Gestational Trophoblastic Diseases
Authors
INTRODUCTION
The prognosis for patients with choriocarcinoma and related gestational diseases has improved dramatically since the 1950s. Cure rates now exceed 90%, even in patients with metastases.1,2,3,4,5,6,7,8,9 Although systemic chemotherapy has provided the major breakthrough in treatment of these neoplasms, more recent investigators indicate combination chemotherapy and adjunctive use of surgery and irradiation are useful.2,5 More data have accrued to suggest chemotherapeutic toxicity can be dramatically reduced by new drug regimens.3,10 Finally, new agents and approaches are being used with some success in patients whose disease is resistant to more traditional therapy.11,12,13
This presentation deals with trophoblastic diseases of gestational origin only. Histologically similar tumors of nongestational origin, such as primary ovarian or testicular tumors, are omitted because of differences in derivation, treatment, and prognosis. Gestational trophoblastic diseases is used to define the spectrum of disease that has at one extreme “benign” hydatidiform mole (even prior to evacuation) and at the other, the highly malignant choriocarcinoma. Such diseases indeed form a spectrum, and to understand and adequately manage a patient with one of these conditions requires knowledge of the entire group.
HISTORY
Ober14 presented an excellent review of the early history of gestational trophoblastic diseases. These diseases have been known since antiquity and have often been poorly understood. In 400 BC, Hippocrates first described hydatidiform mole as “dropsy of the uterus”, while in AD 600 Aetius of Armida described a uterus “filled with bladderlike objects”, which probably also represented this process. In 1700, Smellie first related the terms hydatid and mole, but it was not until 1827 that Velpeau and Boivin first recognized hydatids as cystic dilations of chorionic villi. Sanger, in 1889, coined the term sarcoma uteri deciduocellulare as a malignant tumor derived from the decidua of pregnancy. In 1895, Marchand demonstrated these tumors to be the sequelae of pregnancy, abortion, or hydatidiform mole and described the proliferation of the syncytium and cytotrophoblast. In 1903, Teacher confirmed Marchand's work and negated Sanger's theory of sarcomatous degeneration of the decidua. Finally, Fels, Ernhart, Reossler, and Zondek demonstrated excessive levels of gonadotropic hormone in the urine of patients with these processes.
More recent advances are reviewed in detail later in this chapter, but even a brief review of the history of trophoblastic disease would not be complete without at least the mention of the major contributors of the past 50 years: Hertz and co-workers in the evolution of chemotherapy; Ross, Lipsett, Delfs, Odell, and Vaitukaitus in the application of newer endocrinologic and chemotherapeutic modalities; Hertig, Sheldon, Gore, Brewer, and Park in the detailed pathologic studies performed; and the many general contributions of Bagshawe, Acosta-Sison, Goldstein, Lewis, Brewer, Lurain, and others to the advancement of diagnostic and therapeutic efforts in these diseases.
CLINICAL PATHOLOGY
The term gestational trophoblastic disease (GTD) encompasses a spectrum of disease entities that are often classified together (Fig. 1). Histologically, these diseases include complete and partial hydatidiform moles, which are not true neoplasms but rather represent pathologic conceptuses. Molar pregnancies may be considered to have a modest malignant potential, since aggressive local proliferation, myometrial invasion, and systemic metastasis can occur as a result of molar pregnancy, and women with molar pregnancies are at increased risk for developing gestational choriocarcinoma. Gestational choriocarcinoma and placental site tumors, on the other hand, are true neoplasms. All of these various disease entities are characterized by focal or diffuse proliferation of trophoblast.15, 16, 17, 18, 19
Trophoblast is derived from the outer cell mass of the preimplantation embryo and has several unique properties. It affects the physical implantation of the embryo into the endometrium and produces human chorionic gonadotropin (hCG) in sufficient amounts to maintain early pregnancy. Normal trophoblast lacks expression of transplantation antigens such as HLA and ABO systems, which allows escape from maternal immunologic rejection.20, 21 Normal trophoblastic tissues are uniquely able to invade into maternal decidua, vessels, and myometrium.22 Furthermore, normal trophoblast continuously embolizes from the endometrial sinuses into the maternal venous system where the trophoblastic cells are filtered by the pulmonary circulation and rarely gain access to the remainder of the systemic circulation.23 These properties of normal trophoblast function are exaggerated in all forms of GTD.
Complete and partial hydatidiform mole, invasive mole, and choriocarcinoma all exhibit proliferation of both cytotrophoblast and syncytiotrophoblast cells that maintain secretion of hCG. In contrast, the placental site tumor may be either a benign or a malignant neoplasm derived from the intermediate cytotrophoblast that usually produces low levels of hCG. A functional understanding of the differences in the pathology of the disease processes that comprise GTD is important, even though clinical management is most often based on the individual patient's clinical presentation and pattern of hCG level changes even in the absence of a firm histopathologic diagnosis.
Detailed histopathologic studies coupled with sophisticated cytogenetic techniques have established the presence of two distinct syndromes of molar disease.24 A complete mole is consistently associated with a totally paternally derived diploid genotype (diandric diploidy), while a partial mole is associated with complete triploidy that incorporates an extra haploid paternal chromosomal complement (diandric triploidy).24 Because there can be no transition between these lesions, which are of distinct cytogenetic origin, the older concept of a “transitional” mole must be considered invalid. It is important to recognize distinctions between these two molar syndromes, since they are associated with distinct clinical presentations and different risks for the subsequent development of malignant sequelae (Table 1).
Table 1. Comparison of partial versus complete hydatidiform mole
| Complete Mole | Partial Mole |
Pathology |
|
|
Hydropic villi | Diffuse, often marked | Focal, variable |
Trophoblastic proliferation | Diffuse, variable | Focal, slight |
Fetus, amnion, fetal red blood cells | Absent | Present |
Cytogenetic Analysis | Diploidy; 46,XX most common | Triploidy; 69,XXX most common |
| Paternal origin | Paternal and maternal origin |
Clinical Features |
|
|
“Mole” clinical/ultrasound diagnosis | Common | Rare |
Uterus large for dates | 25–50% | Rare |
Theca lutein cysts | 25–35% | Rare |
Malignant sequelae | 6–32% | <10% |
| Up to one third metastatic | Nonmetastatic |
Compiled from multiple sources.24, 25, 26, 27
Complete hydatidiform mole
A classic triad of histologic features is present in complete hydatidiform mole and is usually easily recognized if sufficient tissue has been sampled. Features diagnostic of a complete mole include (1) generalized diffuse hyperplasia of both cytotrophoblast and syncytiotrophoblast elements; (2) generalized edema of the chorionic villi, including central cistern formation, which results in the macroscopic description of hydatidiform mole characterized by a mass resembling a “bunch of grapes” (Fig. 2); and (3) absence of an embryo, which resorbs before development of the cardiovascular system at an embryonic length of less than 1 mm (Fig. 3).24, 25, 26 Since fetal death and resorption occurs before development of a fetal circulation, fetal vessels generally degenerate soon after formation and no nucleated fetal erythrocytes are observed in the villous capillaries (see Table 1).
|
There is marked variability from patient to patient in the amount of trophoblastic proliferation and cytologic atypia of trophoblast (Fig. 4 and Fig. 5). Although systems have been designed for categorizing the amount of trophoblastic proliferation and attempting to relate this to clinical behavior after molar evacuation,28 several studies have documented that these do not give a reliable guide to the clinical behavior of a mole for an individual patient; therefore, management is based on the pattern of hCG regression after molar evacuation.
|
Invasive hydatidiform mole
Invasive hydatidiform moles are characterized by pathologic features of complete hydatidiform mole in conjunction with invasion beyond the normal placentation site directly into the myometrium,24, 29 often penetrating into the venous system (Fig. 6). Venous metastasis, most frequently to the lower genital tract and lungs, can result. The histologic diagnosis of invasive mole is rarely made now, because most patients with hydatidiform mole are treated by uterine curettage for diagnosis or bleeding and receive treatment based on hCG level regression criteria without resorting to hysterectomy.24, 29 The diagnosis of myometrial invasion is extremely difficult to make on the basis of uterine curettings. Occasionally, noninvasive imaging can suggest the diagnosis of invasive hydatidiform mole. Invasive moles often produce symptoms of uterine subinvolution and bleeding. Local penetration through the myometrium may result in uterine rupture or massive intraperitoneal hemorrhage (Fig. 7). Distant metastasis may result in pulmonary symptoms or hemorrhage from lower genital implants. Although the invasive mole acts clinically in a manner similar to malignancy, it should not be considered an intermediate entity between hydatidiform mole and choriocarcinoma; the natural history of invasive mole does include spontaneous remission.24, 29 Chemotherapy is used to treat the majority of patients who have a rising hCG level after evacuation of hydatidiform mole without distinguishing those patients who have invasive moles from those with choriocarcinoma. Treatment is given to prevent complications from both of these disease entities.
