Amenorrhea
Authors
INTRODUCTION
Amenorrhea, derived from the Greek words men (month) and rein (to flow), denotes the absence or suppression of menstruation. Amenorrhea is a symptom, not a disease, and it has a variety of causes. Ovulatory menstruation requires the physiologically coordinated function of the hypothalamic-pituitary-ovarian (HPO) axis and a normal uterus and outflow tract. Gross interruption in this relation results in amenorrhea.
The mean age of menarche in American girls is 12.8 years.1 The mean for normal ovulatory menstrual intervals is 28 days, and the range is 21 to 44 days.2 The average age for cessation of menses is 51.4 years.3 About half of all women enter menopause between ages 45 and 50; about 25% of women are less than 45 and 25% are greater than 50.4 Any departure from these norms indicates a change in the normal ovulatory cyclicity and fits the definition of amenorrhea.
Classification of amenorrhea is difficult because a multitude of factors may cause dysfunction. Traditionally, amenorrhea is classified as primary (a patient who has never menstruated) or secondary (a patient who previously had normal menstrual function). This distinction is descriptive and artificial, because the underlying pathophysiologic conditions responsible for primary or secondary amenorrhea may be similar. However, it has some practical clinical value in that it may give a clue to etiology and prognosis. Most congenital anomalies involving genetic aberrations and gross developmental anomalies of either the ovarian or müllerian structures present as primary amenorrhea. Secondary amenorrhea is more likely to result from acquired disease, is generally more amenable to treatment, and has a much better prognosis, at least for the restoration of fertility.
Another, and physiologically more logical, classification is based on the patient's hormonal status. By combining these two classifications, the clinician can arrive at the shortest and most fruitful approach to the problem and its solution.
Rational and successful treatment of amenorrhea depends on a precise diagnosis. In most instances, this can be attained by clinical logic and by simple tests. The laboratory aspects of reproductive endocrinology have developed tremendously. Many of the sophisticated tests, however, are extremely expensive and do not add much of practical diagnostic value. It therefore behooves the physician to choose tests carefully, using only those essential for correct diagnosis and rational treatment.
We will discuss amenorrhea as physiologic, primary, or secondary. Although the proper investigation of a patient with primary or secondary amenorrhea is similar, the most probable diagnoses differ. Although the patient with gonadal dysgenesis usually presents with primary amenorrhea, she can present with prior cyclic menses. In addition, chronic functional anovulation can be of pubertal onset. Therefore, the overlap must not be overlooked.
PHYSIOLOGIC AMENORRHEA
Amenorrhea is normal before puberty, during pregnancy and lactation, and after the menopause. Although there are well-defined mean ages for menarche and menopause, menstrual function may begin or cease at ages other than those accepted as physiologically normal. During the first 1 or 2 years after menarche, about 20% of girls have periods of amenorrhea lasting from 2 to 12 months without any ill effects later in life.5 However, girls with longer periods of amenorrhea associated with estrogen-deficient states have been shown to have reduced bone mineral density and should be considered to be at increased risk for osteoporosis.6 On the other hand, menopause, or ovarian failure, can appear at ages much younger than the minimal age of menopause within the physiologic range.
In any event, when amenorrhea is present in a woman of childbearing age, pregnancy must first be ruled out. In most cases, a routine pregnancy test in conjunction with a pelvic examination is sufficient to confirm or exclude gestation. Occasionally, however, ectopically located trophoblastic tissue proliferation may result in a positive test for human chorionic gonadotropin (hCG). In such cases, the use of sonography and determination of the β-subunit of hCG are aids to a correct diagnosis.
In the breastfeeding woman, the duration of lactational amenorrhea is highly variable, even among similar groups with comparable nursing practices. This variance may be secondary to a different sensitivity of the breast-hypothalamus-pituitary system to suckling or differences in the quality of the suckling stimulus.7,8 A smaller increase in prolactin in response to suckling and higher estradiol levels have been demonstrated in women who ovulate within 6 months postpartum.8 In one study, all women resumed normal ovulation while breastfeeding.9 This emphasizes the need for contraception during lactational amenorrhea.
PRIMARY AMENORRHEA
In young girls, abnormal ovarian function manifests clinically as the lack of, or the delayed appearance of, secondary sexual characteristics or delayed menarche.10 For most young girls, menarche occurs between the ages of 9 and 16, with thelarche and pubarche preceding the onset of menses by about 1 year. Absence of signs of puberty by age 14 suggests delayed puberty or abnormal ovarian function and warrants medical consultation.
Etiology
In general, primary amenorrhea may be caused by gonadal or extragonadal malfunction. A detailed description of the various genetic and developmental aberrations is given in the appropriate chapters. Here, such aberrations will be described only in general terms sufficient for a diagnosis. The etiologic classification of primary amenorrhea is summarized as follows:
- Gonadal abnormalities
- Gonadal dysgenesis (Turner's syndrome)
- Pure gonadal dysgenesis
- XY gonadal dysgenesis (Swyer's syndrome)
- Mixed gonadal dysgenesis
- Ovarian insensitivity syndrome (Savage's syndrome)
- 17 α-hydroxylase deficiency
- Chronic functional anovulation of pubertal onset
- Gonadal dysgenesis (Turner's syndrome)
- Extragonadal anomalies
- Congenital absence of the uterus and vagina
- Male pseudohermaphroditism
- Female pseudohermaphroditism
- Abnormal hypothalamic-pituitary function
- Congenital absence of the uterus and vagina
GONADAL ABNORMALITIES.
In about 60% of patients with primary amenorrhea, the cause is failure of gonadal differentiation or inappropriate gonadal function during early fetal and neonatal development.11 As a result, the external genitalia fail to mature, or they are inappropriate for the genetic sex of the patient. In about 40% of women with primary amenorrhea, the cause is either gonadal dysgenesis or gonadal failure in phenotypic females who are actually genetic males.11 The following is a brief review of the clinical conditions in this category.
Gonadal Dysgenesis (Turner's Syndrome).
Gonadal dysgenesis designates the most common error in fetal gonadal differentiation, with an incidence of about 1 in 2,700 newborn phenotypic females.11
A spectrum of sex chromosome karyotypes ranging from 45,X to 45,X/46,XX and 45,X/46,XY has been described in these patients. Short stature (less than 150 cm tall) is the principal clinical finding. Such anomalies as webbed neck, shield chest, a high arched palate, a low hairline on the neck, and cubitus valgus may or may not accompany the diminished stature. Other anomalies seen include cardiovascular and renal malformations. The gonads are replaced by tenuous streaks of fibrous stroma lacking either follicles or tubules containing germ cells. In most instances, sex chromosomal aberrations are found in karyotypes prepared from cultures of somatic cells from such patients. Because of the possible presence of a Y-chromosome component12 and the risk of malignant degeneration of the streak containing the Y chromosome, it is important to screen all patients with gonadal dysgenesis. Use of currently available fluorescent techniques to identify the Y body and immunologic determination of H-Y antigen may help identify patients at risk for dysgenetic gonadal tumors.13
Pure Gonadal Dysgenesis.
This term is used to distinguish sexually immature girls who appear normal and who are taller than 150 cm from those with typical Turner's syndrome. It encompasses a more heterogeneous group of patients, with different etiologies implicated in ovarian failure. Many of these patients have a 46,XX or 46,XY karyotype. Patients with a normal chromosomal component tend to be tall, because the epiphyses stay open in the absence of ovarian steroids. The gonads are similar to the rudimentary streak gonad seen in Turner's syndrome. The distinction between the two is clinically important, because patients with pure gonadal dysgenesis have a higher incidence of presence of a Y chromosome. The syndrome also occurs more frequently in siblings than does classic Turner's syndrome.14
XY Gonadal Dysgenesis (Swyer's Syndrome).
Affected persons have an XY chromosomal component, but phenotypically they are feminine, with normal female internal and external genitalia and streak ovaries. Because of the high frequency of malignant degeneration, persons with XY forms of gonadal dysgenesis should undergo prophylactic excision of the rudimentary gonads.
Mixed Gonadal Dysgenesis.
This term refers to the rare anomaly of asymmetrical gonadal development, with a germ cell tumor or testis on one side and a streak or no gonad on the other side.15 Such persons usually are mosaics with X/XY karyotypes. They have anomalous external genitalia and exhibit virilization at or after puberty. The Y-containing gonad should be removed as soon as possible.
Ovarian Insensitivity Syndrome (Savage's Syndrome).
Patients with ovarian insensitivity syndrome are normally developed but appear to have functional primary ovarian failure.16 On ovarian biopsy, large numbers of primordial follicles are found. It is speculated that because of an ovarian cell membrane gonadotropin-receptor defect or postreceptor protein defect, the ovary cannot respond to the gonadotropic stimulus.17
The resistant ovary syndrome may be an early stage of ovarian failure in which there is maturation arrest of the follicles that will eventually disappear.18
Hypergonadotropic hypogonadism is characterized by decreased gonadal function due to the inability of the gonads to respond to pituitary gonadotropins. Hypergonadotropic hypogonadism in females has many causes, including the gonadal dysgenesis syndromes discussed above and abnormalities of the ovarian receptors for pituitary gonadotropins. Such an abnormality can be associated with a mutation of the luteinizing hormone (LH) receptor gene, leading to LH resistance as a cause of primary amenorrhea.19 On histologic analysis of the ovary, follicles are seen at all developmental stages, suggesting that in humans LH is necessary for ovulation but follicular maturation can occur in the presence of follicle-stimulating hormone (FSH) alone.
17 a-Hydroxylase Deficiency.
Impairment of 17-hydroxylation, an extremely rare cause of primary amenorrhea, results in failure to produce androgens, estrogens, and some adrenal steroids. As a consequence of this abnormality in steroidogenesis, these patients have increased levels of desoxycorticosterone and progesterone. Clinically, these girls exhibit sexual infantilism, primary amenorrhea with elevated gonadotropins, and hypertension with hypokalemic alkalosis.20 The metabolic derangement responds to glucocorticoid replacement therapy, but ovarian function cannot be restored, probably because the joint action of gonadotropins and estrogens is required for normal follicular maturation.21
EXTRAGONADAL ANOMALIES.
Extragonadal anomalies account for about 40% of primary amenorrhea cases.11 The gonads in these patients usually are normal and functioning. If gonads are nonfunctional because of lack of gonadotropic stimulation, ovulation can be induced with exogenous gonadotropins.
Congenital Absence of the Uterus and Vagina.
