback mechanisms of the ovary on pituitary gonadotropin secretion.102,103,104 These changes do suggest that a deterioration of ovarian follicular function lead to the monotropic FSH rise and the subtle changes in the menstrual cycle. Ultimately, hypothalamic-pituitary changes do not seem to initiate the cascade of reproductive failure that is associated with aging, but this idea requires confirmation.
Fallopian Tube Changes
The fallopian tubes represent another reproductive organ influenced by aging, but they are not implicated as a major contributing factor associated with reproductive failure. Studies have demonstrated an increased incidence of ectopic gestation with advancing maternal age.105,106,107 One reason for this may be that tubal function is in part regulated by cyclic ovarian steroid secretion and steroid secretion becomes increasingly irregular as women approach the menopause. Several investigators have studied the electrical activity and contractility of the fallopian tubes, and they have demonstrated a progressive decline in tubal motility with advancing age.108,109,110,111 Although there does seem to be changes in tubal function, the data also suggest that these changes may be related to ovarian dysfunction and inadequate luteal phase steroid secretion rather than inherent changes in the fallopian tubes.108,112 The increased incidence of ectopic pregnancy seen with advancing age may be more related to ovulatory dysfunction than inherent deterioration of fallopian tube function.
Uterine Changes
There has been considerable debate about whether uterine receptivity deteriorates with advancing age. Several explanations have been offered implicating endometrial receptivity as a major contributor to declining fecundity with advancing age. For instance, some investigators have proposed that an age-related decline in uterine perfusion may contribute to the decline in fecundity.113,114 Uterine vascular changes such as fibrosis may lead to changes in blood flow through the uterus.115 Sterzik and coworkers reported an increasing number of out-of-phase endometrial linings in women older than 35 years of age during unsuccessful IVF cycles.116 Others have not found any differences in the expression of receptors, cellular proliferative index, vascular patterns, histology, and other characteristics in women of advancing age compared with younger women.117,118,119 A major obstacle facing investigators studying age-related effects on human reproduction is to distinguish between intrinsic age-related changes in endometrial receptivity and intrinsic changes in other organ systems that result in changes in endometrial receptivity.
Until the advent of donor egg programs, it was impossible to pinpoint the organ system most likely responsible for the age-related changes in fecundity in women of advanced maternal age. The reason for this is that oocyte quality (ovarian derived) could not be separated from endometrial quality (uterine derived) in women of advanced reproductive age using autologous eggs. Convincing data support the hypothesis that oocyte quality decreases with increasing age.120,121,122 Deriving oocytes from young women enabled investigators to study these two variables independently by optimizing the egg quality for all age groups, followed by an evaluation of endometrial receptivity. In the late 1980s, programs using donor eggs for women with ovarian failure began to publish their data on outcomes.123,124 Shortly thereafter, the use of donor eggs spread to perimenopausal women and women who had failed multiple IVF cycles. Initially, investigators used the data from their programs to explain the loss in fecundity in women with advancing maternal age. Natural populations have not been studied; only women undergoing oocyte donation have been studied.
Initial reports were conflicting about whether endometrial receptivity decreased with increasing age. Several independent investigators suggested that the aging uterus and decreased endometrial receptivity were responsible for the decline in fecundity in women of advancing maternal age.125,126,127,128 At the same time, other investigators reported data suggesting that there was no loss of endometrial receptivity in women of advancing maternal age.129,130,131,132,133 Meldrum proposed that one of the reasons for the difference in outcomes might be differences in the protocols used for hormone replacement.134 Differences in populations studied, sample size analyzed, and confounding variables represent additional possible explanations for the conflicting results. It has been substantiated in the literature that there is no decline in endometrial receptivity with aging.120,135,136 Even when postmenopausal women are given appropriate hormone replacement, the uterus maintains its ability to provide a receptive endometrium and demonstrates normal histologic, ultrasonographic, and steroid receptor response.137 Although the process of reproductive aging involves complex mechanisms at the level of the hypothalamus, pituitary gland, fallopian tubes, and endometrium, it is relatively clear that ovarian dysfunction and oocyte depletion is primarily responsible for age-related reproductive failure.
Ovarian Changes
Throughout life, the functional activity of the ovary changes dramatically. Research findings have enabled us to better understand the physiologic basis for the decline in female fecundity and ultimately menopause. Once thought to begin only a few years before menopause, ovarian dysfunction begins well over a decade before the cessation of menses. Long before the menopause, the ovary begins to undergo changes at the cellular level that lead to abnormal steroid and glycoprotein production, abnormal gamete formation, and impaired fertility in women as early as their mid-thirties. Our understanding of the functional components of ovarian follicular physiology has greatly advanced because of the availability of granulosa cells from women undergoing ART.138 Our understanding of oocyte structural mechanics and physiology have improved because of the availability of human oocytes from these women. Access to human granulosa cells and oocytes has allowed investigators to use in vitro methodologies to probe some of the mechanisms responsible for in vivo clinical outcomes. The quantitative changes of the ovarian follicular pool associated with aging have been clearly demonstrated, and we are able to demonstrate changes in quality as well.
The changes that occur within the ovarian cortex throughout a woman's life are primarily responsible for the decrease in her reproductive potential, the increase in her menstrual cycle irregularities, and ultimately the cessation of her menses. Tracing the embryologic development of the ovarian cortex begins to highlight the intricate complexity of this organ. Early in gestation, primordial germ cells, which are of endodermal origin, migrate to the gonadal ridge. As the ovary forms, primordial germ cells are converted to oogonia, which are localized to the cortex. The ovarian cortex consists of tightly packed cells, throughout which are scattered oogonia. Oogonia multiply many times through mitotic activity. After the last mitotic division, DNA replication occurs, and the oogonia are converted to oocytes as meiotic divisions begin. Meiosis results in a reduction of the number of chromosomes, a redistribution of chromosomes of maternal and paternal origin, and an exchange of genetic material between some of the chromosomes. The prophase of meiosis I has been divided into stages: leptotene, zygotene, pachytene, and diplotene. It is in the diplotene stage of meiosis I that the oocyte becomes arrested and remains quiescent for up to 50 years.
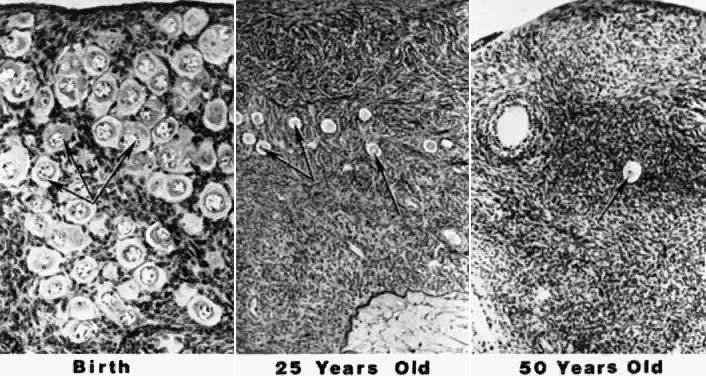
Through continued mitotic and meiotic activity, the maximum number of oocytes is reached at approximately 20 weeks' fetal gestation. That number peaks at 6 to 7 million.139 Intervening cortical stroma, which is of mesodermal origin, migrates around the oocytes and forms the primordial follicle.140 Early on, mitotic activity leads to the profound increase in oogonia, but after meiosis and follicular formation has occurred, follicular atresia begins and causes an enormous depletion of oocytes. Follicular atresia is very similar to apoptosis (i.e. programmed cell death).141 By birth, only 1 to 2 million oocytes remain.142 All of the remaining oocytes progress through to the diplotene stage of prophase meiosis I shortly after birth and remain there until the time of ovulation.143 Follicular atresia continues, and approximately 300,000 oocytes remain at the time of menarche.144 Of the remaining follicles, only 400 to 500 are ovulated over the next 30 to 40 years.145 At the time of menopause, nearly all of the oocytes have been depleted.146 The photomicrograph in Figure 7 illustrates the depletion of follicles occurring with advancing age.147
Follicular atresia is a process that causes follicular depletion, and it occurs in two settings. Monthly follicular atresia, a gonadotropin-dependent process, occurs as a cohort of up to 50 follicles is recruited each month resulting in the selection of a dominant follicle that progresses to ovulate while the remaining follicles are resorbed. This process accounts for less than 1% of the loss of the total endowed primordial oocyte pool. More significantly, a tonic gonadotropin-independent process of atresia occurs, causing hundreds of thousands of primordial follicles to be continuously resorbed. This type of atresia occurs even in the presence of oral contraceptives and pregnancy.148 This process is responsible for the loss of 80% of the endowed primordial oocyte pool from 6 months in utero to birth and is responsible for the loss of 95% of a woman's endowed oocyte pool by the time puberty is reached. Postpubertal follicular atresia is a lifelong process on which monthly cyclic ovulating attrition is superimposed. The age-related depletion of a woman's endowed follicular pool is accompanied by the disruption of follicular granulosa cell function and ovarian steroid production, particularly estrogen. A reduction of ovarian steroid synthesis leads to a disruption of cyclic ovarian function and ultimately to menopause. This is caused by significant age-related changes in ovarian follicular physiology.