Partial hydatidiform mole
As discussed subsequently, the cytogenics of complete and partial hydatidiform moles have established that partial hydatidiform moles are usually derived from a triploid conceptus whose placenta is characterized by focal, variable hydropic villi and usually by focal, slight trophoblastic hyperplasia.24, 25, 26, 27 Other histologic features include scalloping of the villi by trophoblastic inclusions within chorionic villi. The embryo survives much longer than in complete moles, with embryonic deaths typically occurring at or before approximately 8 weeks of gestational age. Frequently there is macroscopic or microscopic evidence of a fetus (Fig. 8).24, 25, 26, 27 Fetal vessels are most often identified and usually contain nucleated fetal erythrocytes. The histologic features may vary, depending on the gestational age of evacuation of a partial hydatidiform mole. Hydropic change progresses in severity throughout gestation. Because of the focal nature of the hydropic changes and trophoblastic proliferation, multiple tissue sections must often be obtained to make the diagnosis of partial hydatidiform mole.
Because triploidy accounts for 1–2% of clinically apparent abortions with a large proportion of these being diandric partial moles, the diagnosis of partial hydatidiform mole should be made more frequently than that of complete hydatidiform mole. However, this has not been the case. A comparison of retrospective and prospective studies confirmed that partial hydatidiform moles are frequently underdiagnosed.30
As discussed subsequently, the focal trophoblastic proliferation and hydropic change observed in partial hydatidiform moles account for the different clinical presentation of partial hydatidiform mole as compared with complete hydatidiform mole and for the decreased risk of malignant sequelae observed in populations of these patients (see Table 1).
Cytogenetics of complete and partial molar syndromes
Since the different genetic constituents of complete and partial mole are established at conception, the current understanding of their genetics does not allow recognition of a “transitional” mole (see Table 1).24
It has been established that complete moles result from diandric diploidy; the egg is usually fertilized by a single sperm and loses the maternal haploid 23,X genetic component by an unknown mechanism. If the paternal haploid set of 23,X chromosomes is reduplicated, the normal component of 46 chromosomes is re-established. Zygotes with a 46,YY genotype are nonviable and not clinically recognized.24 Approximately 5% of complete moles apparently arise from dispermic fertilization of an empty egg, which can result in either a 46,XY or 46,XX genotype.31, 32 Although some studies have suggested that dispermic moles are more prone to develop malignant sequelae, this conclusion has not been statistically validated.33 The issue is further confused by the failure to separate invasive moles and choriocarcinomas in these studies because of the treatment of patients without regard to histologic diagnosis.
Partial moles most often result from dispermic fertilization of an egg with the retention of the maternal haploid set, resulting in diandric triploidy.30, 34 For unknown reasons, 69,XYY partial moles are encountered very rarely, with a ratio of approximately 2:3 for 69,XXX to 69,XXY partial moles.24 Tetraploid partial moles consisting of three paternal and one maternal haploid sets have also been described.24
It is possible that the persistence of the maternal haploid chromosomal complement in partial hydatidiform mole produces attenuation of the complete mole syndrome, resulting in the focal distribution of hydropic villi and trophoblastic hyperplasia as well as in the delay in fetal demise and a milder clinical course.
Gestational choriocarcinoma
Choriocarcinoma is characterized by a dimorphic population of cytotrophoblast and syncytiotrophoblast elements (Fig. 9 and Fig. 10).35 Varying amounts of pleomorphism and anaplasia are present; in the absence of clinical history, the histopathology may be misinterpreted in biopsy specimens of metastatic sites as undifferentiated carcinoma or sarcoma. Chorionic villi are not present, even in primary uterine sites of disease. If villous structures are identified in metastatic deposits, the histologic diagnosis is invasive mole.
|
Gestational choriocarcinoma, similar to other forms of normal and abnormal trophoblast, readily invades into blood vessels, producing metastasis through hematogenous routes of dissemination. The embolic metastatic sites have a tendency to rapidly outgrow their blood supply, producing central necrosis, which can result in massive local hemorrhage.35 Secretion of hCG is retained by the vast majority of gestational choriocarcinomas, because the tumor is derived from elements of both cytotrophoblast and syncytiotrophoblast.35 This tumor marker correlates well with the volume of disease except in a few cases of drug-resistant disease and is a sensitive marker for response to chemotherapy.35 Gestational choriocarcinoma usually progresses rapidly and is fatal without treatment.
Placental site trophoblastic tumor
Placental site trophoblastic tumors are predominately composed of intermediate cytotrophoblast cells arising from the placental implantation site (Fig. 11).35 These neoplasms can result from any type of antecedent pregnancy and are usually locally aggressive, producing myometrial invasion. Because there is a disproportionately small population of syncytiotrophoblast elements, the production of hCG is usually scanty and provides a less reliable marker of tumor volume than for other forms of gestational trophoblastic disease.35 Placental site trophoblastic tumors should be considered to be locally invasive with a low malignant potential because a minority of patients will develop extrauterine metastasis.36, 37, 38, 39, 40 In contradistinction to invasive moles and gestational choriocarcinoma, placental site trophoblastic tumors are usually resistant to conventional chemotherapy used in the treatment of gestational trophoblastic disease.
EPIDEMIOLOGY
The epidemiology of hydatidiform mole and GTD has been confounded by earlier studies that often combined patients with hydatidiform moles, partial moles, and cases of gestational trophoblastic tumors (GTT). Much of the older literature probably exaggerated the incidence of hydatidiform mole in Asia, Africa, and South and Central America because these were largely hospital-based, not population-based, studies adjusted for live births in a tertiary hospital that was likely to receive complicated pregnancies in referral. Furthermore, older studies reporting the epidemiology of hydatidiform moles did not clearly distinguish between complete moles and partial moles, which might obscure potentially important differences in the epidemiology of these two distinct entities. Finally, many older studies reported an incidence of GTD based on the live delivery rate; this would tend to overestimate the incidence of GTD per pregnancy depending on local induced abortion rates.
Hydatidiform mole
Several population-based studies of complete hydatidiform moles have estimated that the incidence ranges between 0.5 and 2.5 per 1000 pregnancies.41 Studies from Saudi Arabia42 and most Asian countries41, 43 indicate an apparent twofold increased risk compared with most white populations. It has long been debated whether these differences in risk of hydatidiform mole are related to racial, socioeconomic, or dietary factors. For example, a study by McCorriston suggested that the incidence of hydatidiform mole in Hawaii was influence by race, with a much higher incidence observed in Japanese and Chinese populations.43 Although the incidence for Hawaiians of Japanese extraction appeared lower than the native rate in Japan,43 analysis of other racial groups revealed no consistent pattern41 and was based on fewer than ten patients in several racial subsets.43 A more recent study from Hawaii confirmed an increased risk among Asians and Filipinos compared with whites and native Hawaiians.44 The incidence of complete moles was similar among immigrant and first-generation women in these racial subgroups.44
Many studies have documented an increased risk for women at the extreme range of reproductive life using both population-based risk estimates and case–control studies. Bagshawe and co-workers reported a 411-fold increased risk of hydatidiform mole for women older than the age of 50 and a six-fold increased risk for women younger than the age of 15 compared with the expected risk in women between the ages of 25 and 29.45 Mazzanti also reported an increasing risk with age, up to a 300-fold increased risk for women older than the age of 50 compared with the regional incidence.46 The majority of these studies have not addressed paternal age; however, Parazzini and co-workers found that paternal age of more than 45 years increased the relative risk for complete mole to 4.9 (2.9 when adjusted for maternal age).47 These age-related differences in the incidence of hydatidiform mole may reflect defective gametogenesis at the extremes of reproductive life that predisposes to the androgenic conceptus giving rise to complete moles. In contrast, Parazzini and co-workers found no maternal or paternal age-associated risk for partial hydatidiform mole.47
Several studies have indicated that a history of previous abnormal gestations increased the risk for hydatidiform moles. Acaia and associates found an incidence of nine moles in 385 pregnancies of 93 women with two previous consecutive spontaneous abortions versus none in 82 control women with normal prior term pregnancies.48 The relative risk of hydatidiform moles increased to 32.1 times the regional frequency of hydatidiform mole in these patients with frequent abortions.48 A case–control study from China by Brinton and colleagues reported a decreased risk for hydatidiform mole with prior term birth but also reported an increased risk for prior therapeutic abortion.49 However, Atrash and associates reported that the type of prior pregnancy had no signficiant effect on the risk of hydatidiform mole among more than 84,000 women undergoing histologically studied legal abortions.50 It is, however, well recognized that a history of prior hydatidiform mole increases the risk of a subsequent mole by approximately ten-fold.41
Several case–control studies have suggested a possible dietary factor in the etiology of hydatidiform mole. Studies from both the New England Trophoblastic Disease Center in the United States51 and from Italy52 found that patients with hydatidiform moles had a significantly lower consumption of foods rich in vitamin A or animal protein and a significantly decreased estimated intake of β-carotene and protein. However, Brinton and colleagues were unable to find any dietary effect on the incidence of hydatidiform mole in China.49 As pointed out by Parazzini and co-workers, the lower carotene and protein intake for women in their study might reflect a nonspecific indication of lower socioeconomic diet.52
Choriocarcinoma
The epidemiology of gestational choriocarcinoma has been less extensively studied, owing to problems in histologic control and fewer cases than those of hydatidiform mole. The single highest risk factor is prior hydatidiform mole; the incidence of choriocarcinoma is increased 1000–2000-fold compared with term pregnancy.35, 41, 53 McAna reported the results of a retrospective 30-year study from New York and a case–control study from upstate New York.54 The 30-year incidence of gestational choriocarcinoma was 2.46 cases per 100,000 pregnancies. There was an increased risk among women older than 45 years old. Nonwhites had an approximately two-fold increased risk of gestational choriocarcinoma compared with whites. In the case–control portion of this study, increased risks were observed in women who had spontaneous abortions before the case pregnancy and among patients who were not married or who held professional/technical jobs.54 Although the risk of choriocarcinoma was increased slightly for nonwhites, this was not statistically significant.