When pubertal changes occur at the appropriate age and secondary sexual characteristics have developed normally but menses have not appeared, primary consideration should be given to the genital tract as the locus of the problem. For unknown reasons, in some genetic females the anlagen of the müllerian ducts or the urogenital sinus do not develop or develop abnormally. In such persons, the external genitalia are normal, but the fallopian tubes, uterus, and upper third of the vagina are absent. The direct cause of congenital anomalies of the female genital tract is unknown. Studies have suggested an association of an autosomal dominant gene, polygenic, or multifactorial factors with müllerian agenesis. Environmental factors, including radiation, intrauterine infections, and medications, have also been linked to genetic anomalies. The possible presence of müllerian-inhibitory-like substance early in development has been suggested.22 An immune etiology or an association with collagen vascular disease has also been suggested.23 In cases of aplastic urogenital sinus, the internal genitalia are normal but the lower two thirds of the vagina fails to develop. Various intermediate anomalies also occur. Congenital absence of the uterus and vagina (Mayer-Rokitansky-Kuster-Hauser syndrome) is the most common defect, with an estimated frequency at birth of 1 per 4,000 females.24 In about 10% of these cases, vaginal aplasia is associated with a functionally normal uterus and cervix. Congenital absence of the cervix associated with a functional uterus is extremely rare.25 Müllerian duct agenesis is often associated with urinary tract anomalies and musculoskeletal malformations.26
When the uterus is absent, the most common complaint is primary amenorrhea. Because of normal ovarian function, the physical development and appearance of these girls is normal. Conversely, when the uterus is present and the vagina or parts of it are absent, menarche appears at the normal age, but the menstrual effluent is retained, producing endometriosis and cyclic abdominal pain without external evidence of menses.25
Diagnosis of such conditions is made during physical examination. When the defect consists of an imperforate hymen only, a simple hymenotomy will restore normal menstrual function. When the defects are more extensive, however, the treatment may be more complex. Transverse vaginal septum, resulting from failure of canalization of the distal third of the vagina, is not always recognized.27 It leads to proximal hematocolpos and hematometra. The symptoms are cyclic lower abdominal pain and bladder irritability. Examination shows a firm pelvic mass that varies in size and is not always symmetrical in outline. Vaginal inspection reveals a short vagina and no cervix. The upper vagina may be quite distended. Treatment involves excision of the septum and mobilization of the upper vagina so that it can be connected to the lower vagina or perineum. Occasionally, a split-thickness skin graft is required.
When the uterine cervix is absent in a patient with vaginal anomalies, the treatment of choice is total abdominal hysterectomy, because attempts at plastic reconstruction of a functional vagina and a patent outflow tract have met with little success.25 Although there are several reports in which preservation of the uterus in association with partial or complete vaginal agenesis resulted in a successful pregnancy, evaluating the condition of the cervix is critical before making a definitive surgical plan. The uterus should be removed in women with cervical atresia, but the uterus can be conserved if the cervix is stenotic. In this procedure, a modification of the McIndoe split-thickness skin graft operation can be used to create a neovagina in conjunction with cervical and vaginal stents.28
Male Pseudohermaphroditism.
Pseudohermaphroditism resulting from inadequate or inappropriate masculinization of the urogenital sinus and external genitalia may be caused by enzymatic deficiencies in gonadal or adrenal steroidogenesis. In such persons, incomplete masculinization of the urogenital sinus and external genitalia gives rise to incomplete male pseudohermaphroditism, which may present clinically as primary amenorrhea. The most common cause of this condition is the testicular feminization syndrome, characterized by normal female external genitalia, a blind vaginal canal, absent uterus, sparse to absent pubic and axillary hair, and well-developed breasts with immature nipples and hypopigmented areolae.29 On physical examination, gonads may be palpable in the inguinal canal, or frequently there is a history of inguinal herniorrhaphy. Subjects with testicular feminization have normal or only slightly elevated serum gonadotropin levels and normal male testosterone levels, but because the cytosol receptors for testosterone are defective, testosterone is biologically ineffective.30 Such persons are always raised as females and come to the physician because of primary amenorrhea or infertility.
Other forms of male pseudohermaphroditism associated with various degrees of virilization31 are discussed in detail elsewhere in these volumes.
Female Pseudohermaphroditism.
Extraovarian sources of androgen may virilize the external genitalia in genetically normal females with normal internal genitalia. Congenital adrenal hyperplasia is the most common cause. Excessive adrenal androgen secretion is usually associated with defective cortisol synthesis due to 21-hydroxylase or 11 β-hydroxylase deficiency.32 In female fetuses with such deficiencies, the excessive adrenocortical androgens virilize the external genitalia to various degrees during fetal life. Generally, these infants show symptoms of adrenocortical insufficiency and are diagnosed and appropriately treated during the neonatal period. Elevated 17-hydroxyprogesterone levels in the blood or increased pregnanetriol in the urine confirms the diagnosis. It is most important to examine the nuclear chromatin or karyotype before assigning a sex to the infant. Although some of these patients may menstruate and ovulate, most are amenorrheic or oligomenorrheic. However, when adequately treated, they resume normal menstrual function.32 Other rare causes of androgen overproduction are virilizing adrenal and ovarian tumors.
Abnormal Hypothalamic-Pituitary Function.
Central nervous system and pituitary lesions in preadolescents may be a cause of primary amenorrhea. Failure of the pituitary to secrete sufficient amounts of gonadotropins results in failure of follicular development and maturation, lack of gonadal steroids, and therefore delayed sexual development and amenorrhea. Such failure may result from inadequate hypothalamic stimulation or primary pituitary disease. Usually these conditions are associated with low gonadotropin levels and are therefore called hypogonadotropic hypogonadism. Several well-known syndromes are included in this category. Kallmann's syndrome (olfactogenital dysplasia),33 pituitary dysfunction manifested by isolated gonadotropin deficiency,34 and destruction of the anterior pituitary by neoplasms or metastases all result in hypogonadotropic hypogonadism. Nonpituitary intracranial space-occupying mass lesions are uncommon causes of primary amenorrhea or galactorrhea.35 Craniopharyngiomas account for one fourth of neoplasms in the pituitary regions. These tumors arise from epithelial remnants of Rathke's pouch and can be associated with hypopituitarism and hyperprolactinemia. The tumors may be found within the third ventricle, hypothalamus, and sella. Pituitary adenomas are a more common cause of secondary amenorrhea, and there is an increased incidence in the fourth decade.
Clinical Evaluation
HISTORY.
A detailed history is paramount in evaluating the patient with amenorrhea. Particular attention should be paid to family and developmental history, the patient's prenatal history, ingestion of drugs, and exposure to chemicals and radiation. Menstrual histories of mother, aunts, and sisters should be recorded. The chronologic development of pubertal changes should be carefully noted, because anomalies may point to the diagnosis. For example, normal thelarche and pubarche are seen with müllerian duct aplasia, whereas lack of axillary and pubic hair in conjunction with good breast development suggests testicular feminization. Information about dietary habits and changes in body weight should be obtained through careful questioning. Psychosocial history is of considerable importance because excessive stress may cause amenorrhea.
PHYSICAL EXAMINATION.
Complete physical and pelvic examinations are essential. Careful clinical observations can provide information that may eliminate the need for expensive laboratory tests. Special attention should be paid to evidence of sex steroid activity (e.g., adequate development of the breasts and pubic and axillary hair indicates normal ovarian function and points to an extragonadal cause for amenorrhea). A simplified clinical assessment of the etiology of primary amenorrhea is presented in Table 1.
TABLE 1. Clinical Differential Diagnosis of Primary Amenorrhea
Presence |
|
|
of Uterus | Breast Development | No Breast Development |
Present | Similar to secondary amenorrhea | Hypothalamic amenorrhea |
|
| Pituitary amenorrhea |
|
| Gonadal dysgenesis |
|
| 17α-hydroxylase deficiency (46,XX) |
Absent | Müllerian agenesis | Testicular regression |
| Androgen insensitivity syndrome | 17,20-desmolase deficiency |
|
| 17α-hydroxylase deficiency (46,XY) |
In general, attention should be paid to the following: androgen overproduction, cortisol overproduction, hypothyroidism, galactorrhea, acromegaly, weight changes, short stature with or without associated anomalies such as webbed neck and cubitus valgus, and sexual infantilism. Obvious signs of Turner's syndrome are easily detectable, but in cases of mosaicism the diagnosis may be difficult.36 Detailed clinical attention to the quality of the skin (oiliness, acne, dryness, abnormal pigmentation, or bruises), hair distribution, general nutritional status, size and consistency of the thyroid, breast development and presence of galactorrhea, and quality of the vaginal mucosa and cervical mucus can alert the physician to possible problems.
Additional important information is gained during the pelvic examination, when obvious genital malformations and anomalies can be detected. Because many patients with primary amenorrhea are youngsters who have never been examined gynecologically, the examination should be carried out with as little trauma as possible; if necessary, it should be done under anesthesia.
PROGESTERONE WITHDRAWAL TEST.
The progesterone withdrawal test, which indirectly reflects HPO activity, is frequently used to challenge the adequacy of endogenous estrogen production. The progesterone challenge must be performed with a “pure” progesterone devoid of intrinsic estrogenic activity. Usually we use 100 mg of progesterone in oil given intramuscularly, or medroxyprogesterone acetate (Provera), 10 mg/day orally for 5 days. Patients with adequate endogenous estrogens will bleed within 3 to 5 days after medication, indicating adequate endogenous estrogen stimulation of the endometrium. Patients with relatively low levels of endogenous estrogens may have a limited response to the progesterone challenge. If bleeding does not occur, more extensive studies are indicated.
LABORATORY TESTS.
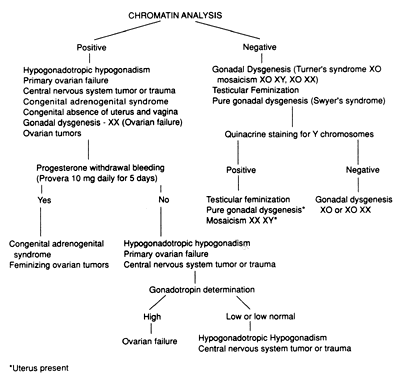
In general, few laboratory tests are needed to establish a diagnosis. Chromatin analysis (buccal smear), karyotyping with quinacrine mustard stain for the presence of a fluorescent chromocenter characteristic of the Y sex chromosome,37 immunologic determination of the H-Y antigen,38 and determination of serum gonadotropins and prolactin by radioimmunoassay are the tests most frequently required. The importance of a karyotype should not be underemphasized. A review of the literature of 33 cytogenetic studies composed of 1,757 phenotypic females with primary amenorrhea revealed a 32% incidence of chromosome abnormalities.22 Figure 1 gives a schematic outline of the laboratory workup.