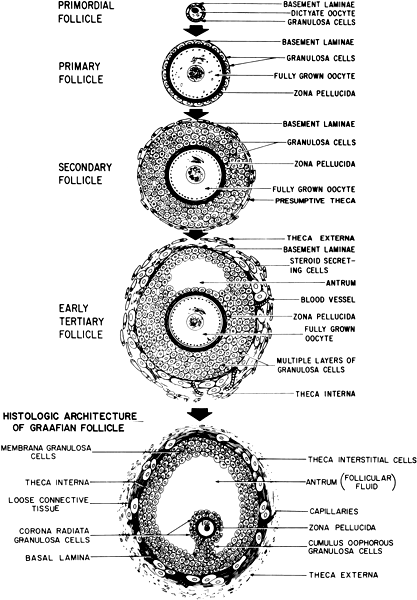
The ovarian cortex contains numerous follicles that vary greatly in stages of growth and degeneration. Figure 8 demonstrates the typical architecture of an ovarian follicle at different stages of development. The complexity and size of the follicles also vary greatly with the stage of development, but each follicle contains an oocyte surrounded by epithelial cells. In the young adult woman, the most commonly found follicles are primordial follicles. They are made up of a primary oocyte and a few flattened follicular cells, pregranulosa cells, enclosed by a basal lamina. Remaining in a resting stage, these follicles are quiescent and essentially unresponsive to gonadotropins. A large number of follicles are continuously leaving this stage and undergoing sequential maturation. After growth is initiated, primordial follicles become primary follicles. The follicular cells differentiate into a complete layer of cuboidal cells, or granulosa cells. The basal lamina separates these cells and the oocyte from the rest of the ovary. The oocyte increases in size but remains in prophase meiosis I. Granulosa cells manufacture and secrete a glycoprotein extracellular matrix, which accumulates between the oocyte and the granulosa cells, ultimately forming the zona pellucida.149
The solid preantral follicle continues to grow as the oocyte increases in size from 10 to 100 μm. At the same time, the granulosa cells are increasing and become multilayered, and the zona pellucida thickens. Before the end of the preantral stage, stromal cells in the cortex that immediately surround the granulosa cells organize into distinct layers called the theca interna and theca externa.
The antral stage follows the preantral stage as the follicle develops a fluid-filled space called the antrum. Follicular fluid originally appears scattered throughout the follicle but coalesces to form the antrum. Granulosa, theca, and interstitial cells all contribute to the production of antral fluid. As the antrum grows in size, it separates the granulosa cells into different layers. The layers of cells surrounding the oocyte, the cumulus oophorus, is connected to the granulosa cells lining the wall of the follicle, the mural granulosa cells, by a stalk of cells between the two layers. The layer of cells immediately surrounding oocyte and zona pellucida is commonly referred to as the corona radiata. Important connections exist between these granulosa cells and the oocyte.150 The mature antral follicle contains a large oocyte that is still arrested in prophase of meiosis I. The cells of the corona radiata share gap junctions with each other and the oocyte. The mural granulosa cells maintain a stratified cuboidal epithelium. The basal layer consists of low columnar cells overlying a dense basement membrane. The theca interna becomes thicker and increasingly vascular. From the cohort of recruited follicles follows a selection of even fewer follicles, leading ultimately to the appearance of a dominant follicle destined to ovulate.151
Ovulation of the dominant follicle is preceded by a series of complex events. During the preovulatory period, the oocyte completes diakinesis within the nucleus, the nuclear envelope disperses, and metaphase spindle organizes at the margin of the oocyte. The nucleus divides, separating the homologous chromosomes and results in a reduction division with very unequal dispersion of the cytoplasm. The result yields a secondary oocyte with a small polar body adjacent to the oocyte, both of which are encased within the zona pellucida. The secondary oocyte proceeds to metaphase of meiosis II and remains there until fertilization occurs.
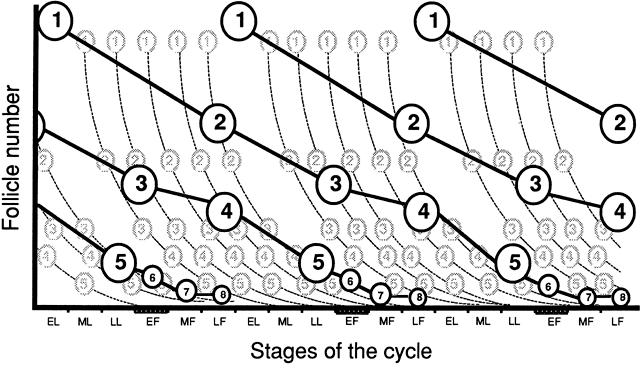
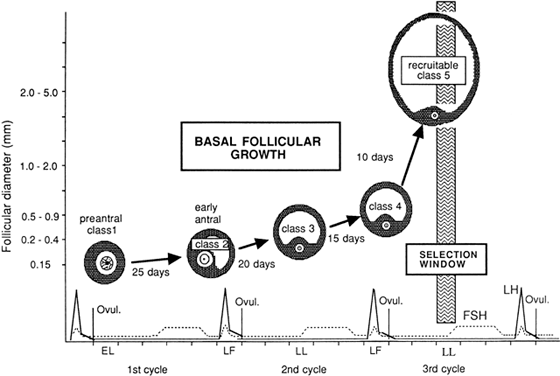
Models of follicular depletion have been previously described. Gougeon described one of the commonly accepted models of oocyte depletion.152 According to this model, at any given time, a given proportion of follicles are moving through various stages of follicle maturation (Fig. 9). Gougeon described a class system for ovarian follicles. Eight classes have been used to describe follicular development through two phases. The phases are tonic (slow) growth phases and gonadotropin-dependent (rapid) growth phases. The time required for a given follicle to pass through all classes is approximately 85 days and spans the length of three menstrual cycles (Fig. 10).
Classes one to four make up the tonic growth phase and represent the transition of preantral follicles up to the early antral follicles less than 2 mm in diameter. Tonic growth is relatively slow but does not imply gonadotropin independence. Gonadotropins are necessary for growth during this phase, but they do not have the same influence on follicles that they have on the gonadotropin-dependent growth phase. Classes 5 to 8 make up the gonadotropin-dependent growth phase. Figure 11 depicts the eight classes and their corresponding developmental sequence. This phase is highly regulated by gonadotropins, and they have profound effects on the follicles. Class 5 follicles represent the group of follicles from which the ovulatory follicle is recruited and selected, leading to dominance and ovulation. The remaining follicles of any given cohort undergo atresia anywhere along the process. Why follicles undergo atresia when they do remains a mystery.
|
Because of the continuous loss of follicles, the follicle pool begins to deplete and show changes in response to the decreasing reserve of follicles. In the past, we defined the perimenopause as the time when ovarian function was so altered by this depletion of follicles that endocrine changes, such as menstrual irregularities, appeared in women.153 We now understand that endocrine changes are occurring at a cellular level, perhaps in response to the depletion of follicles, more than a decade before this time. There is an accelerated loss of follicles from the ovaries around the age of 37 years.154,155 At the same time, women experience an increase in the number of embryonic chromosomal abnormalities, an increase in spontaneous miscarriages, a decrease in bone mineral density,156 and a decrease in fertility rates. It is important for clinicians to identify the progressive deterioration of ovarian function, because someday we may be able to delay the some of the untoward effects of aging in women.
From accumulating data, progressive deterioration of ovarian function seems to be more closely related to the number of follicles remaining in the ovarian cortex than to ovarian age. Figure 12 demonstrates that when a woman enters her middle to upper thirties, the rate of oocyte depletion increases. At that time, approximately 25,000 follicles remain, and the rate of oocyte depletion accelerates. After the oocyte pool reaches approximately 1000 follicles, menopause ensues. Shortly after menopause, there are essentially no follicles remaining.157,158 As the rate of accelerated oocyte depletion begins, we also see an increase in the incidence of aneuploidy, an increase in the incidence of spontaneous abortions, and a decrease in fertility rates.159,160,161 The function of the follicular apparatus is changing during this time and most likely leads to many of these adverse outcomes as women age. These outcomes are similarly found in women who have infertility and are undergoing assisted reproductive technologies.162,163,164 For example, as age increases in women undergoing IVF, so does the incidence of spontaneous abortions, reaching as high as 50% in women older than 40 years.163 Data suggest that these changes are likely to involve follicular cell compromise.