Brinton and co-workers used the SEER database from between 1973 and 1979 and reported an incidence of one choriocarcinoma per 24,096 pregnancies or one per 19,920 live births.55 The risk was increased approximately two-fold in nonwhites compared with whites. Increased risks were seen at the extremes of reproductive age groups, including an 8.6-fold increase among patients older than the age of 40.55 In our experience there has also been an increased risk for malignant and postmolar trophoblastic tumors in these age groups.56
Buckley and colleagues reported a case–control study using 75 survivors of therapy for choriocarcinoma matched with geographic- and age-matched controls.57 They found decreased risks for increasing body mass, a history of dieting, or regular exercise. Increased risks were reported for menarche at age older than 12 years, twins in the cases or a family history of twins, multiple marriages, and infrequent intercourse. They postulated that decreased levels of estrogen below normal were associated with a disruption of ovulation, which might be associated with the development of choriocarcinoma in a subsequent pregnancy.57
As is evident from these studies, the epidemiology of choriocarcinoma is very poorly understood and further efforts are needed to elucidate epidemiologic causal relationships.
HUMAN CHORIONIC GONADOTROPIN
No discussion of GTD would be complete without mentioning the role of hCG monitoring. Virtually all forms of GTD secrete hCG in proportion to the amount of trophoblastic tissue present, with the exception of placental site trophoblastic tumors and the possible exception of some cases of drug-resistant GTT in which hCG can be localized in only a few tumor cells. The ability to measure quantitative hCG values down to physiologic values of hCG-like substances and the development of effective chemotherapy have revolutionized the treatment of patients with GTT.
hCG is a glycoprotein hormone with significant structural similarities to the glycoprotein hormones produced by the pituitary gland.58, 59 It is composed of two distinct glycopeptide subunits that are noncovalently bound, similar to luteinizing hormone (LH), follicle-stimulating hormone (FSH), and thyroid-stimulating hormone (TSH). The α-subunit of all four glycoprotein hormones is essentially identical.58 The β-subunits, although sharing structural similarity, differ sufficiently to impart specific biologic activity on the intact (α-β) hormone. hCG and LH have the most similar β-subunits, with approximately 80% identical amino acids sequences.60 The active binding site for gonadotropin receptors results from conformational changes that require association of the α- and β-subunits together. It is unknown whether the active site involves configuration within the hormone-specific β-subunit or a combination of sites on both subunits. Modification of as few as two amino groups in the α-subunit can cause significant loss of biologic potency without a conformational change of the hormone.61 However, a synthetic fragment of the β-subunit can inhibit binding of hCG to ovarian membrane receptors and stimulate testosterone production by rat Leydig cells.62
The development of polyclonal antibodies specific to multiple epitopes present on the β-subunit chain of hCG resulted in the development of radioimmunoassay (RIA) competitive binding procedures63 to quantitate hormone concentrations in small amounts of body fluids. With the development of sensitive and specific RIA techniques, hCG levels can be detected with sensitivity approximately 100-fold over that of bioassay methods. RIA procedures have become the method of choice for monitoring GTD.
The technical aspects of the β-subunit polyclonal RIA have been reviewed by Vaitukaitis and associates.63 It is important to realize that the β-subunit RIA does not distinguish between free β-subunit and whole-molecule β-subunit fragments. However, because the free β-subunit is more rapidly cleared from circulation, the molecular species measured under most circumstances with this RIA consists of intact hCG.63 The results reflect combined immunologic activity of both intact hCG and its free β-subunit, resulting in exaggeration of the total hCG values, depending on the amount of the subunit that is present. Furthermore, β-subunit RIAs performed with this technique do not exclude high levels of LH and can detect measurable antigenic activity in situations in which serum LH levels are elevated, such as menopause.63 Therefore, sensitivity is limited below a level of approximately 5 mIU/ml.
An immunoradiometric assay (IRMA) has been developed that is similar to the RIA in that it employs radioactive isotopes to quantitate the reaction between hormone antigens and antibodies. However, differences in techniques yield slightly different results. In RIAs, the quantitated hormone competes with radiolabeled hormone for binding to a limited number of antibody sites,63 while in IRMAs, antibody is present in excess; it is the antibody that is labeled rather than the hormone.59 Most systems use antibodies specific for isolated α- and β-subunits in sequence to perform the assay.64 Antibodies against one subunit are fixed to the solid phase to bind hormone from the biologic fluid, effectively concentrating the hormone. Antibodies against the other subunit carry the radioactive label. Because binding to both subunits is required for quantitation, the method identifies only intact hormone or associated partial α-β fragments that include antibody binding sites on each subunit fragment.58, 65 While free subunits may react with their respective antibodies, only the combined subunits link the radiolabeled antibodies to the solid phase and are measured.
Immunoassays that are not dependent on radioactive isotopes offer several advantages over the RIA and IRMA in regard to handling and disposal of radioactive materials. Therefore, both enzyme-linked immunosorbent assays (ELISA) and fluoroimmunoassays (FIA) have been developed.58 Although ELISA methods can be used for quantitative hCG measurements, sensitivity and specificity appear to be less than for RIA or IRMA techniques, particularly in the lower ranges of hCG.58 A technique of FIA for serum hCG has been described using a two-site method with monoclonal antibodies directed against α- and β-subunits.66, 67 Although fundamentally similar to the two-site IRMA techniques, the labeling antibodies are labeled with a rare earth (europium), which forms a highly fluorescent chelate. Similar to the IRMA, this FIA measures only intact hormone without detecting free hCG subunits. A sensitivity of approximately 1 mIU/ml has been achieved with the ability to quantitate hCG over a large range.67 Our experience using the FIA in monitoring patients with GTD suggests that it provides values that are virtually parallel to results achieved with RIA. However, as discussed subsequently, selective measurement only of whole-molecule hCG may not be optimal for monitoring therapy for GTT.
It has been postulated that patients with GTT may secrete abnormal forms of hCG or incomplete molecules (excess free α- or β-subunits). Assays directed against free α- and β-subunits have been investigated. Although Nishimura and co-workers68 and Quigley and colleagues69 reported data suggesting an association between abnormal clearance of free α-subunits and poor outcome in GTT, other studies have found little value in measuring the free α-subunit, with no clear relationship between the detection of free α-subunits and unfavorable disease outcome.70, 71 Unfortunately, the value of measuring free α-subunits is confounded by the cross-reactivity with α-subunits of pituitary origin.
The measurement of free β-subunit in patients with GTT has only recently been investigated because of the difficulties in discriminating between free β-subunit and intact hCG. The recent development of monoclonal antibodies against β-subunit epitopes that are concealed when the β-subunit is associated with the α-subunit has allowed direct quantitation of free β-subunits of hCG. Khazaeli and associates found that patients undergoing evacuation of hydatidiform mole have a higher free β-subunit-total hCG ratio among those who develop persistent disease compared with those who undergo spontaneous remission. Other studies by the same investigators suggested that patients treated with chemotherapy for GTT have a high risk of failure of primary chemotherapy if there is an elevated free β-subunit-hCG ratio early in the course of their disease.72 However, Berkowitz and colleagues were unable to correlate free β-subunit-hCG ratios with disease outcome in patients undergoing evacuation of hydatidiform moles.73 Further studies are needed to clarify the value of free β-subunit monitoring.
As our understanding of hCG metabolism in normal gestations and GTD evolves, it is likely that a panel of assays will be used to quantitate total hCG and free subunit or abnormal fragment levels for monitoring patients with GTD. Alternatively, different assays might be employed to evaluate patients prior to therapy, during active treatment, and during remission of their disease. Despite the limitations of current assays described earlier, hCG remains the model tumor marker for human neoplasms.