At the initial examination, a buccal smear or blood karyotype is taken, blood is drawn for LH, FSH, and prolactin determination, and the patient is given progesterone intramuscularly or medroxyprogesterone orally for the progesterone challenge test. If bleeding occurs within 3 to 5 days, the diagnosis is probably anovulation, because bleeding indicates adequate endogenous estrogen levels and activity along the HPO axis. If bleeding does not occur and a uterus is present, gonadotropin levels and chromosome analysis will determine the diagnosis. High LH and FSH levels in the menopausal range indicate ovarian failure, whereas low or low-normal levels indicate hypothalamic or pituitary dysfunction. Patients with high levels of prolactin should have the pituitary evaluated by either magnetic resonance imaging or computed tomography.
The buccal smear is positive in genotypic females and negative in females with only one chromosome and in genotypic males. Chromatin-negative patients should be karyotyped and, if possible, their blood should be tested for the presence of H-Y antigen. In young chromatin-positive patients whose gonadotropins are elevated, karyotyping of several tissues (leukocytes, skin, gonads) may be helpful in determining the exact genetic aberration, because a normal karyotype from one tissue does not exclude the possibility of sex chromosome mosaicism or gonadal dysgenesis. In such cases, laparoscopic examination and ovarian biopsy and histologic and cytogenetic studies may be used to confirm the diagnosis.
A relatively small number of patients with primary amenorrhea have ambiguous external genitalia with considerable clitoromegaly. Karyotyping with quinacrine stain for the Y chromosome is essential in these cases. A Y chromosome indicates male pseudohermaphroditism, mixed gonadal dysgenesis, or true hermaphroditism.39 A final diagnosis can be made only by inspection and biopsy of the gonadal tissue.
In rare instances, ambiguous external genitalia are found in genetically normal females with congenital adrenal hyperplasia.40 Determination of urinary 17-ketosteroids, pregnanetriol, plasma testosterone, and 17-hydroxyprogesterone establishes the diagnosis. The finding of significantly elevated levels of plasma 17-hydroxyprogesterone and urinary pregnanetriol, suppressible with dexamethasone, confirms the diagnosis. With adequate adrenal suppression and plastic surgery of the external genitalia, these women can function normally.41,42
Virilizing ovarian or adrenal tumors that develop before menarche can cause primary amenorrhea and clitoromegaly. Although such tumors are extremely rare, they should be considered in the diagnosis of amenorrhea.
Treatment
Treatment of primary amenorrhea depends on its cause. The aims of treatment are two: to help the patient attain normal feminine physical development and to restore the patient's fertility potential. When the cause of amenorrhea is a gonadal or severe genital anomaly, little can be done to restore fertility. Estrogen replacement therapy will stimulate maturation of the secondary sexual characteristics, but correction of ambiguous genitalia and excision of the Y chromosome-containing gonads is required. In cases of testicular feminization, the gonads should be removed after puberty because they carry a 22% chance of malignancy.39 The possibility of virilization in testicular feminization is negligible due to inherited androgen insensitivity, and the estrogens produced by the gonads are necessary for physiologic pubertal changes. In all other instances in which a Y chromosome is present, gonadectomy should be performed before puberty to prevent virilization and masculinization after puberty, which may be biologically and psychosocially very distressing to a person raised as a female. Surgical correction of the external genitalia to provide functional and socially acceptable genitalia should be undertaken when feasible and required by the patient.
When amenorrhea is due to hypothalamic or pituitary disorders and the ovaries and genital organs are normal, the prognosis for childbearing is excellent. Estrogen replacement therapy will stimulate maturation and development of the secondary sexual characteristics. When pregnancy is desired, ovulation can be induced by gonadotropin therapy or, in hyperprolactinemic states, by bromocriptine.
Advances in in vitro fertilization-embryo transfer (IVF-ET) have expanded the reproductive alternatives for the woman with gonadal dysgenesis or müllerian agenesis. Ovum donation has allowed patients with Turner's syndrome to undergo IVF-ET with partner's sperm and their own uterus. Patients with müllerian agenesis may be candidates for a surrogate uterus. As there appears to be an increased prevalence of chromosomal aberrations in these patients,22 one should consider karyotyping them before an oocyte retrieval for an embryo transfer to a surrogate uterus.
SECONDARY AMENORRHEA
Amenorrhea is defined as secondary when no menses have occurred for 6 months in a woman who previously had normal menstrual function, or for 12 months if her cycles were irregular. Secondary amenorrhea is an end point in a spectrum of pathophysiologic conditions ranging from apparently normal ovarian function to complete absence of ovarian function. In the early stages of ovarian dysfunction, the cycles may become slightly abnormal, progressing to amenorrhea. Generally, women seek medical attention because of amenorrhea for two reasons: concern for fertility and fear that amenorrhea may be a symptom of some serious underlying disease.
In certain cultures, menstruation is regarded as a sign of femininity, and lack of menses may cause emotional and social problems. Because menses per se are not essential for health, after a serious underlying disease has been ruled out, the patient should participate in the decision about the need for further treatment.
Understanding the normal physiology of ovulation and the functional interaction of the various components of the HPO-genital axis is essential to make a correct diagnosis and select the appropriate treatment.
Etiology
Because multiple factors may play a role in the development of secondary amenorrhea, it is difficult to construct a complete etiologic classification. The brief outline given here, based on assessment of ovarian function, enables one to reach a logical diagnosis and outline a treatment.
- Secondary amenorrhea with normal ovarian function
- Asherman's syndrome
- Endometrial destruction (tuberculosis, schistosomiasis, irradiation)
- Asherman's syndrome
- Secondary amenorrhea with decreased ovarian function
- High gonadotropins
- Premature ovarian failure
- Surgical castration
- Radiation castration
- Premature ovarian failure
- Low or normal gonadotropins
- Functional aberrations of the hypothalamic-pituitary axis
- Psychogenic
- Nutritional (starvation, anorexia nervosa)
- Exercise-induced
- Pseudocyesis
- Central nervous system lesions
- Nongonadal endocrine disorders (thyroid, adrenal, pancreas)
- Pharmacologic (postpill, psychotropic drugs, drug addiction)
- Systemic infectious and chronic diseases
- Idiopathic
- Psychogenic
- Neoplastic, vascular, or traumatic central nervous system disease
- Feminizing ovarian tumors
- Functional aberrations of the hypothalamic-pituitary axis
- High gonadotropins
- Secondary amenorrhea with increased ovarian androgen secretion
- Polycystic ovary syndrome
- Masculinizing ovarian tumors
- Polycystic ovary syndrome
The following is a discussion of the principal causes of secondary amenorrhea classified according to ovarian function.
SECONDARY AMENORRHEA WITH NORMAL OVARIAN FUNCTION.
Intrauterine Synechiae (Asherman's Syndrome).
Ovarian function is normal. Amenorrhea is caused by intrauterine adhesions that obliterate the uterine cavity completely or partially. The adhesions are generally the result of an induced abortion or an overzealous postpartum curettage43 complicated by endometritis and intrauterine scarification. Asherman's syndrome has also been reported to follow metroplasty, myomectomy, cesarean section, dilation and curettage, or severe pelvic infection. The diagnosis is made by history, hysterosalpingography, or hysteroscopy. Generally, these patients do not bleed after progesterone or estrogen-progesterone treatment, although after the latter treatment the patient may experience premenstrual molimina. Treatment consists of lysis of the adhesions under direct vision during hysteroscopy. After the uterine cavity is restored, a Foley catheter or Silastic prosthesis is placed inside the uterus to keep the cavity open. Antibiotics are given for 10 days; the patient is also given a 2-month course of estrogen (Premarin), 5 mg/day for 21 days, followed by medroxyprogesterone acetate (Provera), 10 mg/day for 5 days. The Foley catheter (or prosthesis) is removed after 1 week. The American Fertility Society has established a classification of intrauterine adhesions to be used at the time of surgery for uniformity.44
Ninety percent of women report return of normal menses after hysteroscopic adhesiolysis.45 The successful pregnancy rate after treatment is about 35%,46 and 80% of the patients who conceive report a term pregnancy.45
Endometrial Destruction Due to Infection.
Tuberculosis occasionally causes sufficient endometrial scarification to result in amenorrhea. In some instances, destruction of the endometrium produces sclerotic changes without any significant distortion of the intracavitary portion of the uterus on hysterosalpingography. Genital tuberculosis is rare in the United States. Uterine schistosomiasis, seen occasionally in areas endemic for this disease, is another rare cause of secondary amenorrhea.
SECONDARY AMENORRHEA WITH DECREASED OVARIAN FUNCTION.
Secondary Amenorrhea With High Gonadotropin Levels (Premature Ovarian Failure).
Although premature ovarian failure is a well-defined cause of amenorrhea, accounting for 10% of cases of secondary amenorrhea,45 in most cases its etiology is unknown. Normally, ovarian failure occurs with menopause, but when it occurs before age 40 it is premature and occurs with an incidence of 0.9%.48 It is characterized by low estrogen levels, markedly elevated gonadotropin levels, and amenorrhea. As a result of reports of the frequent association between premature ovarian failure and autoimmune thyroid disease, an autoimmune mechanism has been suggested as the cause.49 Several autoimmune multiglandular endocrinopathies may occur concomitantly with premature ovarian failure.50 The association of an autoimmune disease or other evidence of autoimmunity such as circulating antibodies may be present in 30% to 50% of premature ovarian failure patients.49 Other etiologies include chromosomal abnormalities, galactosemia, and idiopathic or iatrogenic injury of the gonad.51 Chemotherapy or radiation therapy can result in increased atresia and subsequent premature ovarian failure.
Although this condition has been generally considered irreversible, there are several reports of spontaneous resumption of menstrual cyclicity,50 ovulation,52 and conception with normal pregnancy.52,53,54 Thus, elevated FSH levels cannot be considered as prima facie evidence of irreversible ovarian failure. Some of the premature ovarian failure patients had hormone profiles similar to those of perimenopausal women.52 In patients with premature ovarian failure, a normal pregnancy and delivery can result by fertilization of donor eggs and embryo transfer with synchronization and support of the patient's endometrium with exogenous hormones.55
Secondary Amenorrhea With Low or Normal Gonadotropin Levels.