Granulosa cells provide essential metabolic support and participate in intrafollicular communication with their accompanying oocyte. The life cycle of a granulosa cell is composed of periods of proliferation and differentiation followed by quiescence, senescence, or apoptosis. Granulosa cells produce a number of steroids, glycoproteins, and proteins. Changes in the cell cycle or the production of various factors may reflect age-related changes in human granulosa cell competence accompanied by a decline in female fecundity. These changes may also explain the age-related decline in ART success. With increasing age, a woman's estradiol response to ovulation induction, the number of oocytes retrieved, the number of successful implantations, and pregnancy rates decrease.163,165,166,167,168,169,170,171,172 There may be an increase in the resistance of follicles to exogenous gonadotropins, demonstrated by a decreased ovarian response to an increased amount of stimulation in women of advancing reproductive age.
These clinical observations parallel specific physiologic endocrine alterations of the aging ovary. A characteristic shortening of the overall menstrual cycle occurs with advancing age because of a shortening of the follicular phase.97 This is accompanied by a higher early follicular estradiol and rising FSH level (i.e. monotropic FSH rise), as well as a premature rise in progesterone, leading to an earlier LH surge and ovulation.173 Quantitative follicular changes occur within the aging human ovary because of atresia; however, the mechanisms by which this occurs are not well understood. We now believe that qualitative changes also occur in the remaining follicles, contributing to the physiologic changes found clinically. Ongoing clinical investigation has demonstrated that various biomarkers may reflect these qualitative changes. Through intensive study of various biomarkers, we continue to uncover complex physiologic mechanisms that explain the age-related decline in female fecundity.
Through the experience of assisted reproductive technologies, we now believe that various biomarkers may be better predictors of a woman's reproductive potential than her chronologic age. Ovarian reserve describes a woman's reproductive potential as it relates to the processes of follicular depletion and oocyte quality. The first biomarker found to be correlated with predicting ovarian reserve was a measured day 3 serum FSH level.174 Although there may be some degree of intercycle variability, women with elevations of FSH in one cycle usually have elevations in subsequent cycles.175 Generally, the success of ovulation induction and IVF diminishes with declining ovarian reserve, as reflected by rising follicular phase FSH levels.176,177 This is consistent with the fact that serum FSH levels (i.e. monotropic FSH rise) gradually rise in the early follicular phase 8 years before the perimenopausal-menopausal transition in healthy women.90,178 Other investigators have reported that unexpected elevations of basal FSH are associated with unexplained infertility despite regular menses.179 Another basal test that has been examined includes basal estradiol levels. Investigators have consistently demonstrated that elevated day 3 estradiol levels are predictive of poor outcomes in women undergoing ART.180,181,182 Added to these basal measurements, FSH assessment after provocative testing may increase a clinician's ability to detect diminished ovarian reserve.
The use of the clomiphene citrate challenge test (CCCT) has possibly provided additional sensitivity to the predictability of ovarian reserve. The CCCT involves the use of serial measurements of various hormones assayed on specific days before and after the administration of clomiphene citrate. First described by Navot and coworkers in 1987, the CCCT has become increasingly used as a screening test for ovarian reserve.183 The original test consisted of measuring FSH concentration on cycle day 3, administering 100 mg of clomiphene citrate on cycle days 5 through 9, and measuring the serum FSH concentration on cycle day 10. Day 3 FSH levels served as basal levels, and day 10 levels served as provoked levels. The premise behind the CCCT was based on the idea that the day 10 level might unmask diminished ovarian reserve not detected by day 3 FSH levels alone. Scott and other investigators performed follow-up analysis of abnormal CCCT results and found that they were predictive of diminished ovarian reserve and decreased pregnancy rates in women in natural cycles and women undergoing ART.184,185,186 However, Scott also found that age is an independent predictor of poor outcomes despite a normal CCCT. With this in mind, it becomes clear that the CCCT may serve as a useful screening test but fails to explain the physiologic basis for the abnormal rise in FSH levels.
Investigators have identified the physiologic basis of the rising FSH levels in women of advancing reproductive age. Known to modulate the secretion of FSH, inhibin represents a serum marker that may be directly related to functional ovarian reserve.187 Clinical work has shown significantly lower serum inhibin levels in women older than 35 years who are undergoing IVF without showing significant differences in estradiol levels.188,189 This suggests that an age-related reduction in inhibin during maximal ovarian stimulation may be an early index of declining ovarian function with advancing age. It is thought that controlled ovarian hyperstimulation may unmask a condition of “compensated granulosa cell failure” reflected by a decreased serum inhibin, compared with the unchanged serum inhibin levels during spontaneous menses in women with occult ovarian failure (i.e. regular menses and elevated FSH levels). Initial studies measured total inhibin concentration because assays were unable to differentiate inhibin-A from inhibin-B. As new and improved assays for the detection of inhibin-A and inhibin-B have become available, investigators have identified the serum marker responsible for rising FSH levels in women of advancing reproductive age.
Inhibin-B concentrations have been shown to decrease with increasing serum FSH concentrations in women in the follicular phase.103 The physiologic basis of ovarian reserve screening using the CCCT is grounded in the hypothesis that in women with normal ovarian reserve, inhibin production by the developing follicles is sufficient to overcome the effects of the clomiphene citrate on the hypothalamic-pituitary axis. This leads to the suppression of the FSH levels back to within the normal range by cycle day 10. Hoffman and coworkers confirmed this hypothesis in women undergoing ovarian reserve screening.190 They demonstrated lower serum inhibin-B levels on day 3 and day 10 in women with diminished ovarian reserve (elevated FSH levels) compared with women with normal ovarian reserve (normal FSH levels). The data strongly suggest that granulosa cell inhibin-B production in women with diminished ovarian reserve is inadequate to maintain basal FSH levels in the normal range and is unable to suppress FSH levels in the presence of clomiphene citrate. As a result of the data accumulating on inhibin, some investigators have proposed that inhibin-B screening may be a more direct measure of ovarian function and a more sensitive indicator of ovarian reserve than pituitary FSH secretion.
Danforth and coworkers evaluated luteal phase inhibin-A and follicular phase inhibin-B levels and found an inverse relationship with advancing age.102 They also demonstrated a decline in inhibin-A and inhibin-B before the monotropic FSH rise. This is consistent with the findings of Seifer and colleagues that day 3 inhibin-B concentrations are predictive of IVF outcomes.191 Additional data also support the hypothesis that day 3 serum inhibin-B levels decline before the monotropic FSH rise.192,193 Seifer and coworkers studied women undergoing ART who demonstrated a poor response to stimulation as measured by increased ampules of gonadotropins for stimulation yielding a higher cancellation rate, a lower estradiol response, fewer oocytes retrieved, and lower clinical pregnancy rate. They found that this group of women also had lower inhibin-B levels than women undergoing ART with normal ovarian responsiveness, despite both groups having similar nonelevated day 3 FSH levels. In addition to confirming the inverse relationship between declining inhibin levels and rising FSH levels, Santoro and colleagues demonstrated an increase in activin-A, which also contributes to the FSH elevation.194 In the future, day 3 inhibin-B screening may provide a more direct and reliable measure of ovarian reserve. Our understanding of ovarian physiology is evolving through the study of potential biomarkers of aging.
The gonadotropin agonist stimulation test (GAST) is another ovarian reserve test. First described by Padilla in 1990, the GAST is a measure of ovarian responsiveness to GnRH-a gonadotropin stimulation.195 In the early follicular phase, estradiol levels are evaluated in response to GnRH-a, and the data suggest a positive correlation with ART success. Although there are concerns about the expense of the test and the applicability of the test to the general population, some argue that the GAST is excellent marker of ovarian reserve and a sensitive predictor of IVF outcomes.196
Another test introduced as a measure of ovarian reserve is the exogenous FSH hormone ovarian reserve test (EFORT).197 The EFORT test measures basal FSH levels and estradiol levels obtained on cycle day 3, followed by the administration of 300 IU of purified FSH. Twenty-four hours later, estradiol is measured and the change in estradiol (delta E2) is calculated. Women with a low basal FSH level and a high delta E2 were considered normal, demonstrating a good response to gonadotropin therapy. The EFORT, GAST, and CCCT all represent ovarian reserve tests that require ovarian stimulation. Other tests of ovarian reserve have been described that do not require stimulation testing.
In the past, investigators have attempted to quantify potential ovarian responsiveness by using ultrasound measurements of various morphologic ovarian characteristics. One of the characteristics showing some correlation to ART outcomes is antral follicle counts. Investigators have observed that the number of ultrasonically visible antral follicles decreases with advancing age.198 Chang and colleagues showed that, in women undergoing ART, antral follicle counts correlate well with ovarian responsiveness to stimulation and pregnancy outcomes.199
Transvaginal ultrasound ovarian volume measurement represents another morphologic test of ovarian reserve. Syrop and colleagues examined ovarian volumes and found that total ovarian volume and the volume of the smallest ovary were predictive of a woman's response to gonadotropin stimulation and ART success.200 Large ovarian volumes were predictive of good ART outcomes, and small ovarian volumes were associated with poor outcomes. Sharara examined the relationship between ovarian volume and age and FSH levels but did not find any significant correlation.201 However, they also found higher cancellation rates and therefore poorer outcomes in women with smaller ovaries. They suggested that ovarian volumes may be an earlier predictor of diminished ovarian reserve than basal FSH or basal estradiol levels.