DIAGNOSIS AND MANAGEMENT
Hydatidiform mole
The primary management of women with hydatidiform mole encompasses surgical evacuation coupled with close monitoring of subsequent hCG levels. Although the complete and partial hydatidiform moles have distinct cytogenetic, histopathologic, and clinical features, their acute management is very similar and both should be considered clinically in the same category. However, there are several differences in the clinical behavior of these two types of molar pregnancies.24, 27Table 1 is a summary of the cytogenetic, histopathologic, and clinical characteristics of partial and complete hydatidiform moles.
The differences in clinical characteristics of partial and complete hydatidiform mole can be explained in part on the basis of the differing amounts of trophoblastic proliferation in these syndromes.24 Partial hydatidiform moles have focal, irregular hydropic changes of chorionic villi coupled with focal trophoblastic proliferation and frequently have an identifiable fetus up to or beyond 8 weeks' gestation. Because of the only modest increase in placental size and trophoblast mass, the uterus is usually enlarged to a lesser degree than that of the anticipated duration of gestation or is compatible with dates. As noted previously, partial hydatidiform moles are probably underdiagnosed and may comprise up to 1–2% of all clinically recognized spontaneous abortions. A patient with partial hydatidiform mole usually presents with clinical and ultrasound features of a missed or threatened spontaneous abortion. The majority have low pre-evacuation hCG levels and lack theca lutein cysts. Nonmetastatic postmolar GTT is diagnosed in less than 5–10% of patients after evacuation of partial moles.27, 74
In contrast, complete hydatidiform moles have diffuse, often massive, hydropic degeneration of chorionic villi with diffuse trophoblastic proliferation that results in a differing spectrum of clinical signs and symptoms.24 Vaginal bleeding is the most common presenting symptom in patients with complete moles, often producing anemia.75 Up to half of all patients with complete hydatidiform mole will have uterine enlargement beyond the expected gestational age caused by expansion of the uterus by both molar tissue and intrauterine bleeding.75, 76 Unilateral or bilateral ovarian enlargement produced by theca lutein cysts is clinically detected in one quarter to one third of patients with complete hydatidiform mole and is usually associated with hCG levels above 100,000 mIU/ml (Fig. 12).75, 76, 77, 78 Preeclampsia and hyperemesis each occur in approximately one quarter of patients with complete mole. The majority of patients with these symptoms also usually have markedly elevated hCG values.75 The development of pregnancy-induced hypertension before 24 weeks' gestation is almost diagnostic of molar pregnancy. Increases in thyroid hormones are frequently diagnosed in patients with complete hydatidiform moles, but clinical hyperthyroidism is detected in less than 10%.79 There is no consistent relationship between serum hCG values and results of thyroid function tests. Finally, in contrast to partial moles, patients with complete moles have approximately a 20% incidence of trophoblastic tumor after evacuation, with 10–20% of these having metastatic disease.76, 77, 80, 81, 82, 83, 84, 85, 86, 87 The majority of those with trophoblastic tumors have invasive or persistent mole while approximately one quarter to one third have gestational choriocarcinoma.
The diagnosis of hydatidiform mole is no problem after the patient passes molar vesicles. Even then, however, ultrasound should be used to exclude the presence of a fetus (as in partial mole or twin gestation), as well as to further define the presence and size of theca lutein cysts of the ovaries (Fig. 13). A variety of other physical and chemical tests have been used over the years, including amniography, arteriography, computed tomography (CT), and magnetic resonance imaging (MRI), but few are indicated beyond standard pelvic ultrasound. Tests for hCG may occasionally be useful in the differentiation of hydatidiform mole from normal pregnancy. Serum assays may be used, and the results should be compared with the hCG levels of normal pregnancy at the gestational age in question. Although a single result well above normal range for that state of pregnancy suggests molar pregnancy, only the results of serial assays are definitive. Delfs80 has asserted that between days 60 and 100 of pregnancy there is no level of hCG secretions, however high, that could not be caused by a normal pregnancy or some variation thereof. Thus, gonadotropin levels in excess of 100,000–200,000 IU/24-hour urine collection or their equivalent serum levels are compatible with molar pregnancy, with the exception of the peak elevations seen between weeks 9 and 14 of normal pregnancy. Higher levels may also be associated with multiple gestation or toxemia of pregnancy. A continued rise in hCG levels after the 14th week of pregnancy (the hCG level drops at this time in normal pregnancy) is the best evidence of a molar pregnancy that can be obtained by hCG assay (Fig. 14).
|
A variety of other laboratory studies have been investigated in the diagnosis of molar pregnancy. These include determination of serum leucine aminopeptidase level, human placental lactogen, estrogens, and various quantities and ratios of subunits of hCG. The concentrations of all of these vary in normal pregnancy, and, as a rule, are not of significant assistance in the differential diagnosis of normal pregnancy and molar pregnancy, since the values found in well-differentiated hydatidiform moles tend to overlap with those of normal pregnancy.
In summary, the pertinent diagnostic features in hydatidiform mole are as follows:
- Enlargement of the uterus disproportionate to duration of gestation
- Irregular vaginal bleeding to a modest degree, on occasion profuse, beginning after the second month of pregnancy
- Absence of fetal parts on palpation or roentgenogram in a uterus of such a size that one would expect such findings
- Absence of fetal heart tones at a time when, by gestational duration or size, they would be expected to be audible
- Characteristic ultrasonographic patterns
- Symptoms of preeclampsia in the late first or early second trimester
- Cystic enlargement of the ovaries
- High levels of hCG
- Positive findings on ultrasonography (perhaps the best diagnostic feature (see Fig. 13).
Despite the differences between partial and complete hydatidiform moles, the initial management and subsequent surveillance of patients with partial or complete molar gestations are similar. After the diagnosis has been confirmed, the evaluation of a patient with a molar gestation is directed toward screening for metastatic disease and stabilization of the patient for evacuation. Preoperative evaluation consists of a complete physical examination, baseline serum hCG level, chest roentgenogram, hematologic profile, renal and liver function tests, and thyroid function tests. If the uterus is enlarged more than 14–16 weeks' gestational size or the patient has pregnancy-induced hypertension, arterial blood gases should be measured preoperatively because many of these patients will develop respiratory insufficiency after evacuation.
EVACUATION
Techniques for evacuation of hydatidiform mole have included induction of a labor with oxytocin or prostaglandins, hysterotomy, cervical dilation with suction curettage (D&C), and hysterectomy. If the patient desires sterilization, hysterectomy with the mole in situ is our preferred method of evacuation. However, the majority of women with hydatidiform moles can be safely evacuated using suction D&C regardless of uterine size.75
Suction curettage
The patient should be hemodynamically stable with correction of preoperative anemia, stabilization of blood pressure if superimposed pregnancy-induced hypertension is present, and stabilization of systemic manifestations of hyperthyroidism with β-blockers. If the uterus is more than 14–16 weeks' gestational size, a central line should be placed for intraoperative central venous pressure monitoring and rapid administration of fluid or blood products during the procedure. At least 2 units of blood and a laparotomy set should be available in the operating room.
After induction of anesthesia, the cervix is dilated gently with Pratt dilators to allow passage of a suction cannula appropriate for the volume of molar tissue. An oxytocin infusion is begun after introduction of the suction cannula and initiation of the curettage. A 12–14-mm cannula is introduced into the lower to mid endometrial cavity. Because the myometrium is often distended and soft, no effort is made to sound the uterus to the fundus in order to avoid uterine perforation. During suction curettage, the fundus is massaged to assist in stimulating uterine contractions and reduce the risk of perforation. The majority of the molar tissue can be removed by rotating the cannula to evacuate uterine contents. As the uterine fundus involutes, completion of evacuation is performed using gentle curettement with the suction cannula. When the suction evacuation is believed to be complete and the uterus is well contracted, the endometrium is gently curetted using a large sharp curette to ensure complete evacuation. The curettings from suction and sharp curettage should be submitted separately for pathologic review. Oxytocin infusion is continued for 24 hours after molar evacuation or until vaginal bleeding is minimal.
Hysterectomy
Hysterectomy offers the advantage of simultaneous evacuation of hydatidiform mole and sterilization for women who no longer wish to bear a child.77, 88 Additionally, performance of a hysterectomy decreases the risk of malignant sequelae to approximately 3.5% from the 20% anticipated after evacuation with D&C.77However, hysterectomy does not eliminate the potential for malignant sequelae, and these women must have their hCG levels monitored after hysterectomy. We generally perform a simple total abdominal hysterectomy with the mole in situ. Because most women with hydatidiform mole are younger than 40 years of age, the adnexa should not be removed unless the patient is perimenopausal or there is obvious adnexal metastasis. Theca lutein cysts usually regress spontaneously after evacuation or hysterectomy and do not need to be drained or removed unless torsion or intraoperative rupture with hemorrhage occurs.78
Other techniques
Induction of labor with oxytocin or prostaglandins carries the potential increased risk for disseminating trophoblast throughout the systemic circulation caused by uterine contractions against an undilated cervix. Significant blood loss and incomplete evacuation often occur, requiring suction D&C.89 Hysterotomy is also associated with an increased blood loss when compared with suction D&C. The vertical uterine incision frequently results in the requirement for cesarean section in subsequent pregnancies. Because the majority of these patients are in the prime of their reproductive age group, this is an important consideration. Furthermore, Curry and co-workers77 and Tow90 reported that hysterectomy for evacuation of hydatidiform mole resulted in a higher incidence of postmolar malignant sequelae than did suction D&C.