The group with secondary amenorrhea with low or normal gonadotropin levels includes the majority of patients with hypothalamic-pituitary dysfunction and, generally, the majority of patients seen for secondary amenorrhea. The diagnosis of hypothalamic amenorrhea is usually made by exclusion. Most of these patients have a functional derangement of the feedback mechanisms that control HPO interrelations. Functional hypothalamic amenorrhea can be psychogenic, related to weight loss, or induced by exercise. Recent evidence has established that slowing of the hypothalamic gonadotropin-releasing hormone (GnRH) pulse generator is the underlying cause of ovarian acyclicity in women with functional hypothalamic amenorrhea.56,57,58 This mechanism for inhibition of GnRH pulse frequency appears to involve endogenous opioids or dopamine neurona systems.59,60 Associated neuroendocrine abnormalities in hypothalamic amenorrhea include suppressed secretion of reproductive hormones57 and increased cortisol, growth hormone (GH), and nocturnal melatonin secretion.57,61,62,63
Psychogenic Amenorrhea.
It has long been recognized that psychogenic stress can produce amenorrhea. Although the physiologic mechanisms by which psychic stress affects the menstrual cycle are still unclear, developments in neuroendocrinology suggest possible explanations. In various forms of stress, there is an increased release of corticotropin-releasing hormone (CRH). The effects of stress include a decrease in circulating LH levels due to the effects of CRH on the hypothalamic-pituitary axis.64 Central administration of CRH decreases LH levels, and a synthetic CRH antagonist blocks the inhibitory action of stress on LH levels.64 Psychogenic amenorrhea may occur in association with either acute or chronic emotional stress and may vary in duration from several months to many years. There are no specific diagnostic tests for psychogenic amenorrhea, and the diagnosis is usually made by exclusion. Women with stress-related hypothalamic amenorrhea have been shown to have not only elevated basal plasma cortisol, but a blunted cortisol response to CRH administration.62
Studies support the hypothesis of a cause-and-effect relation between stressful life events and the onset of secondary amenorrhea of the hypogonadotropic subtype. The number, quality, and objective negative impact of stressful life events were higher among this group than in a control group of healthy menstruating women.65
The prognosis for these patients is quite favorable. In many instances, normal menstrual function returns after the psychological problems are overcome. However, when menstrual function does not return spontaneously, ovulation can be induced with clomiphene citrate or gonadotropins, and pregnancy can be achieved.
Weight Loss-Related Amenorrhea.
Adolescent girls on crash diets may cease to menstruate. Such weight loss-related amenorrhea is probably hypothalamic in origin. Sometimes menses return after weight is regained, but frequently the patient remains amenorrheic for years. According to Frisch, a woman's weight, or more precisely the ratio between body fat and lean mass, plays a critical role in the onset and maintenance of menses; once this ratio is altered, amenorrhea develops.66 In this study, Frisch showed that a minimum of 17% body fat was required for the onset of menarche and 22% body fat for maintenance or resumption of normal menstrual function. However, discrepancies can occur when calculating body fat, because height and weight alone do not accurately reflect lean muscle mass. Using several methods to estimate body fat, a 1991 study debated low body fat as a direct cause of amenorrhea.67 The mechanism by which acute weight loss affects hypothalamic-pituitary function is unknown.
The extreme of this condition, anorexia nervosa, is psychogenic in origin. This condition, usually seen in women under 25 years of age, is characterized by emaciation, amenorrhea, constipation, hypothermia, bradycardia, low blood gonadotropin levels, and other endocrine and hematologic abnormalities.68 These persons have inappropriate perceptions of their body image and exhibit a distorted attitude toward eating and weight gain.
Gonadotropin levels are low to low-normal. Patients exhibit failure of pulsatile LH output, especially during sleep,69 and a blunted LH response to GnRH stimulation.70 The adrenocorticotropic hormone (ACTH)-adrenal axis is similarly altered by decreased pulsatile ACTH output, and there is some loss of diurnal variation in plasma cortisol levels, which are elevated. There is a marked decrease in the conversion of estradiol to estriol and an increase in the conversion of estradiol to catecholestrogens; in obese patients, the reciprocal changes in estrogen metabolism are observed.69 This provides strong evidence that the alteration in estradiol metabolism in anorexia nervosa is not specific to this disorder but is related to body fat and nutrition. Catecholestrogen can induce a hypothalamic suppression of gonadotropin output,71 which may be responsible for the low level of gonadotropins observed in anorexia nervosa. Underweight patients diagnosed with anorexia nervosa exhibit a blunted pituitary response to CRH stimulation tests.72 In addition, analysis of cerebrospinal fluid shows an increase in CRH in these patients, suggesting a central defect in the physiology of CRH. The response to the CRH stimulation test normalizes with weight gain.
Other physiologic sequelae of this self-induced starvation include the development of osteoporosis.73 Patients with anorexia nervosa have reduced bone density and increased risk for vertebral compression fractures. Although most physiologic consequences of anorexia nervosa are reversible with weight gain, alterations in bone mass may not be so readily reversible. Vigorous exercise in these patients appears to protect bone mass despite estrogen deficiency and low calcium intake.
In these patients with amenorrhea associated with reduced body mass index due to weight loss, the GH-releasing hormone-induced GH response was higher than in healthy control subjects.74
Exercise-Induced Amenorrhea.
The growing involvement of women in sports and athletics, while enhancing general physical fitness, has led to an awareness of certain disorders of menstrual cyclicity as evidenced by delay of menarche, oligomenorrhea, amenorrhea, and abnormalities of luteal phase function.75 Exercise-induced amenorrhea has been attributed to a complex interplay of physical, hormonal, nutritional, psychological, and environmental factors that include the stress of competition, decreased protein consumption, and altered lean/fat ratio. Pure physiologic interpretation is complicated by the frequent accompaniments of weight loss and emotional stress that occur often in persons undergoing physical training and athletic competition. As noted in stress-related amenorrhea, cortisol levels have been demonstrated to be increased in amenorrheic athletes.76 This increase is greater than that in eumenorrheic athletes, which is greater than in nonathletic women.76 This suggests that a person's response to the CRH-cortisol axis is one of the underlying etiologies of exercise-associated amenorrhea. A persistent depression of circulating levels of estrogen, progesterone, LH, and FSH is characteristic of exercise-induced amenorrhea. Vigorous exercise, particularly if compounded by weight loss, can disturb menstrual cyclicity in initially normal untrained women.75 Cross-sectional studies report that 6% to 18% of women who are recreational runners and up to 50% of competitive runners training about 80 miles/week may be amenorrheic. LH pulse frequency can also be decreased, as evidenced in a group of eumenorrheic runners compared with sedentary women.77 It has been shown that amenorrheic athletes resume menstrual function during states of inactivity, despite minimal weight change.78
The direct association of endogenous opioids and exercise-related amenorrhea has been reviewed in several studies, with variable results.79 Many of the studies showed an increase in endorphin at the time of stress. Measurement of peripheral endorphin, however, is not valid, because the control levels and effect on the hypothalamus are pertinent. One study on amenorrheic athletes revealed that naloxone, an opioid receptor antagonist, did not enhance gonadotropin release in amenorrheic athletes.80 The relation between endogenous opioids and exercise remains to be clarified and could lead to a more accurate evaluation of the athlete with amenorrhea.
Physical exertion plays a major role in bone mass and bone remodeling. Surprisingly, bone loss rather than an increase in bone density can result from vigorous exercise in amenorrheic athletes as compared with eumenorrheic athletes.81,82 Thus, although physical activity has a beneficial effect on maintaining bone mineral, this effect can be overridden by other factors associated with long-term or prolonged strenuous activities in these amenorrheic athletes. Warren and co-workers identified a compromise in the exercise-induced increase in bone mass in a stressed bone of amenorrheic dancers.83 The risk of developing osteoporosis and the incidence of stress fractures are further emphasized by the association of scoliosis, stress fractures, and amenorrhea in ballet dancers.84
Pseudocyesis.
Pseudocyesis is one of the most dramatic examples of psychogenic amenorrhea. The patient, overanxious to conceive, develops amenorrhea, morning sickness, abdominal enlargement, breast changes, and even perception of fetal movements. Hormonal studies in individual cases have revealed that the pulsatile pattern of LH and prolactin is markedly elevated, while FSH remains normal.85 Mean basal concentrations of LH, FSH, estrone, and estradiol detected in these patients, particularly the LH/FSH ratio, are similar to those observed in polycystic ovarian disease.86
Postpill Amenorrhea (Hypothalamic Oversuppression Syndrome).
Some women taking oral contraceptives develop amenorrhea when the pills are discontinued. The incidence of postpill amenorrhea is not known, but it is estimated that less than 1% of women using oral contraceptives develop it. There is no relation to the length of time the pill was taken or to the type of pill, although most of the reported cases involved the combined estrogen-progestin preparation. In these women, the hypothalamic-pituitary axis is suppressed by the exogenous steroids and remains so after the pills are discontinued. About 25% of women recover menstrual function spontaneously within several months to several years. In others, amenorrhea may persist for prolonged periods,87,88 but ovulation and pregnancy can be brought about by ovulation-inducing drugs. The use of oral contraceptives has been associated with increased conception delays,89 particularly in older women.90
Central Nervous System Lesions.
Pituitary or parapituitary tumors are the central nervous system lesions most frequently associated with amenorrhea. Galactorrhea is frequently, but not always, seen in patients with pituitary tumors. Because amenorrhea and galactorrhea may precede neurologic symptoms by years, a central nervous system lesion must be considered in any patient with the syndrome of amenorrhea, galactorrhea, and hyperprolactinemia. Although galactorrhea remains the clinical hallmark of this disorder, increased prolactin levels may be present without recognizable clinical symptoms. Only 33% of women with hyperprolactinemia have a history of galactorrhea.91 Some 13% to 20% of amenorrheic patients have elevated prolactin levels,92 but only about 75% of female patients with elevated prolactin levels have galactorrhea. On the other hand, many patients with gross galactorrhea have normal prolactin levels.93 Because pituitary tumors have been found in 50% of patients with significantly elevated prolactin levels,94 determination of prolactin levels is an integral part of the diagnostic procedure.