Research is ongoing to discover the ideal biomarker of ovarian reserve, with the hope of providing clinicians the tools to accurately predict the impact of age on a couple's chance for success when undergoing ART. Wh
CONCLUSIONS
Age has a profound effect on human reproduction. Genetic, environmental, social, and other external factors contribute to the age-related decline in human reproduction. However, the ovarian follicle seems to be the pacemaker of female reproduction, and it is ovarian follicular dysfunction that has the most powerful influence on the age-related decline in fertility. If oocyte quality is in part determined by follicular granulosa cell quality, it may be possible to improve oocyte quality by replacing or supplementing cells with specific steroid or growth factors that are lacking in aging granulosa cells. If researchers eventually develop the technique of in vitro maturation of immature oocytes from women with diminished ovarian reserve, we may then learn the exact requirements of the follicular apparatus, allowing the creation of an “artificial” follicular apparatus for oocyte maturation. Ultimately, we may be able to retard end-organ compromise rather than palliate end-organ failure.
Women should be educated about the profound impact of age on reproduction before it is too late. Communicating to young women that they will experience diminished ovarian reserve beginning in their mid-thirties will enable them to plan childbearing. Preventative or therapeutic strategies implemented by physicians may reduce associated short-term and long-term morbidity associated with age-related ovarian dysfunction.
REFERENCES
Johnson L, Petty CS, Neaves WB: Influence of age on sperm production and testicular weights in men. J Reprod Fertil 70: 211, 1984 |
|
Johnson L, Zane RS, Petty CS, Neaves WB: Quantification of the human Sertoli cell population: Its distribution, relation to germ cell numbers, and age-related decline. Biol Reprod 31: 785, 1984 |
|
Macleod J, Gold R: The male factor in fertility and infertility. Fertil Steril 4: 194, 1953 |
|
Silber S. Effect of age on male fertility. Semin Reprod Endocrinol 9:241, 1991 |
|
Nieschlag E, Lammers U, Freischem C et al: Reproductive functions in young fathers and grandfathers. J Clin Endocrinol Metab 55: 676, 1982 |
|
MacLeod J, Gold R: The male factor in fertility and infertility. VII. Semen quality in relation to age and sexual activity. Fertil Steril 4: 10, 1953 |
|
Schwartz D, Mayoux M, Spira A et al: Semen characteristics as a function of age in 833 fertile men. Fertil Steril 39: 530, 1983 |
|
American College of Obstetricians and Gynecologists Committee Opinion, Committee on Genetics: Advanced paternal age: Risks to the fetus. Number 189, October 1997. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet 59:271, 1997 |
|
Hook E: Rates of chromosomal abnormalities at different maternal ages. Obstet Gynecol 58: 282, 1981 |
|
Hassold T, Chiu D: Maternal age-specific rates of numerical chromosome abnormalities with specific reference to trisomy. Hum Genet 70: 11, 1985 |
|
Hassold T: Nondisjunction in the human male. Curr Topics Dev Biol 37: 383, 1998 |
|
de Michelena M, Burstein E, Lama J, Vasquez J: Paternal age as a risk factor for Down syndrome. Am J Med Genet 45: 679, 1993 |
|
Hook E, Regal R: A search for a paternal-age effect upon cases of 47,+ 21 in which the extra chromosome is of paternal origin. Am J Hum Genet 36: 413, 1984 |
|
Eichenlaub-Ritter U: Genetics of oocyte ageing. Maturitas 30: 143, 1998 |
|
Erickson J: Down syndrome, paternal age, maternal age and birth order. Ann Hum Genet Lond 41: 289, 1978 |
|
Roth M, Feingold J, Baumgarten A et al: Reexamination of paternal age effect in Down's syndrome. Hum Genet 63: 149, 1983 |
|
Friedman JM: Genetic diseases in the offspring of older fathers. Obstet Gynecol 57: 745, 1981 |
|
Modell B, Kuliev A: Changing paternal age distribution and the human mutation rate in Europe. Hum Genet 86: 198, 1990 |
|
Riccardi V, Dobson C, Chakraborty R, Bontke C: The pathophysiology of neurofibromatosis: IX. Paternal age as a factor in the origin of new mutations. Am J Med Genet 18: 169, 1984 |
|
McIntosh G, Olshan A, Baird P: Paternal age and the risk of birth defects in offspring. Epidemiology 6: 282, 1995 |
|
Olshan A, Schnitzer P, Baird P: Paternal age and the risk of congenital heart defects. Teratology 50: 80, 1994 |
|
Federation CECOS, Schwartz D, Mayoux MJ: Female fecundity as a function of age: Results of artificial insemination in 2,193 nulliparous women with azospermic husbands. N Engl J Med 306:404, 1982 |
|
Virro M, Shewchuk A: Pregnancy outcome in 242 conceptions after artificial insemination with donor sperm and effects of maternal age on the prognosis for successful pregnancy. Am J Obstet Gynecol 148: 518, 1984 |
|
Mosher W, Pratt W: Fecundity and infertility in the United States: Incidence and trends. Fertil Steril 56: 192, 1991 |
|
Toulemon L: Historical overview of fertility and age. Maturitas Suppl 1: 5, 1988 |
|
Ventura S: Trends and variations in first births to older women, 1970-86. Vital Health Stat 47: 1, 1989 |
|
Tietze C: Reproductive span and rate of reproduction among Hutterite women. Fertil Steril 8: 89, 1957 |
|
Hendershot GE: Maternal age and overdue conceptions. Am J Public Health 74: 35, 1984 |
|
Henry L: Some data on natural fertility. Eugenics Q Z 8: 81, 1961 |
|
Menken J, Trussell J, Larsen U: Age and infertility. Science 233: 1389, 1986 |
|
James W: The causes of the decline in fecundability with age. Soc Biol 26: 330, 1979 |
|
Wood C, Calderon I, Crombie A: Age and fertility: Results of assisted reproductive technology in women over 40 years. J Assist Reprod Genet 9: 482, 1992 |
|
Maroulis GB: Effect of aging on fertility and pregnancy. Semin Reprod Endocrinol 9: 165, 1991 |
|
Hansen J: Older maternal age and pregnancy outcome: A review of the literature. Obstet Gynecol Surv 41: 726, 1986 |
|
Creswell J, Egger P, Fall C et al: Is the age of menopause determined in utero? Early Hum Dev 49: 143, 1997 |
|
De Bruin J, Dorland M, Bruinse H et al: Fetal growth retardation as a course of impaired ovarian development. Early Hum Dev 51: 39, 1998 |
|
Anasti J: Premature ovarian failure: An update. Fertil Steril 70: 1, 1998 |
|
Torgerson D, Avenell A, Russell I, Reid D: Factors associated with the onset of menopause in women aged 45–49. Maturitas 19: 83, 1994 |
|
Cramer D, Huijuan X, Harlow B: Family history as a predictor of early menopause. Fertil Steril 64: 740, 1995 |
|
Verkauf B: Myomectomy for fertility enhancement and preservation. Fertil Steril 58: 1, 1992 |
|
Buttram V Jr, Reiter R: Uterine leiomyomata: Etiology, symptomatology, and management. Fertil Steril 36: 433, 1981 |
|
Goldenberg M, Sivan E, Sharabi Z et al: Outcome of hysteroscopic resection of submucous myomas for infertility. Fertil Steril 64: 714, 1995 |
|
Sudik R, Husch K, Steller J, Duame E: Fertility and pregnancy outcome after myomectomy in sterility patients. Eur J Obstet Gynecol 65: 209, 1996 |
|
Darai E, Dechaud H, Benifla J et al: Fertility after laparoscopic myomectomy: Preliminary results. Hum Reprod 12: 1931, 1997 |
|
Eldar-Geva T, Meagher S, Healy D et al: Effect of intramural, subserosal, and submucosal uterine fibroids on the outcome of assisted reproductive technology treatment. Fertil Steril 70: 687, 1998 |
|
Olive D, Schwartz L: Endometriosis. N Engl J Med 328: 1759, 1993 |
|
Cramer D: Epidemiology of endometriosis. In Wilson EA (ed): Endometriosis, pp 5–22. New York: Alan R Liss, 1987 |
|
Halme J, Surrey ES: Endometriosis and infertility: The mechanisms involved. In Chadha DR, Buttram VC (eds): Current Concepts in Endometriosis, pp 157–178. New York: Alan R Liss, 1990 |