THECA LUTEIN CYSTS
Clinically evident (greater than 5–6 cm) theca lutein cysts of the ovary are detected in approximately one quarter to one third of women with hydatidiform mole, with additional smaller cysts often detected by ultrasound alone.76, 77, 78 Ovarian enlargement correlates with marked elevation of serum hCG levels greater than 100,000 mIU/ml. Histologically and physiologically these cysts are similar to iatrogenic ovarian hyperstimulation produced by exogenous gonadotropin/hCG administration for induction of ovulation. Although theca lutein cysts are usually detected before molar evacuation, they often develop within the first week after evacuation.76, 78 The mean time for disappearance of theca lutein cysts is approximately 8 weeks. It is very rare for a patient to develop overt ovarian hyperstimulation with fluid retention and/or ascites, but an occasional patient will develop ovarian torsion or rupture and bleeding from the cyst, requiring oophorectomy.78 Theca lutein cysts are associated with an increased incidence of postmolar trophoblastic tumor; in particular, Montz and colleagues reported a 75% incidence of postmolar sequelae among women with bilateral theca lutein cysts.78 Although theca lutein cysts usually regress spontaneously with falling hCG levels after molar evacuation, approximately 30% will develop secondary enlargement in response to rising hCG levels associated with postmolar sequelae.78 Occasionally, these cysts will persist for several months after hCG level remission has been achieved.
RESPIRATORY DISTRESS SYNDROME
During evacuation of hydatidiform moles there are many potential causes for respiratory distress, including trophoblastic deportation, high-output congestive heart failure caused by anemia or hyperthyroidism, preeclampsia, and iatrogenic fluid overload.91 Pulmonary complications are observed in approximately one quarter of patients with uterine size more than 16 weeks' gestation. Although the syndrome of trophoblastic embolization has been emphasized in the past as an underlying cause for respiratory distress syndrome,92, 93 Hankins and associates detected only scanty amounts of trophoblastic cells in the pulmonary artery blood among a small series of women undergoing evacuation of large molar pregnancies.94 Furthermore, Cotton and co-workers documented a transient impairment of left ventricular function during general anesthesia in a small series of patients studied with invasive central monitoring performed during suction D&C for molar evacuation.95 This might contribute to the development of pulmonary edema in unmonitored patients given large volumes of crystalloid during the procedure. In general, pulmonary complications should be managed with appropriate ventilator support and central monitoring with a Swan-Ganz catheter to accurately determine fluid status and the need for fluids, blood products, or diuresis. All patients should have a chest roentgenogram after evacuation of hydatidiform mole to rule out significant trophoblastic deportation, pulmonary metastasis, or development of pulmonary edema.
UTERINE PERFORATION
Uterine perforation should rarely occur as an acute complication during primary suction D&C for hydatidiform mole. If perforation is recognized, the suction should be immediately discontinued, the cannula removed, and the rate of oxytocin infusion increased. Laparoscopy or laparotomy should be performed to access the site of perforation. If hemostasis is adequate and there is no damage to gastrointestinal organs, curettage can be completed under laparoscopic visualization.
Rarely, uterine perforation occurs during or after suction D&C through a focus of deep myometrial penetration by invasive mole. Surgical management should be individualized based on the site and extent of perforation. Although some patients will require hysterectomy, small series have suggested that individual patients with invasive moles can be treated with segmental resection and repair of the affected myometrium.96, 97 Most frequently, these will occur in the midline of the uterine fundus.
MANAGEMENT OF COEXISTENT FETUS
Rare cases of twin pregnancies consisting of normal conceptus and complete hydatidiform mole have been reported.98, 99, 100 It is important that all cases suggesting these entities be carefully studied both cytogenetically and histopathologically to avoid confusion with a partial hydatidiform mole. In rare cases, a fetus has been carried to viability.100
We have been involved in the care of several women in whom the differential diagnosis has included partial hydatidiform mole versus twin gestation with coexistent mole and normal pregnancy. In these circumstances, we recommend a thorough obstetric ultrasound to rule out fetal malformations and to fully characterize the placenta. On several occasions the presumed mole has subsequently been confirmed to be either a nonviable twin, retroplacental hematoma, or other nonmolar placental abnormality. In patients whose pregnancies appear to consist of a viable fetus with molar changes in a portion of the placenta, we have attempted to use either chorionic villous sampling in an effort to prove or disprove the existence of a triploidy or amniocentesis later in gestation to assess the fetal karyotype. However, the majority of these cases can be resolved through a careful ultrasonographic study of the placenta and histopathologic examination of the products of conception after delivery or spontaneous abortion. Although the persistence of a marked elevation (more than 100,000 mIU/ml) in the level of serum hCG is consistent with the diagnosis of hydatidiform mole, we have observed several anecdotal cases in which this diagnosis was entertained during the second trimester of pregnancy and subsequently disproved at delivery. Therefore, we do not encourage overmanagement of these unusual pregnancies because the majority of patients in whom this diagnosis is entertained will not have the diagnosis of twin viable conceptus–molar gestation confirmed.
RISK FACTORS FOR POSTMOLAR GESTATIONAL TROPHOBLASTIC TUMOR
Several clinicopathologic factors have been associated with an increased risk for the development of postmolar GTT. Many investigators have reported that increasing maternal age is associated with an increased risk of trophoblastic tumor.72, 76, 77 This risk appears to increase as the patient enters the perimenopausal age range. In contrast, teenagers do not appear to have a consistently increased risk for the development of postmolar GTT. Likewise, gestational age at diagnosis of molar pregnancy has been found to have conflicting associations with the development of postmolar GTT.76, 77
Hertig and Sheldon reported that the amount and characteristics of trophoblastic proliferation observed histologically in the primary mole roughly correlated with the subsequent development of postmolar GTT.28 However, others have been unable to document an increased risk for patients with increasing amounts of trophoblastic proliferation.77 Unfortunately, the identification of marked amounts of trophoblastic proliferation or anaplasia may be, in part, dependent on the number of histologic sections obtained from the primary mole. Other clinical factors related to an increased amount of trophoblastic proliferation have been documented to affect outcome after molar evacuation.
Curry and co-workers77 and Morrow and associates76 reported similar adverse effects for uterine enlargement and the presence of theca lutein cysts. The presence of uterine enlargement beyond that appropriate for dates was associated with an increased risk of postmolar GTT to between 25% and 48%, respectively, while the presence of clinically detected theca lutein cysts increased the risk to approximately 50%.76, 77 The combination of these factors identified populations with a risk of approximately 60% for developing postmolar GTT. Other investigators have also reported adverse effects of uterine enlargement and theca lutein cysts. In particular, Montz and associates reported a markedly increased risk for women with bilateral theca lutein cysts.78
Other clinical risk factors for postmolar GTT have been reported, including the development of pulmonary complications during molar evacuation and uterine subinvolution with hemorrhage following evacuation. Although these clinical features are observed in a minority of patients, they do identify high-risk subsets of patients. In particular, Morrow and associates reported that postmolar GTT was subsequently diagnosed in all six women with postevacuation hemorrhage in their series.78
Although individual clinical factors can be used to identify women at an increased risk for the development of postmolar GTT, they lack the ability to predict the course of disease for individual patients. Some investigators have used clinicopathologic factors to identify high-risk patients who might benefit from prophylactic chemotherapy. However, even using multivariate analysis, Parazzini and co-workers were able to retrospectively assign only 69% of their patients to high- and low-risk groups.101 The low-risk group had a 4% and the high-risk group had a 32% incidence of postmolar GTT. Unfortunately, the high-risk group accounted for only six (15%) of 39 patients who developed postmolar GTT in this study.101 This study underlines the necessity for following each individual patient with serial hCG monitoring, rather than depending on clinical risk factors to assign therapy.