If a galactorrheic patient has a normal prolactin level, a prolactin-secreting tumor is extremely unlikely unless it is associated with amenorrhea; then, a nonfunctioning or poorly functioning tumor should be excluded. As long as the prolactin level is outside the normal range, a pituitary tumor should be excluded under any circumstances. The likelihood of finding a pituitary adenoma does not correlate with the level of hyperprolactinemia.95
Pituitary tumors or elevated prolactin levels probably interfere with normal hypothalamic-pituitary function. Numerous pharmacologic and pathologic conditions are associated with inappropriate prolactin secretion (Table 2).96 The results are a decrease in the amount or cyclicity of gonadotropin secretion (thus causing amenorrhea) and a decrease in prolactin-inhibiting-factor secretion (thus causing galactorrhea). Recognition that prolactin is a distinct pituitary hormone and development of specific and sensitive radioimmunoassays to measure it have enabled early diagnosis of many such lesions. Hyperprolactinemia associated with amenorrhea and gonadal steroid insufficiency is associated with reduction in bone mass, whereas eumenorrheic hyperprolactinemic patients are not believed to be at increased risk.97 This emphasizes the importance of treating the hyperprolactinemic patient with amenorrhea.
TABLE 2. Conditions Associated with Inappropriate Prolactin Secretion
Pharmacologic Causes | Pathologic Causes |
Estrogen Therapy | Hypothalamic Lesions |
| Craniopharyngioma |
Anesthesia | Glioma |
| Granulomas |
DA receptor Blocking Agents | Histocytosis disease |
Phenothiazines | Sarcoid |
Haloperidol | Tuberculosis |
Metoglopramide | Stalk transection |
Domperidone | Postsurgical or head injury |
Pimozide | Irradiation damage of the hypothalamus |
Sulpiride | Pseudocyesis (functional) |
DA Re-uptake Blocker | Pituitary Tumors |
Nomifensine | Cushing's disease |
CNS-DA Depleting Agents | Acromegaly |
Reserpine | Prolactinoma |
α-methyldopa | Mixed GH, or ACTH- and PRL-secreting adenomas |
Monoamine oxidase inhibitor | “Nonfunctional” adenomas |
Inhibition of DA Turnover | Reflex Causes |
Opiates | Chest wall injury and herpes zoster neuritis |
Stimulation of Serotoninergic System | Upper abdominal surgery |
Amphetamines |
|
Hallucinogens | Hypothyroidism |
Histamine H2-Receptor Antagonists | Renal Failure |
Cimetidine | Ectopic Production |
| Bronchogenic carcinoma |
| Hypernephroma |
DA = dopamine; CNS = central nervous system; GH = growth hormone; ACTH = adrenocorticotropic hormone; PRL = prolactin
(Yen SSC, Jaffe RB [eds]: Reproductive Endocrinology; Physiology, Pathophysiology, and Clinical Management, 3rd ed, p 364. Philadelphia, WB Saunders, 1991)
Nonfunctioning pituitary tumors that do not secrete prolactin may cause amenorrhea. Gummas of the pituitary, tuberculomas, aneurysms of the internal carotid artery, and postpartum pituitary ischemia and necrosis are other causes of pituitary insufficiency and amenorrhea. In addition, neoplastic lesions of the hypothalamus or higher cortical centers may cause amenorrhea, but in most such conditions the neurologic symptoms precede amenorrhea. Nonpituitary intracranial space-occupying mass lesions may also cause hyperprolactinemia by interrupting the flow of prolactin-inhibiting factor to the pituitary.
Amenorrhea With Other Endocrinopathies.
Amenorrhea may occur as a result of systemic endocrinopathies involving an extragonadal gland.5 Abnormalities in thyroid function—either hyperthyroidism or hypothyroidism—may produce amenorrhea; amenorrhea is more often seen in hyperthyroidism with exophthalmos. Adrenal cortical overproduction (Cushing's syndrome) or underproduction (Addison's disease) may also interfere with normal menstrual function. Juvenile diabetes mellitus is associated with amenorrhea in 50% of cases. Generally, in all of these conditions, effective treatment for the basic condition results in return of normal menstrual function.
Drug-Induced Amenorrhea.
Various drugs can induce amenorrhea by virtue of their hypothalamic or central nervous system effects. Phenothiazine derivatives, reserpine, and ganglionic blocking agents affect the hypothalamus and may cause amenorrhea, sometimes associated with galactorrhea. These effects are usually reversible once the drug is discontinued.99 Fewer than 10% of patients have a return of menses if there is associated hormonal evidence of ovarian hypofunction, absent oocytes, or poor follicular maturation. Younger women treated for less time with alkylating agents may have potentially reversible amenorrhea. In a group of patients with early breast cancer treated with adjuvant chemotherapy (cyclophosphamide, methotrexate, and 5-fluorouracil), the incidence of drug-induced amenorrhea was greater in older patients and also required a shorter time to develop.100 Independent of age, number of involved nodes, tumor size, and number of chemotherapy cycles, patients who develop drug-induced amenorrhea during adjuvant therapy have a longer disease-free survival.100 There is no documented increase in congenital anomalies, spontaneous abortions, or pregnancy complications in patients who conceive after chemotherapy.
Amenorrhea is also associated with various types of drug addiction. Medroxyprogesterone acetate suspension (Depo-Provera), used as an injectable, long-acting contraceptive or as a supplementary progestational agent, may also cause prolonged periods of amenorrhea.
Acute and Chronic Disease.
The precise role of general systemic illnesses in the etiology of amenorrhea is not understood. Amenorrhea is often associated with generalized, systemic illnesses such as lung disease, cardiac disease, renal disease with chronic uremia, severe infections, malabsorption syndromes involving poor nutrition, and neoplasms. Amenorrhea in these conditions is probably caused by interference with the hypothalamic-pituitary axis, but it is difficult to determine whether the amenorrhea is directly related to the illness or to the accompanying psychogenic factors, to drugs, or to the general metabolic condition. Once the disease is cured or improved, menses usually return.
Chronic iron overload, secondary to idiopathic hemochromatosis or transfusion therapy with consequent hemosiderosis, can result in both pituitary and gonadal damage with subsequent amenorrhea.101,102 These patients usually have low levels of estradiol, LH, and FSH.
Estrogen-Producing Ovarian Tumors.
Women in the reproductive age range who develop estrogen-producing ovarian tumors may experience menstrual irregularities and periods of amenorrhea. Although such tumors are very rare, they should be considered in the diagnosis.
SECONDARY AMENORRHEA WITH ANDROGEN EXCESS.
Androgen overproduction by either the adrenals or the ovaries may lead to amenorrhea. Adrenal androgen overproduction is seen in congenital adrenal hyperplasia and virilizing adrenal tumors. The former is usually diagnosed in infancy, and, when adequately treated, menstrual function is normal. Virilizing adrenal tumors are quite rare and are treated surgically.
Polycystic Ovary Syndrome.
The major cause of ovarian androgen overproduction is the polycystic ovary syndrome (PCOS). This syndrome is probably caused by derangement of the physiologic relation along the HPO axis. Patients with PCOS have irregular menses, obesity, hirsutism, and in many instances infertility. They are oligo-ovulatory or anovulatory and have oligomenorrhea or amenorrhea. In typical cases, the ovaries are enlarged, with white, thickened capsules beneath which are multiple cystic follicles in various developmental stages. This appearance, however, is not a prerequisite for the diagnosis. PCOS has been reported with Cushing's syndrome, congenital adrenal hyperplasia, and several central nervous system lesions. It has also been seen in amenorrheic women with hyperprolactinemia. Serum LH levels are chronically elevated and characteristically show frequent peaks of low magnitude; FSH levels are lower than normal.103 The constant, tonic LH stimulation of the ovaries results in abnormal follicular stimulation—hence the polycystic appearance—and increased levels of androstenedione, which is converted to estrone and testosterone, accounting for the slightly elevated estrogen levels and hirsutism. Urinary 17-ketosteroids are high-normal or only slightly elevated. There are apparent insulin-binding and postreceptor defects in adipocytes in amenorrheic PCOS subjects.104
Late-Onset Congenital Adrenal Hyperplasia.
Late-onset congenital adrenal hyperplasia is a mild defect in steroidogenesis that can present with hirsutism, menstrual irregularity, or amenorrhea. Nonclassical disease is more prevalent in Eastern European Jews (1 in 30), Hispanics (1 in 40), and Yugoslavs (1 in 50).105 It should be considered in the differential diagnosis of presumed PCOS. The ACTH-stimulating test provides a definitive diagnosis.
Masculinizing Ovarian Tumors.
The initial symptoms of masculinizing ovarian tumors in young women may be amenorrhea followed by defeminization, hirsutism, and virilization. Although these tumors are extremely rare, they must be kept in mind when diagnosing amenorrhea.
Diagnosis
When making a diagnosis, the physician must answer three basic questions. One, do the ovaries secrete normal amounts of estrogens? Two, is the patient ovulatory (i.e., is progesterone present)? Three, do the ovaries secrete normal or abnormal amounts of androgens? In most instances, the answer to these questions can be obtained by simple clinical observations.
A careful history is essential in evaluating the patient with secondary amenorrhea, especially because the physical appearance of most patients is normal. The physician should look for signs of lack of estrogens, androgen overproduction, cortisol overproduction, galactorrhea, hypothyroidism, and weight changes. An accurate basal body temperature chart, vaginal cytology, the quality and quantity of cervical mucus, and a properly timed endometrial biopsy provide sufficient information on the status of estrogenic activity, ovulation, and, indirectly, the HPO axis.
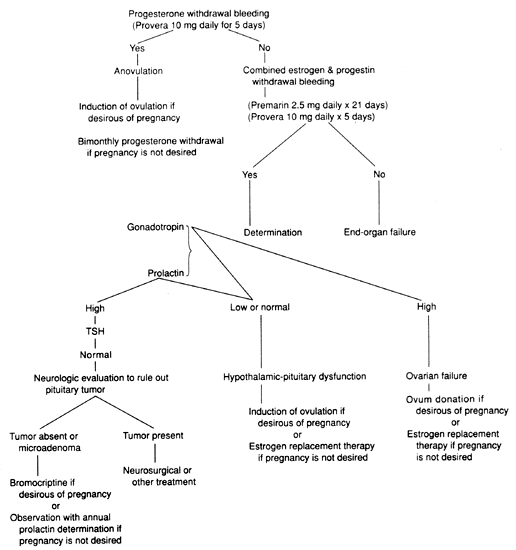
The following simple strategy for investigating amenorrhea, which requires a limited number of tests and in most instances leads to a diagnosis, is appropriate for patients whose initial examination is negative. Figure 2 provides a diagram of the steps in this strategy.