|
Houston D: Evidence for the risk of pelvic endometriosis by age, race, and socioeconomic status. Epidemiol Rev 6: 167, 1984 |
|
Meigs J: Endometriosis: Etiologic role of marriage, age and parity; conservative treatment. Obstet Gynecol 2: 46, 1953 |
|
Westrom L: Incidence, prevalence, and trends of acute pelvic inflammatory disease and its consequences in industrialized countries. Am J Obstet Gynecol 138: 880, 1980 |
|
Doyle M, DeCherney A, Diamond M: Epidemiology and etiology of ectopic pregnancy. Obstet Gynecol Clin North Am 18: 1, 1991 |
|
Chow W-H, Daling V, Cates WJ et al: Epidemiology of ectopic pregnancy. Epidemiol Rev 9: 70, 1987 |
|
Klein R, Klein B, Moss S et al: Prevalence of diabetes mellitus in southern Wisconsin. Am J Epidemiol 119: 54, 1984 |
|
Keiding N, Holst C, Green A: Retrospective estimation of diabetes incidence from information in a prevalent population and historical mortality. Am J Epidemiol 130: 588, 1989 |
|
Chang R, Nakamura R, Judd H, Kaplan S: Insulin resistance in non-obese patients with polycystic ovarian disease. J Clin Endocrinol Metab 57: 356, 1983 |
|
Buyalos R, Geffner M, Bersch N et al: Insulin and insulin-like growth factor-I responsiveness in polycystic ovarian syndrome. Fertil Steril 57: 796, 1992 |
|
Tunbridge W, Caldwell G: The epidemiology of thyroid diseases. In Braverman L, Utiger R (eds): Werner and Ingbar's the Thyroid: A Fundamental and Clinical Text, pp 578–587. 6th ed. Philadelphia: JB Lippincott, 1991 |
|
Robuschi G, Safran M, Braverman L et al: Hypothyroidism in the elderly. Endocr Rev 8: 142, 1987 |
|
Thomas R, Reid R: Thyroid disease and reproductive dysfunction: A review. Obstet Gynecol 70: 789, 1987 |
|
Wilansky D, Greisman B: Early hypothyroidism in patients with menorrhagia. Am J Obstet Gynecol 160: 673, 1989 |
|
Sharara F, Seifer D, Flaws J: Environmental toxicants and female reproduction. Fertil Steril 70: 613, 1998 |
|
National Academy of Sciences Steering Committee on Identification of Toxic and Potentially Toxic Chemicals for Consideration by the Toxicology Program, Board on Toxicology and Environmental Health Hazards, Commission on Life Sciences, National Research Council Toxicity Testing: Strategies to Determine Needs and Priorities, Washington, D.C., 1984 |
|
Fielding J: Smoking and women: Tragedy of the majority. N Engl J Med 317: 1343, 1987 |
|
Van Voorhis B, Dawson J, Stovall D et al: The effects of smoking on ovarian function and fertility during assisted reproduction cycles. Obstet Gynecol 88: 785, 1996 |
|
Hughes E, Brennan B: Does cigarette smoking impair natural or assisted fecundity? Fertil Steril 66: 679, 1996 |
|
Weinberg C, Wilcox A, Baird D: Reduced fecundability in women with prenatal exposure to cigarette smoking. Am J Epidemiol 129: 1072, 1989. |
|
Cooper G, Baird D, Hulka B et al: Follicle-stimulating hormone concentrations in relation to active and passive smoking. Obstet Gynecol 85: 407, 1995 |
|
Sharara F, Beatse S, Leonardi M et al: Cigarette smoking accelerates the development of diminished ovarian reserve as evidenced by the clomiphene citrate challenge test. Fertil Steril 62: 257, 1994 |
|
Adeno M, Gallagher H: Cigarette smoking and the age of menopause. Ann Hum Biol 9: 121, 1982 |
|
Howell S, Shalet S: Gonadal damage from chemotherapy and radiotherapy. Endocrinol Metab Clin North Am 27: 927, 1998 |
|
Counts D, Pescovitz O, Barnes K et al: Dissociation of adrenarche and gonadarche in precocious puberty and in isolated hypogonadotropic hypogonadism. J Clin Endocrinol Metab 64: 1174, 1987 |
|
Sulcova J, Hill M, Hampl R, Starka L: Age and sex related differences in serum levels of unconjugated dehydroepiandrosterone and its sulphate in normal subjects. J Endocrinol 154: 57, 1997 |
|
Crilly R, Marshall D, Nordin B: Effect of age on plasma androstenedione concentration in oopherectomized women. Clin Endocrinol 10: 199, 1979 |
|
Zumoff B, Strain G, Miller L, Rosner W: Twenty-four-hour mean plasma testosterone concentration declines with age in normal premenopausal women. J Clin Endocrinol Metab 80: 1429, 1995 |
|
Sherman B, Wysham C, Pfohl B: Age-related changes in the circadian rhythm of plasma cortisol in man. J Clin Endocrinol Metab 61: 439, 1985 |
|
Blichert-Toft M, Blichert-Toft B, Jensen H: Pituitaryadrenocortical stimulation in the aged as reflected in levels of plasma cortisol and compound S. Acta Chir Scand 136: 665, 1970 |
|
Purifoy F, Koopmans L, Tatum R: Steroid hormones and aging: Free testosterone, testosterone and androstenedione in normal females aged 20–87 years. Hum Biol 52: 181, 1980 |
|
Longcope C, Franz C, Morello C et al: Steroid and gonadotropin levels in women during the perimenopausal years. Maturitas 8: 189, 1986 |
|
Zumoff B, Rosenfeld R, Strain G et al: Sex differences in the twenty-four-hour mean plasma concentrations of dehydroisoandrosterone (DHA) and dehydroisoandrosterone sulfate (DHAS) and the DHA to DHAS ratio in normal adults. J Clin Endocrinol Metab 51: 330, 1980 |
|
Rannevik G, Carlstrom K, Jeppson S et al: A prospective long-term study in women from pre-menopause to post-menopause: Changing profiles of gonadotrophins, oestrogens, and androgens. Maturitas 8: 297, 1986 |
|
Orentreich N, Brind J, Rizer R, Vogelman J: Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab 59: 551, 1984 |
|
Bird C, Tremblay J, Masters V, Clark A: Δ5-androstenediol: Kinetics of metabolism and binding to plasma proteins in normal post-menopausal women. Acta Endocrinol 99: 309, 1982 |
|
Longcope C, Jaffee W, Griffing G: Metabolic clearance rates of androgens and oestrogens in aging women. Maturitas 2: 283, 1980 |
|
Longcope C, Bourget C, Flood C: The production and aromatization of dehydroepiandrosterone in postmenopausal women. Maturitas 4: 325, 1982 |
|
Longcope C: Androgen and estrogen conversion ratios in aging women. Maturitas 2: 13, 1979 |
|
Meldrum D, Davidson B, Tartaryn I, Judd H: Changes in circulating steroids with aging in postmenopausal women. Obstet Gynecol 7: 624, 1981 |
|
Parker L, Lifrak E, Ramadan, Lai M: Aging and the human zona reticularis. Arch Androl 10: 17, 1983 |
|
Metcalf M, Livesey J. Gonadotropin excretion in fertile women: Effect of age and the onset of the menopausal transition. J Endocrinol 105: 357, 1985 |
|
Sherman B, West J, Korenman S: The menopausal transition: Analysis of LH, FSH, estradiol, and progesterone concentrations during menstrual cycles of older women. J Clin Endocrinol Metab 42: 629, 1976 |
|
Wildt L, Hausler A, Marshall G et al: Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology 109: 376, 1981 |
|
Klein N, Battaglia D, Clifton D et al: The gonadotropin secretion pattern in normal women of advanced reproductive age in relation to the monotropic FSH rise. J Soc Gynecol Invest 3: 27, 1996 |
|
Nippoldt T, Reame N, Kelch R, Marshall J: The roles of estradiol and progesterone in decreasing luteinizing hormone pulse frequency in the luteal phase of the menstrual cycle. J Clin Endocrinol Metab 69: 67, 1989 |
|
Alexander S, Askel S, Hazelton J et al: The effect of aging on hypothalamic function in oopherectomized women. Am J Obstet Gynecol 162: 446, 1990 |
|
Parker C Jr, Porter J: Luteinizing hormone-releasing hormone and thyrotropin-releasing hormone in the hypothalamus of women: Effects of age and reproductive status. J Clin Endocrinol Metab 58: 488, 1984 |
|
Klein N, Battaglia D, Fujimoto V et al: Reproductive aging: Accelerated ovarian follicular development associated with a monotropic follicle-stimulating hormone rise in normal older women. J Clin Endocrinol Metab 81: 1038, 1996 |
|
Treolar A, Boynton R, Behn B, Brown B: Variation of the human menstrual cycle through reproductive life. Int J Fertil 12: 77, 1967 |
|