Newer laboratory methods may improve the ability to predict the development of postmolar GTT. Assays that measure free β-subunits in the presence of intact hCG have been developed. Khaezaeli and co-workers reported preliminary evidence to suggest that elevations in the free β-subunit fraction are observed more frequently at the time of evacuation in patients with hydatidiform moles destined to develop postmolar GTT.97 The use of the free β-subunit assays was prospectively evaluated in a Gynecologic Oncology Group study.102 Furthermore, the identification of aneuploidy in the primary mole using flow cytometry appears to identify patients at higher risk for postmolar GTT.103, 104
PROPHYLACTIC CHEMOTHERAPY
The role of prophylactic chemotherapy, given at or prior to the time of molar evacuation to prevent postmolar GTT remains controversial. The rationale for the use of a limited course of methotrexate or dactinomycin is clear: systemic levels of chemotherapy would theoretically prevent the establishment of locally invasive disease or metastasis that might occur as a result of embolization of trophoblast at the time of D&C and would perhaps increase the rate of regression of molar tissue in patients with a large volume of disease. Several investigators, however, have expressed concerns regarding the use of prophylactic chemotherapy around the time of molar evacuation when anecdotal reports of deaths caused by prophylactic chemotherapy were reported in the 1970s.77 Furthermore, large series reported in the early 1970s from Singapore reported that although the use of prophylactic methotrexate resulted in a nonsignificant decrease in the incidence of choriocarcinoma following evacuation of hydatidiform mole there was a paradoxically significant increase in the mortality rate with one death from drug toxicity and two from choriocarcinoma among the treated patients.105 Therefore, many investigators have believed that routine application of prophylactic chemotherapy is not warranted in the management of most patients with hydatidiform moles.
On the other hand, several comparative series have reported a significantly decreased risk of postmolar GTT among patients treated with prophylactic chemotherapy.75, 106, 107 Specifically, Kim and associates conducted a prospective randomized trial using prophylactic methotrexate with folinic acid at the time of molar evacuation.106 In this series the use of prophylactic chemotherapy reduced the incidence of postmolar GTT from 47% to 14% in patients with high-risk moles but did not significantly decrease the low incidence of postmolar GTT in those with low-risk moles.106 In contrast to other studies using conventional courses of methotrexate and dactinomycin, the methotrexate/folinic acid regimen appears to have quite limited toxicity, making it safer for a prophylactic regimen. However, because methotrexate is the most frequently used agent for first-line therapy in patients with postmolar GTT, we believe it may be important to use a different cytotoxic agent for chemoprophylaxis to prevent the development of drug resistance in patients who fail chemoprophylaxis.
Further randomized studies are needed to define the ideal regimens and patient populations that would benefit from chemotherapeutic prophylaxis after evacuation of hydatidiform mole. From the available data, patients with high-risk hydatidiform moles would appear to benefit from prophylactic chemotherapy, but the risk of postmolar GTT is not eliminated; therefore, these patients still require surveillance with serial hCG testing. At present, we cannot recommend the indiscriminate use of prophylactic chemotherapy after evacuation of hydatidiform mole because of the nearly universal availability of sensitive hCG assays for monitoring patients at least in the USA.
SURVEILLANCE AFTER MOLAR EVACUATION
Surveillance using serial, highly sensitive and accurate quantitative serum hCG levels is the only reliable means for the early detection of malignant sequelae after evacuation of hydatidiform mole. One of any number of sensitive assays employing polyclonal or monoclonal antibodies to either whole-molecule or total (free and bound) β-hCG fragments can be used. A baseline level should be obtained within 48 hours of evacuation and serial levels followed at 1-week intervals until normal hCG levels are obtained (Fig. 14).75, 76, 77, 81, 82, 83, 84, 85, 86, 87 Levels should then be followed at 1–2-month intervals to ensure that spontaneous remission is sustained beyond 6–12 months. Although some have recommended that patients with partial hydatidiform moles can stop surveillance after hCG level remission has been achieved, the approximately 5–10% incidence of trophoblastic tumor after evacuation of partial moles74 reported by the New England Trophoblastic Disease Center is of concern; we generally recommend at least 3–6 months of normal hCG levels in these patients before surveillance is discontinued. It is rare to observe reelevation of hCG levels caused by postmolar GTT after more than 6 months of normal hCG levels without an intercurrent pregnancy. Virtually all cases of postmolar GTT reported in adequately monitored patients have occurred within the first 6 months after molar evacuation;75, 76, 77, 80, 81, 82, 83, 84, 85, 86, 87 therefore, we believe that a minimum of 6 months of hCG remission should be recommended for patients after an evacuation of a complete hydatidiform mole.
Pelvic examinations should be repeated every 2 weeks and chest roentgenograms every month until the hCG level has declined to less than 1000 mIU/ml. Patients who have not undergone hysterectomy should use contraception during the interval of hCG level monitoring until sustained remission has been documented. This avoids confusion caused by an elevated hCG level associated with an intercurrent pregnancy. Although studies from the United Kingdom suggested an increased risk for postmolar GTT in women who used oral contraceptives,108 several studies from the United States and Canada,109, 110, 111 including a randomized Gynecologic Oncology Group study,112 have failed to demonstrate any increased risk for postmolar GTT in women using moderate- to low-dose oral contraceptives. The differing results may reflect different criteria used to diagnose postmolar GTT or, alternatively, may reflect use of different formulations of oral contraceptives in the studies cited. We routinely recommend the use of oral contraceptives with a low estrogen content unless there are specific contraindications to their use, because they are the most effective means of reversible contraception.
DIAGNOSIS OF POSTMOLAR GESTATIONAL TROPHOBLASTIC TUMORS
Approximately 20% of patients undergoing evacuation of a complete hydatidiform mole will develop postmolar GTT,80, 81, 82, 83, 84, 85, 86, 87 70–90% of these consist of histologically defined persistent or invasive moles, while 10–30% are choriocarcinomas. Because the historical mortality for patients with invasive moles ranged around 20%,113 most investigators in the United States have used conservative criteria for initiating chemotherapy in patients after evacuation of hydatidiform mole in an attempt to reduce the morbidity caused by local proliferation, infection, and hemorrhage and to prevent mortality from local disease or systemic metastasis. The vast majority of patients are therefore treated on the basis of hCG level regression patterns without a firm histologic diagnosis.
Before the development of effective chemotherapy, Delfs noted that approximately 9% of patients with molar pregnancies developed proliferative sequelae and required hysterectomy.80 Series reported since the introduction of chemotherapy have had a wide variation in the frequency of postmolar GTT, with 6–25% of the patients who developed postmolar GTT having metastatic disease.76, 77, 80, 81, 82, 83, 84, 85, 86, 87 These observed differences in the frequency of postmolar GTT likely reflect inclusion of partial moles in some series, a different incidence of metastatic disease in patient populations, or, most significantly, different hCG level regression criteria used to define postmolar GTT and assign therapy in the various studies.
Before the development of sensitive hCG assays, clinical risk factors alone were often used to follow patients after evacuation of hydatidiform moles. Histologic assessment of trophoblastic proliferation can yield high- and low-risk groups of molar gestations28 but are of little use in determining the need for therapy in the individual patients.77 Excessive uterine enlargement, theca lutein cysts, development of respiratory distress syndrome after uterine evacuation, and postevacuation uterine bleeding are also associated with a higher frequency of postmolar GTT.75 In contrast, prompt uterine involution and regression of theca lutein cysts are favorable signs. However, monitoring of hCG levels, as discussed earlier, is the most sensitive and accurate method for predicting the development of postmolar GTT.
Criteria for the diagnosis of malignant postmolar GTT include high levels of hCG (serum level >20,000 mIU/ml) more than 4 months after evacuation of a hydatidiform mole, progressively increasing hCG values, histologic evidence of choriocarcinoma or placental site trophoblastic tumor, or evidence of metastatic disease. Most American centers will administer chemotherapy to patients who exhibit a plateau of serial hCG values. Additionally, some investigators have recommended instituting therapy based on persistence of detectable hCG at some arbitrary interval following molar evacuation.
Bagshawe and colleagues have used extremely conservative criteria for instituting therapy after molar evacuation and treated only approximately 6% of their 280 patients.83 Treatment was administered to patients with vaginal or pulmonary metastases only if the hCG levels rose or the patient developed complications from metastatic disease. Likewise, patients with a hCG level plateau were observed for several weeks and were not treated unless the hCG level actually rose.83 These criteria are in sharp contrast to the more frequent recommendations that all patients with any metastatic disease, or even a plateau of hCG level persisting for 3 consecutive weeks, be treated.
Kohorn has also suggested that patients with plateauing hCG levels might be safely followed beyond 2 weeks if reliable hCG follow-up is available.86 Nine per cent of 131 patients followed after molar evacuation in his series had hCG plateaus for more than 2 weeks during surveillance and subsequently resumed a pattern of declining hCG levels. Six of these achieved spontaneous hCG level regression. Additionally, five patients who were started on chemotherapy for a hCG level plateau were subsequently found to have an immediate pretherapy hCG level fall of more than 25% from their sustained plateau.86 These data suggest that patients with plateauing hCG levels may be safely monitored with serial hCG values over several weeks and that a significant percentage of these patients will enter spontaneous remission.