After blood is drawn for determination of LH, FSH, and prolactin, a course of medroxyprogesterone acetate, 10 mg/day orally for 5 to 7 days, or an intramuscular injection of 100 mg progesterone in oil is given. Occurrence of bleeding within 1 week indicates activity along the HPO axis, estrogen production, a responsive endometrium, and a normal outflow tract. The diagnosis is probably anovulation.
Patients who fail to bleed after progestin should be challenged with estrogens and progestin. Failure to bleed after this maneuver suggests that the endometrium is unresponsive, and further studies should be undertaken to detect endometrial pathology (Asherman's syndrome or endometrial destruction). If withdrawal bleeding occurs, the uterus is normal and able to respond to the hormonal challenge, and the defect is along the HPO axis. Determination of gonadotropin and prolactin levels will help establish a diagnosis.
The response to the progestin challenge test can be predicted from the endometrial thickness measured sonographically.106 Endometrial thickness of 6 mm or more predicted the occurrence of withdrawal bleeding with an accuracy of 95.5%. Endometrial thickness was superior to the serum E2 level in predicting withdrawal bleeding.
Measurement of blood or urinary estrogens has little practical value in quantifying endogenous estrogens, nor does a single measurement of a specific estrogen have much value for patient management. In most instances, estrogen levels are low or within the normal range. Unusually high levels of total estrogens or estradiol suggest an estrogen-producing ovarian tumor, but these are extremely rare.
When there is clinical evidence of decreased ovarian secretion, determination of serum gonadotropins is essential. Significantly elevated levels indicate ovarian failure; low or low-normal levels indicate hypothalamic or pituitary failure.
The distinction between ovarian and hypothalamic-pituitary failure is of major diagnostic and prognostic importance. Whereas ovarian failure is usually irreversible, spontaneous recovery is common in hypothalamic-pituitary dysfunction, and even if there is no resumption of menses, restoration of fertility is possible.
The GnRH stimulation test can assess the status of gonadotropin secretion by the pituitary gland. Women with elevated prolactin levels should have magnetic resonance imaging or computed axial tomography of the pituitary gland95 and visual field examination to facilitate the diagnosis of pituitary tumors, because many of these are prolactin-secreting.107,108 Forty percent of patients with hyperprolactinemia have small prolactin-secreting pituitary microadenomas.108 Because the secretion of prolactin by the pituitary is stimulated by estrogen and hyperprolactinemic amenorrhea is associated with subnormal secretion of estradiol by the ovary, clinical evaluation of serum prolactin concentrations must be made in relation to the patient's estrogen status.
Because not all pituitary tumors secrete prolactin, patients with low gonadotropin levels who do not bleed after progesterone should have studies done to rule out intracranial lesions that might compromise hypothalamic-pituitary function without concomitant excessive prolactin secretion.
Treatment
EUESTROGENIC AMENORRHEA.
Even if no serious pathology is uncovered, therapy for euestrogenic amenorrhea is warranted to prevent sequelae of the amenorrheic state. Euestrogenic women are at risk of developing endometrial hyperplasia from chronic unopposed estrogen.109 Therefore, euestrogenic anovulatory women warrant therapy for endometrial protection, which can be achieved by periodic progestin-induced withdrawal bleeding. Progestins given every 6 to 8 weeks will induce withdrawal bleeding at regular intervals. If spontaneous bleeding occurs, the progestin is not taken for 6 to 8 weeks; if another period does not appear, the medication is taken again. Medroxyprogesterone acetate, 5 mg/day for 12 consecutive days, is usually sufficient in euestrogenic women who are not interested in fertility. If the patient is sexually active, oral contraception can be prescribed. Women desirous of pregnancy can be treated with various forms of ovulation induction.
HYPOESTROGENIC AMENORRHEA.
Estrogen decreases bone resorption of calcium, increases intestinal absorption of calcium, and increases renal tubular reabsorption of calcium, all of which promote bone preservation. The manifestations of estrogen deficiency in patients with premature menopause are familiar, but the degree of estrogen deficiency in patients with weight-related amenorrhea may be even more severe, because there is added loss of extraovarian production of estrone. Patients with exercise-induced amenorrhea also have less fat tissue, and weight loss can compound their potential hypoestrogenic state.75,110 Development of osteoporosis and loss of bone density in anorexia nervosa,73 exercise-induced amenorrhea,81,82 and weight loss-related amenorrhea73 provide evidence to standardize estrogen replacement therapy in all hypoestrogenic amenorrheic women regardless of the age at onset. Such women should be treated with daily estrogen, adding a 12- to 14-day course of progestin therapy during the last 10 to 14 days of estrogen. Doses of 0.625 mg of conjugated estrogens and 5 mg of medroxyprogesterone acetate can be given, or other preparations in comparable doses. These patients will then have cyclic menses and receive adequate estrogen replacement. In addition, calcium supplementation is advised. Those desirous of pregnancy can be treated by ovulation induction.
HYPERPROLACTINEMIC AMENORRHEA.
Some women with untreated hyperprolactinemia suffer from such symptoms as dyspareunia, as well as impairment of libido independent of estrogen levels. Hyperprolactinemia is also associated with decreased bone density and development of osteoporosis, primarily secondary to the hypoestrogenic state. Hyperprolactinemia in eumenorrheic women does not appear to lead to a reduction in bone mass.100 The use of bromocriptine both reduces elevated prolactin levels and restores menses in 80% to 90% of women.111 The recent mainstay of therapy to correct infertility associated with hyperprolactinemia is bromocriptine therapy.112 Pituitary microadenomas (i.e., prolactinomas) shrink well on treatment with bromocriptine, which has also been used favorably in treating macroadenomas.113 During pregnancy it is clear that tumor expansion is so rare with small microadenomas that medical suppression is the indicated treatment if needed. Patients with macroadenomas are at greater risk of expansion during pregnancy. When a large pituitary tumor is found, adequate treatment by surgery or irradiation is advised before pregnancy is attempted.114 Once the patient conceives, we discontinue therapy with bromocriptine and reintroduce it only in the rare situation of tumor growth, as assessed by frequent visual field examinations. In general, for the hyperprolactinemic amenorrheic patient, the dose of bromocriptine selected is gradually built up to 2.5 mg three times daily with meals to bring the prolactin level to within the normal range. Individual cases may require more or less bromocriptine to induce ovulation and to normalize prolactin levels.
SUMMARY
Pregnancy is the most common cause of amenorrhea and must be ruled out before proceeding with a diagnostic evaluation. A careful history and physical examination may reveal evidence of androgen excess, estrogen deficiency, or other endocrinopathies. Serum prolactin and thyroid-stimulating hormone (TSH) levels should be checked in all women who are not pregnant. Galactorrhea by history or on examination or an elevated prolactin level should be investigated with an imaging study to rule out a pituitary adenoma. If serum prolactin and TSH levels are normal, a progesterone challenge test should be performed to determine outflow tract patency and estrogen status. In women with hypoestrogenic amenorrhea, indicated by a negative challenge test and a competent outflow tract, serum gonadotropins may be measured to determine whether amenorrhea represents ovarian failure or pituitary or hypothalamic dysfunction. Hypothalamic amenorrhea is common in women with a history of weight loss, stress, or vigorous exercise. GnRH stimulation testing can be useful to differentiate hypothalamic from pituitary central dysfunction.
ADVANCES IN THE MANAGEMENT OF INFERTILITY ASSOCIATED WITH AMENORRHEA
The mainstay of ovulation induction has been clomiphene citrate and human menopausal gonadotropin (HMG)-hCG, especially in clomiphene failures or hypoestrogenic persons.115 Pulsatile GnRH administered by portable pump to induce ovulation and conception has been introduced and has been successful, especially in hypoestrogenic women.116,117 Naltrexone, an opioid antagonist, may have a future role for ovulation induction in functional hypothalamic amenorrhea.118,119 Naltrexone administration has been reported to produce increased gonadotropins and steroid plasma concentrations and to induce ovulation.120,121,122
A purified preparation of urinary FSH can be of value in the treatment of patients with PCOS123 who have had poor success with HMG-hCG or pulsatile GnRH therapy. In addition, pretreatment with GnRH agonists to dampen aberrant endogenous gonadotropin release and then treatment with exogenous gonadotropins offer increased success in the PCOS patient.124
Progress in biotechnology has provided a way to develop recombinant gonadotropins for injection without the impurities associated with urinary preparations. The bioactivities of the new recombinant gonadotropins and current urinary preparations are indistinguishable both in vivo and in vitro.125 However, because of the greater purity of the recombinant products, subcutaneous administration is possible, without the batch-to-batch variability of the urinary products. In the future, it is likely that the recombinant gonadotropins will be used instead of the urinary preparations to induce follicular development.
Fertility treatment in patients with hyperprolactinemia has already been discussed.
Lastly, patients with primary premature ovarian failure should not be automatically considered hopelessly infertile. In addition to spontaneous recovery of fertility,52,53,54 the success of in vitro fertilization with ovum donation53 has made this a viable option.