Lenton E, Landgren B, Sexton L, Harper R: Normal variation in the length of the follicular phase of the menstrual cycle: Effect of chronological age. Br J Obstet Gynaecol 91: 681, 1984 |
|
Odell W, Swerdloff R: Progesterone-induced luteinizing and follicle-stimulating hormone surge in postmenopausal women: A stimulated ovulatory peak. Proc Natl Acad Sci USA 61: 529, 1968 |
|
Tashima T, Kitamoto T, Tateishi J et al: Incidence and characterization of age related amyloid deposits in the human anterior pituitary gland. Virchows Arch A 412: 323, 1988 |
|
Greenberg S: The pathogenesis of hypophyseal fibrosis in aging: Its relationship to tissue iron deposition. J Gerontol 30: 531, 1975 |
|
Danforth D, Arbogast L, Mroueh J et al: Dimeric inhibin: A direct marker of ovarian aging. Fertil Steril 70: 119, 1998 |
|
Klein N, Illingworth P, Groome N et al: Decreased inhibin B secretion is associated with the monotropic FSH rise in older, ovulatory women: A study of serum and follicular fluid levels of dimeric inhibin A and B in spontaneous menstrual cycles. J Clin Endocrinol Metab 81: 2742, 1996 |
|
Reame N, Wyman T, Phillips D et al: Net increase in stimulatory input resulting from a decrease in inhibin B and an increase in activin A may contribute in part to the rise in follicular phase follicle-stimulating hormone of aging cycling women. J Clin Endocrinol Metab 83: 3302, 1998 |
|
Macintosh M: Trends in ectopic pregnancy in New Zealand. Aust N Z J Obstet Gynaecol 26: 145, 1986 |
|
Westrom L, Bengtsson L, Mardh P: Incidence, trends and risks of ectopic pregnancy in a population of women. Br J Obstet Gynecol 282: 15, 1981 |
|
Makinen J, Erkkola R, Laippala P: Causes of the increase in the incidence of ectopic pregnancy. Am J Obstet Gynecol 160: 642, 1989 |
|
Talo A, Pulkkinen M: Electrical activity in the human oviduct during the menstrual cycle. Am J Obstet Gynecol 142: 135, 1982 |
|
Pulkkinen M, Jaakkola U: Low serum progesterone levels and tubal dysfunction—A possible cause of ectopic pregnancy. Am J Obstet Gynecol 161: 934, 1989 |
|
Lindblom B, Hamberger L, Ljung B: Contractile patterns of isolated oviductal smooth muscle under different hormonal conditions. Fertil Steril 33: 283, 1980 |
|
Helm G, Owman C, Sjoberg N, Walles B: Motor activity of the human fallopian tube in vitro in relation to plasma concentration of estradiol and progesterone, and the influence of noradrenaline. J Reprod Fertil 64: 233, 1982 |
|
Milwidsky A, Segal S, Menashe M et al: Corpus luteum function in ectopic pregnancy. Int J Fertil 29: 244, 1984 |
|
Goswamy R, Williams G, Steptoe P: Decreased uterine perfusion: A cause of infertility. Hum Reprod 3: 955, 1988 |
|
De Ziegler D, Bessis R, Frydman R: Vascular resistance of uterine arteries: Physiological effects of estradiol and progesterone. Fertil Steril 55: 755, 1991 |
|
Crawford B, Davis J, Harrigill K: Uterine artery atherosclerotic disease: Histologic features and clinical correlation. Obstet Gynecol 90: 210, 1997 |
|
Sterzik K, Dallenbach C, Schnieder V et al: In vitro fertilization: The degree of endometrial insufficiency varies with the type of ovarian stimulation. Fertil Steril 50: 457, 1988 |
|
Noci I, Borri P, Chieffi O et al: Aging of the human endometrium: A basic morphological and immunohistochemical study. Eur J Obstet Gynecol Reprod Biol 63: 181, 1995 |
|
Noci I, Gheri G, Bryk S et al: Aging of the human endometrium: Peri-implantation phase endometrium does not show any age-dependent variation in lectin binding. Eur J Obstet Gynecol Reprod Biol 64: 11, 1996 |
|
Batista M, Cartledge T, Zellmer A et al: Effects of aging on menstrual cycle hormones and endometrial maturation. Fertil Steril 64: 492, 1995 |
|
Stolwijk A, Zielhuis G, Sauer M et al: The impact of the woman's age on the success of standard and donor in vitro fertilization. Fertil Steril 67: 702, 1997 |
|
Schattman G, Davis O, Rosenwaks Z: Patient selection and screening for assisted reproductive technology. Infertil Reprod Med Clin North Am 4: 619, 1993 |
|
Rotsztejn D, Asch R: Effect of aging on assisted reproductive technologies (ART): Experience from egg donation. Semin Reprod Endocrinol 9: 272, 1991 |
|
Sauer M, Macaso T, Hernandez M: Establishment of a nonanonymous donor oocyte program: Preliminary experience at the University of Southern California. Fertil Steril 52: 433, 1989 |
|
Borrero C, Remohi J, Ord T et al: A program of oocyte donation and gamete intrafallopian transfer. Hum Reprod 4: 275, 1989 |
|
Yaron Y, Botchan A, Amit A et al: Endometrial receptivity: The age-related decline in pregnancy rates and the effect of ovarian function. Fertil Steril 60: 314, 1993 |
|
Levran D, Ben-Schlomo I, Dor J et al: Aging of the endometrium and oocytes: Observations on conception and abortion rates in an egg donation model. Fertil Steril 56: 1091, 1991 |
|
Flamigni C, Borini A, Violini F et al: Oocyte donation: Comparison between recipients from different age groups. Hum Reprod 8: 2088, 1993 |
|
Cano F, Simon C, Remohdi J, Pellicer A: Effect of aging on the female reproductive system: Evidence for a role of uterine senescence in the decline in female fecundity. Fertil Steril 64: 584, 1995 |
|
Sauer M, Paulson R, Lobo R: Reversing the natural decline in human fertility: An extended clinical trial of oocyte donation to women of advanced reproductive age. JAMA 268: 1275, 1992 |
|
Navot D, Bergh P, Williams M et al: Poor oocyte quality rather than implantation failure as a cause of age-related decline in female fertility. Lancet 337: 1375, 1991 |
|
Navot D, Drews M, Bergh P et al: Age related decline in female fertility is not due to diminished capacity of the uterus to sustain embryo implantation. Fertil Steril 61: 97, 1994 |
|
Sauer M, Paulson R, Lobo R: A preliminary report on oocyte donation: Extending reproductive potential to women over 40. N Engl J Med 323: 1157, 1990 |
|
Sauer M, Paulson R, Lobo R: Pregnancy after 50: Application of oocyte donation to women after natural menopause. Lancet 341: 321, 1993 |
|
Meldrum D: Female reproductive aging: Ovarian and uterine factors. Fertil Steril 59: 1, 1993 |
|
Paulson R, Hatch I, Lobo R, Sauer M: Cumulative conception and live birth rates after oocyte donation: Implications regarding endometrial receptivity. Hum Reprod 12: 835, 1997 |
|
Legro R, Wong I, Paulson R et al: Recipient's age does not adversely affect pregnancy outcome after oocyte donation. Am J Obstet Gynecol 172: 96, 1995 |
|
Sauer M, Miles R, Dahmoush L et al: Evaluating the effect of age on endometrial responsiveness to hormone replacement therapy: A histologic, ultrasonographic, and tissue receptor analysis. J Assist Reprod Genet 10: 47, 1993 |
|
Seifer D, Berlinsky D: The human granulosa cell model—Lessons gleaned from assisted reproductive technologies. Assist Reprod Rev 3: 49, 1993 |
|
Baker T: A quantitative and cytological study of germ cells in human ovaries. Proc R Soc Lond 158: 417, 1963 |
|
Peters H, Himelstein-Braw R, Faber M: The normal development of the ovary in childhood. Acta Endocrinol 82: 617, 1976 |
|
Adashi E: The ovarian follicular apparatus. In Adashi EY, Rock JA, Rosenwaks Z (eds): Reproductive Endocrinology, Surgery, and Technology, pp 18–40. Philadelphia: Lippincott-Raven, 1996 |
|
Himelstein-Braw R, Byskov A, Peters H, Faber M: Follicular atresia in the infant human ovary. J Reprod Fertil 46: 55, 1976 |
|
Byskov A: Primordial germ cells and regulation of meiosis. In Austin C, Short R (eds): Reproduction in mammals, vol 1, pp 1–16. Cambridge, UK: Cambridge University Press, 1982 |
|
Block E: Quantitative morphological investigations of the follicular system in women: Variations at different ages. Acta Anat 14: 108, 1952 |
|