Therapy has usually been initiated if hCG levels remained elevated beyond an arbitrary length of time after molar evacuation in some series. For example, Morrow and colleagues instituted chemotherapy if hCG was detectable at 8 weeks after molar evacuation,76 and Hatch and associates treated most patients if levels were elevated 12 weeks after evacuation.84 Although these reports may review different patient populations, the proportion of treated patients is somewhat higher in their studies than in other reports in which therapy was instituted on the basis of hCG level alone and not based on time from molar evacuation.
Before the development of effective chemotherapy, Delfs noted that 22% of her patients had an elevated hCG level more than 60 days after molar evacuation and, of these, 42% required hysterectomy.80 Other reports have also observed that 36–40% of patients with persistent hCG elevations more than 60 days after molar evacuation required therapy.72, 87 However, no deaths were observed in the study by Lurain and colleagues even among patients who had therapy instituted more than 60 days after evacuation.87 These studies indicate that although patients with hCG elevations persisting after evacuation of hydatidiform mole are at an increased risk for postmolar GTT, the majority can be safely followed using serial hCG testing.
We recommend that patients with hydatidiform mole have therapy instituted according to the following criteria: (1) hCG level rise, (2) hCG level plateau (± 10%) for three or more consecutive weekly measurements (x, x + 7 days, x + 14 days); (3) appearance of metastases; or (4) histologic evidence of choriocarinoma, placental site trophoblastic tumor, or invasive mole. Using these criteria, we have continued to treat approximately 20% of our patients after molar evacuation.
PREGNANCY AFTER HYDATIDIFORM MOLE
A majority of women who have been treated for hydatidiform mole are in their prime reproductive years, and many desire future child bearing. Physicians from the New England Trophoblastic Disease Center reported a large series of women whose reproductive outcomes were studied after evacuation of complete hydatidiform moles.114 The risk for stillbirth, prematurity, spontaneous abortion, and congenital malformation was similar to that in the general population. Recurrent molar gestations were observed in 1.3% of their patients.79 Other series have also reported an increased risk for repetitive complete and partial moles ranging between 1% and 2%.115, 116 Furthermore, the risk of a third molar gestation increases to 28% after a second mole.117
Therefore, after a woman has had a molar pregnancy, she should be reassured as to the likely normal outcome of future pregnancies but she should be aware of the increased risk of a repetitive molar gestation. We recommend obtaining an ultrasound scan early in pregnancy to confirm normal fetal and placental development, combined with a chest roentgenogram to screen for occult metastasis from choriocarcinoma masked by the hCG level rise of pregnancy. The placenta or products of conception should be examined histologically at the time of delivery or pregnancy evacuation. Additionally, a hCG level should be obtained 6–8 weeks after delivery of any future pregnancy to exclude the rare occurrence of choriocarcinoma.
Gestational trophoblastic tumors
The diagnosis of GTT is established when a woman has rising or plateauing hCG levels or develops metastatic disease after evacuation of a hydatidiform mole. The diagnosis of invasive mole, placental site tumor, or choriocarcinoma is a histologic criterion for GTT. Approximately one half to two thirds of cases of GTT follow molar pregnancies, while gestational choriocarcinomas derived from term pregnancies, spontaneous abortions, and tubal pregnancies account for the remainder.35 Often patients who develop GTT after nonmolar gestations may present with nongynecologic signs and symptoms, including gastrointestinal or urologic hemorrhage, hemoptysis, or cerebral hemorrhage.118, 119 Irregular uterine bleeding or amenorrhea may also be observed. Under these circumstances, the diagnosis of GTT is facilitated by a high index of suspicion coupled with hCG level testing and the exclusion of a normal pregnancy. It should be stressed that the possibility of metastatic GTT should be considered in any woman of the reproductive age-group presenting with metastatic disease involving the lungs or distant sites from an unknown primary site of malignancy.
GTT invade into the myometrium and penetrate small uterine vessels. Venous metastasis then occurs, resulting in retrograde metastasis to the lower genital tract through the vaginal venous plexus, with direct spread to the parametrium and distant spread to the lungs. Usually systemic hematogenous metastases occur only after pulmonary metastases have become established.120 Small pulmonary metastases might not be detected by conventional chest roentgenography but can be detected using computed tomography scans of the lungs in approximately 40% of patients treated for “nonmetastatic” GTT.121 Therefore, we recommend that all women with GTT should have a complete metastatic survey before initiating treatment, consisting of chest roentgenography or CT scan of the lungs and CT scan of the brain, abdomen, and pelvis. An immediate pretreatment hCG value should be obtained in addition to performance of a complete blood cell count and renal and liver function tests. We obtain a pelvic ultrasound to exclude the possibility of intrauterine pregnancy before beginning chemotherapy. The role of MRI studies for the evaluation of women with GTT is not yet fully defined, however, small series of patients indicate that these scans may help localize small foci of intrauterine disease and identify myometrial invasion.122, 123
Arteriography is not routinely used for initial radiographic evaluation since false-negative and false-positive findings are encountered more frequently than with CT scans. Angiographic changes can persist in the uterus of women with GTT long after hCG remission has been achieved.124 Therefore, selective arteriography is reserved only as an optional diagnostic tool to delineate lesions of unclear etiology detected by other imaging techniques.
Before CT or MRI scans of the brain were available, Bagshawe and Harlan reported that occult central nervous system (CNS) metastases may be detected using lumbar puncture with simultaneous serum and cerbrospinal fluid (CSF) hCG level determinations.125 The plasma–CSF hCG level ratio is normally greater than 60:1 in the absence of CNS metastases from GTD and is usually less than 60:1 in those patients with CNS metastases.125 However, some investigators have reported falsely lowered plasma–CSF hCG ratios among women undergoing first-trimester abortions without trophoblastic disease and in patients with nonmetastatic GTT.126 Because CT scans of the brain with contrast enhancement have excellent sensitivity and specificity for metastatic disease and can be used in conjunction with MRI scans to evaluate questionable lesions detected on CT scans, we have not routinely obtained CSF hCG levels in the initial evaluation of patients with GTT. Most frequently, we have used CSF hCG levels to evaluate patients with resistance to chemotherapy who have an obscure site of persistent disease.
Although operative procedures may be useful in the therapy for women with GTT, they are rarely indicated for staging or diagnosis alone. Although histologic evaluation of tissue obtained by D&C may yield prognostic information relating to response to first-line chemotherapy,127 secondary D&C in patients with rising hCG levels after molar evacuation will rarely result in spontaneous remission when used without chemotherapy.123
The therapeutic routine efficacy of pretreatment D&C combined with chemotherapy has never been formally evaluated in a randomized study. Berkowitz and associates127 evaluated routine pretreatment D&C in 37 patients with nonmetastatic postmolar GTT. Twenty (54%) had no trophoblastic tissue detected by pretreatment D&C; 19 of these patients developed sustained remission with limited chemotherapy. Patients having intrauterine disease with a worsened histology by Hertig and Sheldon criteria28 were at risk for failure of initial chemotherapy.127 None of the patients in this study suffered uterine perforation or other complications. However, Schlaerth and associates documented an 8.1% incidence of uterine perforation during D&C performed in this setting, requiring hysterectomy in two patients.128 The effect of pretreatment D&C on hCG level regression has never been established. Furthermore, others have documented that results of postevacuation curettage rarely affect the management of postmolar GTT.129, 130 Therefore, we prefer to reserve secondary D&C for patients who experience significant uterine bleeding during chemotherapy.
Laparoscopy, craniotomy, and thoracotomy are rarely justified to establish the primary diagnosis of GTT since this diagnosis can be made on the basis of elevated hCG level coupled with radiographic evidence of metastatic disease after excluding pregnancy. Consideration of the possibility of GTT in these settings would render the majority of these diagnostic surgical procedures unnecessary.
Although we have found that the vast majority of patients with high-risk metastases from GTT almost always have radiographic evidence of pulmonary metastases,131 we remain reluctant to recommend a less than compulsive radiographic evaluation before initiation of treatment. Selection of the initial therapy and subsequent survival is largely dependent on identification of poor prognostic factors in patients with metastatic disease. It would be a tragedy to miss the diagnosis of a high-risk metastatic site in a patient with a negative chest roentgenogram who might otherwise be salvaged with aggressive therapy.
CLASSIFICATION AND STAGING
The International Federation of Gynecology and Obstetrics (FIGO) has developed a staging system for women with GTT that is based on anatomic site involvement, conforming to FIGO staging systems used for all other gynecologic cancers (see Table 2).132 Although this system recognizes the stepwise progression of metastases in GTT, we believe that it fails to take into account other factors that are important in determining the prognosis for individual patients with GTT. Assignment of therapy on the basis of anatomic involvement alone is not warranted: although essentially all of the patients in stage I have low-risk disease and all of the patients in stage IV have high-risk disease, there is considerable overlap in stage II and III, with a substantial minority of patients in these groups who have significant risk factors beyond the anatomic site of involvement. This aspect of GTT has long been recognized, and several prognostic classifications have been used for grouping patients with GTT.