REFERENCES
Harlan WR, Harlan EA, Grillo GP: Secondary sex characteristics of girls 12 to 17 years of age: The US health examination survey. J Pediatr 96: 1074, 1980 |
|
Ross GT, Cargill CM, Lipsett MB et al: Pituitary and gonadal hormones in women during spontaneous and induced ovulatory cycles. Recent Prog Horm Res 26: 1, 1970 |
|
Jaszmann LJB: Epidemiology of the climacteric syndrome. In Campbell J (ed): The Management of the Menopause and Postmenopausal Years, p 12. Baltimore, University Park, 1976 |
|
Jones HW Jr, Cohen EJ, Wilson RB: Clinical aspects of the menopause, p 3. DHEW Publication No. (NIH) 73, 1972 |
|
Jeffcoate N: Principles of Gynaecology. London, Butterworth, 1975 |
|
Davies MC, Hall ML, Jacobs HS: Bone mineral loss in young women with amenorrhea. Br Med J 301: 790, 1990 |
|
Diaz S, Seron-Ferre M, Cardenas H et al: Circadian variation of basal plasma prolactin, prolactin response to suckling, and length of amenorrhea in nursing women. J Clin Endocrinol Metab 68: 946, 1989 |
|
Diaz S, Cardenas H, Brardeis A: Early difference in the endocrine profile of long and short lactational amenorrhea. J Clin Endocrinol Metab 72: 196, 1989 |
|
Short RV, Lewis PR, Renfree MB et al: Contraceptive effects of extended lactational amenorrhea: Beyond the Bellagio Consensus. Lancet 337: 715, 1991 |
|
Root AW, Reiter EO: Evaluation and management of the child with delayed pubertal development. Fertil Steril 27: 745, 1976 |
|
Williams RH (ed): Textbook of Endocrinology, 5th ed. Philadelphia, WB Saunders, 1974 |
|
McDonough PG, Byrd JR, Tho PT: Phenotypic and cytogenetic findings in 82 patients with primary ovarian failure: Changing trends. Fertil Steril 28: 638, 1977 |
|
Saenger P, Levince LS, Wachtel SS et al: Presence of H-Y antigen and testis in 46,XX true hermaphroditism, evidence for Y-chromosomal function. J Clin Endocrinol Metab 43: 1234, 1976 |
|
McDonough PG, Byrd JR: Gonadal dysgenesis. Clin Obstet Gynecol 20: 565, 1977 |
|
Sohval AR: Mixed gonadal dysgenesis: A variety of hermaphroditism. Am J Hum Genet 15: 155, 1963 |
|
Jones GS, Moraes-Rhusehsen MD: A new syndrome of amenorrhea in association with hypergonadotropism and apparently normal ovarian follicular apparatus. Am J Obstet Gynecol 104: 597, 1969 |
|
Konicnakx PR, Broseus IA: The “gonadotropin-resistant ovary” syndrome as a cause of secondary amenorrhea and infertility. Fertil Steril 28: 926, 1977 |
|
Jewelewicz RJ, Schwartz M: Premature ovarian failure. Bull NY Acad Med 62: 219, 1986 |
|
Toledo S, Brunner H, Kraaij R et al: An inactivating mutation of the luteinizing hormone receptor causes amenorrhea in a 46XX, female. J Clin Endocrinol Metab 81: 3850, 1996 |
|
DeLange WE, Weeke A, Artz W et al: Primary amenorrhea with hypertension due to 17-hydroxylase deficiency. Acta Med Scand 193: 565, 1973 |
|
Ross GT: Gonadotropins and preaantral follicular maturation in women. Fertil Steril 25: 522, 1974 |
|
Jaffe SB, Loucopoulos A, Jewelewicz R: Cytogenetics of Mullerian agenesis. J Reprod Med 37: 242, 1992 |
|
Harger JH, Archor DF, Marchese SG et al: Etiology of recurrent pregnancy losses and outcome of subsequent pregnancies. Obstet Gynecol 62: 574, 1983 |
|
Griffin JE, Edwards C, Madden JD et al: Congenital absence of the vagina: The Mayer-Rokitansky-Kuster-Hauser syndrome. Ann Intern Med 85: 224, 1976 |
|
Niver DH, Barrette G, Jewelewicz R: Congenital atresia of the uterine cervix and vagina: Three cases. Fertil Steril 33: 25, 1980 |
|
Fore SR, Hammond CB, Parker RT et al: Urologic and genital anomalies in patients with congenital absence of vagina. Obstet Gynecol 46: 410, 1975 |
|
Akinkuge A: Vaginal atresia and cryptomenorrhea. Obstet Gynecol 46: 317, 1975 |
|
Bates G, Wiser W: A technique for uterine conservation in adolescents with vaginal agenesis and a functional uterus. Obstet Gynecol 66: 290, 1985 |
|
Morris JM: The syndrome of testicular feminization in male pseudohermaphrodites. Am J Obstet Gynecol 65: 1192, 1953 |
|
Bardin CW, Bullock SP, Sherins RJ et al: Androgen metabolism and mechanism of action in male pseudohermaphroditism: A study of testicular feminization. Recent Prog Horm Res 29: 65, 1973 |
|
Wilson JD, Goldstein JL: Classification of hereditary disorders of sexual development. In Bergsons D (ed): Genetic Forms of Hypogonadism. New York, Grune & Stratton, 1975 |
|
Neu M, Levine L: Congenital adrenal hyperplasia. Pediatr Ann 3: 27, 1974 |
|
Tagatz G, Fialkow PJ, Smith O et al: Hypogonadotropic hypogonadism associated with anosmia in the female. N Engl J Med 283: 1326, 1970 |
|
Spitz IM, Diamant Y, Rosen E et al: Isolated gonadotropin deficiency. N Engl J Med 290: 10, 1974 |
|
Dastur D, Lalitha V: Pathological analysis of intracranial space-occupying lesions in 1000 cases including children: Pituitary adenomas, developmental tumors, parasitic and developmental cysts. J Neurol Sci 4: 397, 1972 |
|
Federman DP: Abnormal Sexual Development, p 42. Philadelphia, WB Saunders, 1967 |
|
Miller OJ, Miller DA, Warburton D: Application of new techniques to the study of human chromosomes. In Steinberg AD, Bearn AD (eds): Progress in Medical Genetics. New York, Grune & Stratton, 1973 |
|
Ohno S: H-Y antigen and chromosomal determination of primary (gonadal) sex. In Ohno S (ed): Major Sex-Determining Genes, p 15. New York, Springer Verlag, 1979 |
|
Jones HW Jr, Scott WW: Hermaphroditism, Genital Anomalies and Related Endocrine Disorders, 2nd ed. Baltimore, Williams & Wilkins, 1971 |
|
Simpson JL, Christakos AC, Horwith M et al: Gonadal dysgenesis in individuals with apparently normal chromosomal complements: Tabulation of cases and compilation of genetic data. Birth Defect 7: 215, 1971 |
|
Satiropoulas A, Morishima A, Hormsy J et al: Long-term assessment of genital reconstruction in female pseudohermaphrodites. J Urol 115: 599, 1976 |
|
Jones HW, Garcia SC, Klingensmith GJ: Secondary surgical treatment of the masculinized external genitalia of patients with virilizing adrenal hyperplasia. Obstet Gynecol 48: 73, 1976 |
|
Asherman JG: Amenorrhea traumatica (atretica). J Obstet Gynaecol Br Emp 55: 23, 1948 |
|
The American Fertility Society classifications of adrenal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, mullerian anomalies and intrauterine adhesions. Fertil Steril 49:944, 1988 |
|
March CM, Israel R: Gestational outcome following hysteroscopic lysis of adhesions. Fertil Steril 36: 455, 1981 |
|
Jewelewicz R, Kahalaf S, Neuwirth RS et al: Obstetrical complications after treatment of intrauterine synechiae (Asherman's syndrome). Obstet Gynecol 47: 701, 1976 |
|
Jacobs H, Knuth U, Hull M et al: Post-“pill” amenorrhea: Cause or coincidence? Br Med J 2: 940, 1977 |
|
Coulam C, Adamson S, Annegers J: Incidence of premature ovarian failure. Obstet Gynecol 67: 604, 1986 |
|
Alper M, Garner P: Premature ovarian failure: Its relationship to autoimmune disease. Obstet Gynecol 66: 27, 1985 |
|
Tan S, Hagne W, Becker F et al: Autoimmune premature ovarian failure with polyendocrinopathy and spontaneous recovery of ovarian follicular activity. Fertil Steril 45: 421, 1986 |
|
Mishell DR Jr, Davajan V: Infertility, Contraception and Reproductive Endocrinology, 2nd ed, p 268. Oradell, NJ, Medical Economics, 1986 |
|
Rebar R, Erickson G, Yen SSC: Idiopathic premature ovarian failure: Clinical and endocrine characteristics. Fertil Steril 37: 35, 1982 |
|
Aiman J, Smentek C: Premature ovarian failure. Obstet Gynecol 66: 9, 1985 |
|
Wright C, Jacobs H: Spontaneous pregnancy in a patient with hypergonadotrophic ovarian failure. Br J Obstet Gynaecol 86: 389, 1979 |
|
Chan CLK, Cameron IT, Findlay JK et al: Oocyte donation and in vitro fertilization for hypergonadotropic hypogonadism. Clinical state of the art. Obstet Gynecol Surv 42: 350, 1987 |
|
Reame NE, Saunder SE, Case GD et al: Pulsatile gonadotropin secretion in women with hypothalamic amenorrhea: Evidence that reduced frequency of gonadotropin secretion is the mechanism of persistent anovulation. J Clin Endocrinol Metab 61: 851, 1985 |
|
Berga SL, Mortola JF, Girton L et al: Neuroendocrine aberrations in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab 68: 301, 1989 |
|
Crowley WF Jr, Filicori M, Spratt D et al: The physiology of gonadotropin-releasing hormone (GnRH) secretion in men and women. Recent Prog Horm Res 41: 473, 1985 |
|
Berga SL, Loucks AB, Rossmanith WG et al: Acceleration of luteinizing hormone pulse frequency in functional hypothalamic amenorrhea by dopaminergic blockade. J Clin Endocrinol Metab 72: 151, 1991 |
|
Liu JH: Hypothalamic amenorrhea: Clinical perspectives, pathophysiology, and management. Am J Obstet Gynecol 163: 1732, 1990 |
|
Suh SY, Liu JH, Berga SL et al: Hypercortisolism in patients with functional hypothalamic amenorrhea. J Clin Endocrinol Metab 66: 733, 1988 |
|
Biller BMK, Federoff HJ, Koenig JI et al: Abnormal cortisol secretion and responses to corticotropin-releasing hormone in women with hypothalamic amenorrhea. J Clin Endocrinol Metab 70: 311, 1990 |
|
Brzenzinski A, Lynch HJ, Seibel MM et al: The circadian rhythm of plasma melatonin during the normal menstrual cycle and in amenorrheic women. J Clin Endocrinol Metab 66: 891, 1988 |
|