Franchi L, Mandl A, Zuckerman S: The development of the ovary and the process of oogenesis. In Zuckerman S, Mandl AM, Eckstein P (eds): The Ovary, pp 1–88. London: Academic Press, 1962 |
|
Costoff A, Mahesh V: Primordial follicles with normal oocytes in the ovaries of post-menopausal women. Am Geriatr Soc 23: 193, 1975 |
|
Erickson G: An analysis of follicle development and ovum maturation. Semin Reprod Endocrinol 4: 233, 1986 |
|
Kenigsberg D, Hodgen G: Regulation of ovarian function. In Riddick DH (ed): Reproductive Physiology in Clinical Practice, pp 1–21. New York: Thieme, 1987 |
|
Chiquoine A: The development of the zona pellucida of the mammalian ovum. Am J Anat 106: 149, 1960 |
|
Anderson E, Albertini D: Gap junctions between the oocyte and companion follicle cells in the mammalian ovary. J Cell Biol 71: 680, 1976 |
|
Hodgen G: The dominant ovarian follicle. Fertil Steril 38: 281, 1982 |
|
Gougeon A: Dynamics of follicular growth in humans: A model from preliminary results. Hum Reprod 1: 81, 1986 |
|
Brambilla D, McKinlay S: A prospective study of factors affecting age at menopause. J Clin Epidemiol 42: 1031, 1989 |
|
Faddy M, Gosden R, Gougeon A et al: Accelerated disappearance of ovarian follicles in mid-life: Implications for forecasting menopause. Hum Reprod 7: 1342, 1992 |
|
Gougeon A, Ecochard R, Thalabard J: Age-related changes of the population of human ovarian follicles: Increase in the disappearance rate of non-growing and early-growing follicles in aging women. Biol Reprod 50: 653, 1994 |
|
Rannevik G, Jeppson S, Johnell O et al: A longitudinal study of the perimenopausal transition. Altered profiles of steroid and pituitary hormones: SHBG and bone mineral density. Maturitas 21: 103, 1995 |
|
Gosden R: Follicular status at menopause. Hum Reprod 2: 617, 1987 |
|
Richardson S, Senikas V, Nelson J: Follicular depletion during the menopausal transition: Evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab 65: 1231, 1987 |
|
Tietze C: Introduction to the statistics of abortion. In Engle ET (ed): Pregnancy Wastage. Springfield: CC Thomas, 1953 |
|
Mosher W: Fecundity and fertility in the United States. Am J Public Health 78: 181, 1988 |
|
Hook EB, Cross PK: Interpretation of recent data pertinent to genetic counseling for Down syndrome. In Willey AM, Cater TP, Kelly S, Porter IH, (eds): Clinical Genetics: Problems in Diagnosis and Counseling, pp 119–145. New York: Academic Press, 1982 |
|
Bopp B, Alper M, Thompson I, Mortola J: Success rates with gamete intrafallopian transfer and in vitro fertilization in women of advanced maternal age. Fertil Steril 63: 1278, 1995 |
|
Padilla S, Garcia J: Effect of maternal age and number of in vitro fertilization procedures on pregnancy outcome. Fertil Steril 52: 270, 1989 |
|
Assisted reproductive technology in the United States: 1996 results generated from the American Society for Reproductive Medicine/Society for Assisted Reproductive Technology Registry. Fertil Steril 71:798, 1999 |
|
Toner J, Philput C, Jones G, Muasher S: Basal follicle-stimulating hormone level is a better predictor of in vitro fertilization performance than age. Fertil Steril 55: 784, 1991 |
|
Jacobs S, Metzger D, Dodson W, Haney A: Effect of age in response to human menopausal gonadotropin stimulation. J Clin Endocrinol Metab 71: 1525, 1990 |
|
Piette C, Mouzon J, Bachelot A, Spira A: In vitro fertilization: Influence of woman's age on pregnancy rates. Hum Reprod 5: 56, 1990 |
|
Sharma V, Riddle A, Mason B et al: An analysis of factors influencing the establishment of a clinical pregnancy in an ultrasound based ambulatory in vitro fertilization program. Fertil Steril 49: 468, 1988 |
|
Casron S, Dickey R, Goeial B et al: Outcome in 242 in vitro fertilization embryo replacements of gamete intrafallopian transfer-induced pregnancies. Fertil Steril 51: 644, 1989 |
|
Craft I, Al-Shawaf T, Lewis P et al: Analysis of 1071 GIFT procedures: The case for a flexible approach to treatment. Lancet 1: 1094, 1988 |
|
Hughes E, King C, Wood E: A prospective study of prognostic factors in in vitro fertilization and embryo transfer. Fertil Steril 51: 838, 1989 |
|
Penzias A, Thompson I, Alper M et al: Successful use of gamete intrafallopian transfer does not reverse the decline in fertility in women over 40 years of age. Obstet Gynecol 77: 37, 1991 |
|
Hoffman G, Scott Jr. R, Horowitz G et al: Evaluation of the reproductive performance of women with elevated day 10 progesterone levels during ovarian reserve screening. Fertil Steril 63: 979, 1995 |
|
Scott R Jr, Hofmann G: Prognostic assessment of ovarian reserve. Fertil Steril 63: 1, 1995 |
|
Scott Jr R, Hofmann G, Oehninger S, Muasher S: Intercycle variability of day 3 follicle-stimulating hormone levels and its effect on stimulation quality in in vitro fertilization. Fertil Steril 54: 297, 1990 |
|
Scott R Jr, Toner J, Muasher S et al: Follicle-stimulating hormone levels on cycle day 3 are predictive of in vitro fertilization outcome. Fertil Steril 51: 651, 1989 |
|
Pearlstone A, Fournet N, Gambone J et al: Ovulation induction in women age 40 and older: The importance of basal follicle-stimulating hormone level and chronological age. Fertil Steril 58: 674, 1992 |
|
Lenton E, Sexton L, Lee S et al: Progressive changes in LH and FSH and LH:FSH ratio on women throughout reproductive life. Maturitas 10: 35, 1988 |
|
Cameron I, O'Shea F, Rolland J et al: Occult ovarian failure: A syndrome of infertility, regular menses, and elevated follicle stimulating hormone concentrations. J Clin Endocrinol Metab 67: 1190, 1988 |
|
Licciardi F, Liu H, Rosenwaks Z: Day 3 estradiol serum concentrations as prognosticators of ovarian stimulation response and pregnancy outcome in patients undergoing in vitro fertilization. Fertil Steril 64: 991, 1995 |
|
Smotrich D, Widra E, Gindoff P et al: Prognostic value of day 3 estradiol on in vitro fertilization outcome. Fertil Steril 64: 1136, 1995 |
|
Buyalos R, Daneshmand S, Brzechffa P: Basal estradiol and follicle-stimulating hormone predict fecundity in women of advanced reproductive age undergoing ovulation induction therapy. Fertil Steril 68: 272, 1997 |
|
Navot D, Rosenwaks Z, Margalioth E: Prognostic assessment of female fecundity. Lancet 2: 645, 1987 |
|
Scott R, Leonardi M, Hoffman G et al: A prospective evaluation of clomiphene citrate challenge test screening in the general infertility population. Obstet Gynecol 82: 539, 1993 |
|
Scott R, Opsahl M, Leonardi M et al: Life table analysis of pregnancy rates in a general infertility population relative to ovarian reserve and patient age. Hum Reprod 10: 1706, 1995 |
|
Tanbo T, Dale P, Ludne O et al: Prediction of response to controlled ovarian hyperstimulation: A comparison of basal and clomiphene citrate-stimulated follicle stimulating hormone levels. Fertil Steril 53: 295, 1990 |
|
Ying S-Y: Inhibins, activins, and follistatins: Gonadal proteins modulating the secretion of follicle-stimulating hormone. Endocr Rev 9: 267, 1988 |
|
Hughes E, Robertson D, Handelsman D et al: Inhibin and estradiol responses to ovarian hyperstimulation: Effects of age and predictive value for in vitro fertilization outcome. J Clin Endocrinol Metab 70: 358, 1990 |
|
Buckler H, Evans C, Mamtora H et al: Gonadotropin, steroid, and inhibin levels in women with incipient ovarian failure during anovulatory and ovulatory rebound cycles. J Clin Endocrinol Metab 72: 116, 1991 |
|
Hoffman G, Danforth D, Seifer D: Inhibin-B: The physiologic basis of the clomiphene citrate challenge test for ovarian reserve screening. Fertil Steril 69: 474, 1998 |
|
Seifer D, Lambert-Messerlain G, Hogan J et al: Day 3 serum inhibin-B is predictive of assisted reproductive technologies outcome. Fertil Steril 67: 110, 1997 |
|
Seifer D, Scott R, Bergh P et al: Women with declining ovarian reserve may demonstrate a decrease in day 3 serum inhibin-B prior to a rise in day 3 FSH. Fertil Steril 72: 63, 1999 |
|
Welt C, McNicholl D, Taylor A, Hall J: Female reproductive aging is marked by decreased secretion of dimeric inhibin. J Clin Endocrinol Metab 84: 105, 1999 |
|
Santoro N, Tovaghgol A, Skurnick J: Decreased inhibin tone and increased activin A secretion characterize reproductive aging in women. Fertil Steril 71: 658, 1999 |
|
Padilla S, Bayati J, Garcia J: Prognostic value of early serum estradiol response to leuprolide acetate in in vitro fertilization. Fertil Steril 53: 288, 1990 |
|
Ranieri D, Quinn F, Makhlouf A et al: Simultaneous evaluation of basal follicle-stimulating hormone and 17β-estradiol response to gonadotropin-releasing hormone analogue stimulation: An improved predictor of ovarian reserve. Fertil Steril 70: 227, 1998 |
|
Fanchin R, de Ziegler D, Olivennes F et al: Exogenous follicle stimulating hormone ovarian reserve test (EFFORT): A simple and reliable screening test for detecting “poor responders” in in-vitro fertilization. Hum Reprod 9: 1607, 1994 |
|
Reuss M, Kline J, Santos R et al: Age and the ovarian follicle pool assessed with transvaginal ultrasonography. Am J Obstet Gynecol 174: 624, 1996 |
|
Chang M, Chiang C, Hsieh T et al: Use of the antral follicle count to predict the outcome of assisted reproductive technologies. Fertil Steril 69: 505, 1998 |
|
Syrop C, Willhoite A, Van Voorhis B: Ovarian volume: A novel outcome predictor for assisted reproduction. Fertil Steril 64: 1167, 1995 |
|
Sharara F, McClamrock H: The effect of aging on ovarian volume measurements in infertile women. Obstet Gynecol 94: 57, 1999 |
|
Grifo J, Rosenwaks Z, Cohen J, Munne S: Implantation failure of morphologically normal human embryos is due largely to aneuploidy [Abstract 0–003], p S2. Presented at the 50th Annual Meeting of the American Fertility Society, San Antonio, 1994 |
|
Benadiva C, Kligman I, Munne S: Aneuploidy 16 in human embryos increases significantly with maternal age. Fertil Steril 66: 248, 1996 |
|
Keefe D, Niven-Fairchild T, Powell S, Buradagunta S: Mitochondrial DNA deletions in oocytes and reproductive aging in women. Fertil Steril 64: 577, 1995 |
|
Eppig JJ: Intercommunication between mammalian oocytes and companion somatic cells. Bioessays 13: 569, 1991 |
|
Terranova P: Regulation of the granulosa cell: Growth factor interactions. Semin Reprod Endocrinol 9: 313, 1991 |
|
Eppig JJ: Mammalian oocyte development. In Hillier SG (ed): Ovarian Endocrinology, pp 107–131. Oxford: Blackwell Scientific, 1991 |
|
Shultz RM: Meiotic maturation of mammalian oocytes. In Wasserman P (ed): Elements of Mammalian Fertilization, pp 78–104. Boca Raton: CRC Press, 1991 |
|
O W-S, Robertson D, deKretser D: Inhibin as an oocyte meiotic inhibitor. Mol Cell Endocrinol 62: 307, 1989 |
|
Eppig J, Shultz R, O'Brien M, Chesnel F: Relationship between the developmental programs controlling nuclear and cytoplasmic maturation of mouse oocytes. Dev Biol 164: 1, 1994 |
|
Ducibella T, Buetow J: Competence to undergo normal, fertilization-induced cortical activation develops after metaphase I of meiosis in mouse oocytes. Dev Biol 165: 95, 1994 |
|
Eppig J, Schroeder A, O'Brien M: Developmental capacity of mouse oocytes matured in vitro: Effects of gonadotropic stimulation, follicular origin, and oocyte size. J Reprod Fertil 95: 119, 1992 |
|
Pavlok A, Lucas-Hahn A, Niemann H: Fertilization and developmental competence of bovine oocytes derived from different categories of antral follicles. Mol Reprod Dev 31: 63, 1992 |
|
Vanderhyden B: Species differences in the regulation of cumulus expansion by an oocyte-secreted factor(s). J Reprod Fertil 98: 219, 1993 |
|
Coskun S, Uzumcu M, Lin Y et al: Regulation of cumulus cell steroidogenesis by the porcine oocyte and preliminary characterization of oocyte-produced factor(s). Biol Reprod 53: 668, 1995 |
|
Seifer D, Gardiner A, Ferreira K, Peluso J: Apoptosis as a function of ovarian reserve in women undergoing in vitro fertilization. Fertil Steril 66: 593, 1996 |
|
Chun S, Billig H, Tilly J et al: Gonadotropin suppression of apoptosis in cultured preovulatory follicles: Mediatory role of endogenous insulin-like growth factor I. Endocrinology 135: 1845, 1994 |
|
Luciano A, Pappalardo A, Ray C, Peluso J: Epidermal growth factor inhibits large granulosa cell apoptosis by stimulating progesterone synthesis and regulating the distribution of intracellular free calcium. Biol Reprod 51: 646, 1994 |
|
Pellicer A, Mari M, de los Santos M et al: Effects of aging on the human ovary: The secretion of immunoreactive alpha-inhibin and progesterone. Fertil Steril 61: 663, 1994 |
|
Seifer D, Gardiner A, Lambert-Messerlain G, Schneyer A: Differential secretion of dimeric Inhibin in cultured luteinized granulosa cells as a function of ovarian reserve. J Clin Endocrinol Metab 81: 736, 1996 |
|
Klein N, Battaglia D, Miller P et al: The ovarian follicular fluid environment in reproductive aging: Changes in hormones and growth factors. J Soc Gynecol Invest 2: 141, 1995 |
|
Gaulden ME: Maternal age effect: The enigma of Down syndrome and other trisomic conditions. Mutat Res 296: 69, 1992 |
|
Van Blerkom J: The influence of intrinsic and extrinsic factors on the developmental potential and chromosomal normality of the human oocyte. J Soc Gynecol Invest 3: 3, 1996 |
|
Friedman C, Danforth D, Herbosa-Encarnacion C et al: Follicular fluid vascular endothelial growth factor concentrations are elevated in women of advanced reproductive age undergoing ovulation induction. Fertil Steril 68: 607, 1997 |
|
King K, Cidlowski J: Common pathways to life and death. J Cell Biochem 58: 175, 1995 |
|
Melkrantz W, Schlegel R: Apoptosis and the cell cycle. J Cell Biochem 58: 160, 1995 |
|
Seifer D, Honig J, Penzias A et al: Flow cytometric analysis of deoxyribonucleic acid in human granulosa cells as a function of chronological age and ovulation induction regimen. J Clin Endocrinol Metab 75: 636, 1992 |
|
Seifer D, Charland C, Berlinsky D et al: Proliferative index of luteinized granulosa cells varies as a function of ovarian reserve. Am J Obstet Gynecol 169: 1531, 1993 |
|
Battaglia D, Goodwin P, Klein N, Soules M: Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod 11: 2217, 1996 |
|
Battaglia D, Klein N, Soules M: Changes in centrosomal domains during meiotic maturation in the human oocyte. Mol Hum Reprod 2: 845, 1997 |
|
Angell R: Aneuploidy in older women. Hum Reprod 9: 1199, 1994 |
|
Munne S, Alikani M, Giles T et al: Embryo morphology, developmental rates, and maternal age are correlated with chromosomal abnormalities. Fertil Steril 64: 382, 1995 |
|
Nasseri A, Mukherjee T, Grifo J et al: Elevated day 3 serum follicle stimulating hormone and/or estradiol may predict fetal aneuploidy. Fertil Steril 71: 715, 1999 |
|
Seifer D, Naftolin F: Moving toward an earlier and better understanding of perimenopause. Fertil Steril 69: 387, 1998 |
|
Bianco A, Stone J, Lynch L et al: Pregnancy outcome at age 40 and older. Obstet Gynecol 87: 917, 1996 |
|
Cohen W, Newman L, Friedman E: Risk of labor abnormalities with advancing maternal age. Obstet Gynecol 55: 414, 1980 |
|
Adashek J, Peaceman A, Lopez-Zeno J et al: Factors contributing to the increased cesarean birth rate in older parturient women. Am J Obstet Gynecol 169: 936, 1993 |
|
Fretts R, Usher R: Causes of fetal death in women of advanced maternal age. Obstet Gynecol 89: 40, 1997 |
|
Rochat R, Koonin L, Atrash H et al: Maternal mortality in the United States: Report from the maternal mortality collaborative. Obstet Gynecol 72: 91, 1988 |