Rivier C, Rivier J, Vale W: Stress-induced inhibition of reproductive functions: Role of endogenous corticotropin-releasing factor. Science 231: 607, 1986 |
|
Fioroni L, Fava M, Genazzani A et al: Life events' impact in patients with secondary amenorrhoea. J Psychosom Res 38: 617, 1994 |
|
Frisch RE, McArthur JW: Menstrual cycles: Fatness as a determinant of minimum weight for height necessary for their maintenance or onset. Science 185: 949, 1974 |
|
Ross JS: Perinatal care: The Kuranko context of choice. Health Care for Women International 12: 249, 1991 |
|
Warren MP, Vande Wiele RL: Clinical and metabolic features of anorexia nervosa. Am J Obstet Gynecol 117: 435, 1973 |
|
Fishman J, Boyar RM, Hellman L: Influence of body weight on estradiol metabolism in young women. J Clin Endocrinol Metab 41: 989, 1975 |
|
Warren MP, Jewelewicz R, Dyrenfurth I et al: The significance of weight loss in the evaluation of pituitary response to LH-RH women with secondary amenorrhea. J Clin Endocrinol Metab 40: 601, 1975 |
|
Adashi E, Rakoff J, Divers W et al: The effect of acutely administered 2-hydroxyestrone on the release of gonadotropins and prolactin before and after estrogen priming in hypogonadal women. Life Sci 25: 2051, 1979 |
|
Gold P, Gwirtsman H, Avgerinos P et al: Abnormal hypothalamic-pituitary-adrenal function in anorexia nervosa: Pathophysiologic mechanisms in underweight and weight-corrected patients. N Engl J Med 314: 1335, 1986 |
|
Rigotti N, Nussbaum S, Herzog D et al: Osteoporosis in women with anorexia nervosa. N Engl J Med 311: 1601, 1984 |
|
Genazzani A, Petraglia F, Gastaldi M et al: Growth hormone (GH)-releasing hormone-induced GH response in hypothalamic amenorrhea: Evidence of altered central neuromodulation. Fertil Steril 65: 935, 1996 |
|
Bullen B, Skrinar G, Beitins I et al: Induction of menstrual disorders by strenuous exercise in untrained women. N Engl J Med 312: 1349, 1985 |
|
Ding J-H, Sheckter CB, Drinkwater BL et al: High serum cortisol levels in exercise-associated amenorrhea. Ann Intern Med 108: 530, 1988 |
|
Cumming D, Vickovic M, Wall S et al: Defects in pulsatile LH release in normally menstruating runners. J Clin Endocrinol Metab 60: 810, 1985 |
|
Henley K, Vaitukaitis JL: Exercise-induced menstrual dysfunction. Ann Rev Med 39: 443, 1988 |
|
Sforzo GA: Opioids and exercise: An update. Sports Medicine 7: 109, 1988 |
|
Samuels MH, Sanborn CF, Hofeldt F et al: The role of endogenous opiates in athletic amenorrhea. Fertil Steril 55: 507, 1991 |
|
Drinkwater B, Nilson K, Chestnut C et al: Bone mineral content of amenorrheic and eumenorrheic athletes. N Engl J Med 311: 277, 1984 |
|
Linberg J, Fears W, Hunt M et al: Exercise-induced amenorrhea and bone density. Ann Intern Med 101: 647, 1984 |
|
Warren MP, Brooks-Gunn J, Fox RP et al: Lack of bone accretion and amenorrhea: Evidence for a relative osteopenia in weight-bearing bones. J Clin Endocrinol Metab 72: 847, 1991 |
|
Warren M, Brooks-Gunn J, Hamilton L et al: Scoliosis and fractures in young ballet dancers—relation to delayed menarche and secondary amenorrhea. N Engl J Med 315: 1417, 1986 |
|
Yen SSC, Rebar RW, Quensenberry W: Pituitary function in pseudocyesis. J Clin Endocrinol Metab 43: 132, 1976 |
|
DeVane G, Vera M, Buhi W et al: Opioid peptides in pseudocyesis. Obstet Gynecol 65: 183, 1985 |
|
Dillon RS: Handbook of Endocrinology, p 477. Philadelphia, Lea & Febiger, 1973 |
|
Tyson SE, Andreasson B, Herth J et al: Neuroendocrine dysfunction in galactorrhea-amenorrhea after oral contraceptive use. Obstet Gynecol 46: 1, 1975 |
|
Linn S, Schoenbaum S, Monson R et al: Delay in conception for former pill users. JAMA 247: 629, 1982 |
|
Harlap S, Baras M: Conception waits in fertile women after stopping oral contraceptives. Int J Fertil 29: 73, 1984 |
|
Chang RJ, Keye WR, Young JR et al: Detection, evaluation, and treatment of pituitary microadenomas in patients with galactorrhea and amenorrhea. Am J Obstet Gynecol 128: 356, 1977 |
|
Bergh T, Nillius SJ, Wide L: Hyperprolactinemia in amenorrhea: Incidence and clinical significance. Acta Endocrinol 86: 683, 1977 |
|
L'hermite M, Caufriez A, Vekemans M et al: Pharmacological and pathological aspects of human prolactic secretion. Prog Reprod Biol 2: 244, 1977 |
|
Klinberg DL, Noel GL, Frantz AG: Galactorrhea: A study of 235 cases including 48 with pituitary tumors. N Engl J Med 296: 589, 1977 |
|
Brenner S, Lessing J, Quagliarello J et al: Hyperprolactinemia and associated pituitary prolactinomas. Obstet Gynecol 65: 661, 1985 |
|
Yen SSC, Jaffe RB: Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management, 3rd ed, p 364. Philadelphia, WB Saunders, 1991 |
|
Klibanski A, Biller BMK, Rosenthal DI et al: Effects of prolactin and estrogen deficiency in amenorrheic bone loss. J Clin Endocrinol Metab 67: 124, 1988 |
|
Tolis G, Summa M, Van Campenhout J: Prolactin secretion in 65 patients with galactorrhea. Am J Obstet Gynecol 118: 91, 1974 |
|
Damewood M, Grochow L: Prospects for fertility after chemotherapy or radiation for neoplastic disease. Fertil Steril 45: 443, 1986 |
|
Bianco AR, De Mastro L, Gallo C et al: Prognostic role of amenorrhea induced by adjuvant chemotherapy in premenopausal patients with early breast cancer. Br J Cancer 63: 799, 1991 |
|
De Sanctis V, Vullo C, Katz M et al: Hypothalamic-pituitary-gonadal axis in thalassemic patients with secondary amenorrhea. Obstet Gynecol 72: 643, 1988 |
|
Goldschmidt H, Spiera H, Schumacher HR, Zaroulis CG: Idiopathic hemochromatosis presenting as amenorrhea and arthritis. Am J Med 82: 1057, 1987 |
|
Yen SSC, Vela P: Inappropriate secretion of FSH and LH in polycystic ovarian disease. J Clin Endocrinol Metab 30: 435, 1970 |
|
Marsden P, Murdoch A, Taylor R: Severe impairment of insulin action in adipocytes from amenorrheic subjects with polycystic ovary syndrome. Metabolism 43: 1536, 1994 |
|
Speroff L, Glass RH, Kase NG: Clinical Gynecologic Endocrinology and Infertility, p 391. Baltimore, Williams & Wilkins, 1989 |
|
Nakamuras F, Douchi T, Okit et al: Relationship between sonographic endometrial thickness and progestin-induced withdrawal bleeding. Obstet Gynecol 87: 722, 1996 |
|
Zimmerman CA, Defendini R, Frantz AG: Prolactin and growth hormone in patients with pituitary adenomas: Correlative study of hormone in tumor and plasma by immunoperoxidase technique and radioimmunoassay. J Clin Endocrinol Metab 38: 577, 1974 |
|
Franks S, Jacobs H: Hyperprolactinemia. J Clin Endocrinol Metab 12: 641, 1983 |
|
Coulam C, Annegers J, Kranz J: Chronic anovulation syndrome and associated neoplasia. Obstet Gynecol 61: 403, 1983 |
|
Shangold M: Exercise and amenorrhea. Semin Reprod Endocrinol 3: 35, 1985 |
|
Vance M, Evans W, Thorner M: Bromocriptine. Ann Intern Med 100: 78, 1984 |
|
Molitch M: Pregnancy and the hyperprolactinemic woman. N Engl J Med 312: 1364, 1985 |
|
Molitch M, Elton R, Blackwell R et al: Bromocriptine as primary therapy for prolactin-secreting macroadenomas: Results of a prospective multicenter study. J Clin Endocrinol Metab 60: 698, 1985 |
|
Grossman A, Besser G: Prolactinomas. Br Med J 290: 182, 1985 |
|
Schwartz M, Jewelewicz R: The use of gonadotropins for induction of ovulation. Fertil Steril 35: 3, 1981 |
|
Loucopoulos A, Ferin M, Vande Wiele RL et al: Pulsatile administration of gonadotropin-releasing hormone for induction of ovulation. Am J Obstet Gynecol 148: 895, 1984 |
|
Archer D: Use of luteinizing hormone-releasing hormone for ovulation induction. Semin Reprod Endocrinol 4: 285, 1986 |
|
Remorgida V, Venturini PL, Anserini P et al: Naltrexone in functional hypothalamic amenorrhea and in the normal luteal phase. Obstet Gynecol 76: 1115, 1990 |
|
Wildt L, Leyendecker G: Induction of ovulation by the chronic administration of naltrexone in hypothalamic amenorrhea. J Clin Endocrinol Metab 64: 1334, 1987 |
|
Genazzani A, Gastaldi M, Petraglia F et al: Naltrexone administration modulates the neuroendocrine control of luteinizing hormone secretion in hypothalamic amenorrhoea. Hum Reprod 10: 2868, 1995 |
|
Genazzani A, Petraglia F, Gastaldi M: Naltrexone treatment restores menstrual cycles in patients with weight loss-related amenorrhea. Fertil Steril 64: 951, 1995 |
|
Leyendecker G, Waibel-Treber S, Wildt L: Pulsatile administration of gonadotrophin-releasing hormone and oral administration of naltrexone in hypothalamic amenorrhoea. Hum Reprod 8: 184, 1993 |
|
Polan M, Daniele A, Russell J et al: Ovulation induction with human menopausal gonadotropin compared to human urinary follicle-stimulating hormone results in a significant shift in follicular fluid androgen levels without discernible differences in granulosa luteal cell function. J Clin Endocrinol Metab 63: 1284, 1986 |
|
Filicori M, Ferrari P, Paresch A et al: Gonadotropin-releasing hormone analog (GnRH-a)—induced pituitary desensitization renders polycystic ovarian disease patients more susceptible to pulsatile GnRH to induce ovulation [Abstract 502]. Presented at the 68th Annual Meeting of the Endocrine Society, Anaheim, CA, 1986 |
|
Tarlatzis, B: Development and clinical applications of new gonadotropin preparations in assisted reproduction techniques. Assist Reprod Rev 7: 2, 1997 |