Table 2. International Federation of Gynecology and Obstetrics (FIGO) staging for gestational trophoblastic tumors
Stage | Description |
I | Strictly confined to uterine corpus |
II | Extends outside the uterus, but limited to genital structures |
III | Extends to the lungs with or without genital tract involvement |
IV | All other metastatic sites |
(Modified from Pettersson F, Kolstad P, Ludwig H et al: Annual Report on the Results of Treatment in Gynecologic Cancer, vol 19. Stockholm, International Federation of Gynecology and Obstetrics, 1985)
After the development of single-agent methotrexate and actinomycin therapy and initial reports of successful chemotherapy in patients with GTT, it was observed that essentially all patients with nonmetastatic disease could be cured with single-agent chemotherapy but that certain patients with metastatic disease were at higher risk for failure of treatment with these agents. In 1965, the National Institutes of Health (NIH) group identified several factors in patients with GTT that predicted resistance to single-agent chemotherapy, including high pretreatment hCG level, prolonged duration of disease, brain or liver metastases, and previous failed or inadequate therapy.133 In the late 1960s and early 1970s, Hammond and co-workers134 and others135 recognized that patients with these risk factors were more likely to be cured if they were treated initially with combination rather than single-agent chemotherapy.
A clinical classification system based on these risk factors is most frequently used in the United States to determine initial treatment and report results (Table 3).134 Gestational trophoblastic tumors are divided into three categories: nonmetastatic, low-risk/good-prognosis metastatic, and high-risk/poor-prognosis metastatic. Essentially all patients with nonmetastatic GTT can be cured using initial single-agent chemotherapy; therefore, they are not assigned to a prognostic category and are all initially treated with single-agent chemotherapy. The presence of any single high-risk factor in a patient with metastatic GTT places her in the high-risk/poor-prognosis metastatic GTT category. A similar system is used at the Memorial Sloan-Kettering Cancer Center, which, however, subdivides patients into low-, moderate-, and high-risk groups based on the criteria used in the clinical classification system.136 This system recognizes that patients who have high pre-treatment hCG levels or long duration of disease as the only prognostic risk factors are at lesser risk of treatment failure than patients with metastases involving the brain and/or liver or those who have failed prior chemotherapy.
Table 3. Clinical classification of gestational trophoblastic tumors
- Nonmetastatic: No evidence of disease outside uterus
- Metastatic
- Good prognosis
- Short duration of symptoms (<4 months)
- Low hCG level (<40,000 mIU/ml serum β-hCG)
- No metastases to brain or liver
- No antecedent term pregnancy
- No prior chemotherapy
- Short duration of symptoms (<4 months)
- Poor prognosis (any high-risk factor)
- Long duration of symptoms (>4 months)
- High pretreatment hCG level (>40,000 mIU/ml serum β-hCG)
- Brain or liver metastases
- Antecedent term pregnancy
- Prior chemotherapy (unsuccessful)
- Long duration of symptoms (>4 months)
- Good prognosis
Compiled from multiple sources134, 137, 138, 139
The main virtue of the clinical classification system is that it allows prompt identification of patients who would be likely to be successfully cured using simple forms of chemotherapy and identifies patients who would be unlikely to be cured using single-agent treatment. In the past, the majority of patients who were treated with combination chemotherapy received methotrexate, dactinomycin, and chlorambucil (or cyclophosphamide) combinations, commonly referred to as MAC.134, 135, 136 With the development of other agents active against GTT and of combinations that are perhaps more active against GTT than MAC, several investigators have reported on the failure of the clinical classifications system to identify patients at risk for failing MAC-type regimens.
A more complicated prognostic scoring system has been adopted by the World Health Organization (WHO, Table 4).140 This system is based on Bagshawe's experience at the Charing Cross Hospital in London during 1957–1973.141 Multiple factors were found to have prognostic significance when analyzed separately, including patient age, parity, type of antecedent pregnancy, time interval between antecedent pregnancy and development of GTT, pretreatment hCG level, paternal and maternal blood type, number and site of metastases, size of largest tumor, and treatment with prior chemotherapy. The WHO prognostic index score applies a weighted score to each factor; each factor is assumed to act independently, and the sum of individual component scores is used to determine the individual patient's risk. Patients with a score of 4 or less are considered low risk, those with a score of 5–7 are intermediate risk, and those with scores 8 or greater are high risk.140 Unfortunately, paternal blood type is not uniformly available for many patients treated in the United States; therefore, several investigators have modified this system to omit blood group information.142, 143, 144 Using modifications of the WHO prognostic index score, several researchers have reported that the WHO prognostic index score correlates with survival in patients with high-risk disease by the clinical classification system who are treated with MAC-based chemotherapy.142, 143, 144 It is important to consider the individual prognostic factors and their impact on the survival of patients with GTT.
Table 4. World Health Organization prognostic index score for gestational trophoblastic tumors
| Score* | |||
Prognostic Factors | 0 | 1 | 2 | 4 |
Age (years) |
| >39 |
|
|
Antecedent pregnancy | Hydatidiform mole | Abortion | Term |
|
Interval† | <4 | 4–6 | 7–12 | >12 |
hCG (IU/liter) | <103 | 103–104 | 104–105 | >105 |
ABO groups (female × male) |
| O × A | B |
|
Largest tumor, including uterine tumor |
| 3–5 cm | >5 cm |
|
Site of metastases |
| Spleen, kidney | Gastrointestinal tract, liver | Brain |
Number of metastases identified |
| 1–4 | 4–8 | >8 |
Prior chemotherapy |
|
| Single drug | Two or more |
* The total score for a patient is obtained by adding the individual scores for each prognostic factor.
Total score 0–4 = low risk, 5–7 = intermediate risk, >8 = high risk.
† Interval: time (months) between end of antecedent pregnancy and start of chemotherapy.
(Modified from World Health Organization Scientific Group on Gestational Trophoblastic Disease. Technical Report Series No. 692. Geneva, World Health Organization, 1983)
Nonmetastatic disease
The majority of patients in this category have GTT diagnosed after molar evacuation. This category is probably heavily weighted toward those patients with proliferative moles or invasive moles rather than true choriocarcinoma. Virtually all series of patients reported since Hammond and associates145 described the use of methotrexate and dactinomycin in the treatment of these patients have essentially 100% sustained remission rates after primary treatment with single-agent regimens. Therefore, we do not assign patients with nonmetastatic GTT to prognostic risk groups.
hCG levels
With the exception of placental site trophoblastic tumors, hCG is a sensitive and specific marker for the monitoring of patients with GTT. Levels presumably reflect the total body burden of viable tumor. It should be specified that all of the classification systems use the immediate pretreatment hCG level to assign treatment, rather than the hCG level at the time of molar evacuation. Ross and colleagues initially reported that women with metastatic GTT who had hCG levels more than 100,000 IU/24 h urine collection had a remission rate of 41% versus 91% in those with lower levels.133 This finding has been confirmed in several subsequent studies.135, 141 In particular, Bagshawe noted a graduated risk associated with increasing levels of serum hCG, ranging from 4% when the hCG level was less than 10,000 mIU/ml to 61% when the level was greater than 1 million mIU/ml.141 However, when analyzing patients with metastatic GTT who had not received previous treatment, we noted that hCG level alone did not predict outcome.137 This undoubtedly reflects the fact that almost all patients with high hCG levels in this report were initially treated with combination chemotherapy regimens,137 thus negating the prognostic importance of the hCG level alone that was observed among patients treated with single-agent therapy.
Duration of disease
The duration of GTT, as measured by time from termination of the previous pregnancy or duration of symptoms until the initiation of treatment, indirectly reflects the potential for the development of spontaneous drug resistance and tumor growth. Ross and colleagues first reported a worse outcome in patients with a duration of disease in excess of 4 months.133 Bagshawe found a progressively increasing fatality rate with time, ranging from 3% at less than 3 months to 63% at more than 24 months.141 Significantly lower remission rates for patients with lengthy duration of disease have also been reported by other investigators,135, 137, 138, 142, 146 even among previously untreated patients who receive combination chemotherapy for high-risk factors.
Metastatic site
Detailed studies by Sung and co-workers have documented that the progression of GTT proceeds initially into the myometrium with pulmonary and vaginal metastases occurring earlier and brain metastases or other disseminated metastases occurring later in the progression of disease.120, 147 As noted previously, the absence of metastases is a favorable prognostic factor. Metastatic sites form the basis for the anatomic FIGO staging system.132 Site of metastatic involvement is an important prognostic factor in determining survival. Several studies have documented the high-risk nature of brain, liver, and renal metastases. However, when initial treatment is individualized, the effect of initial metastatic site of involvement appears to be lessened.137
Type of antecedent pregnancy
The type of antecedent gestation probably has two indirect effects on outcome. First, patients who are treated for GTT after a nonmolar pregnancy almost always have choriocarcinoma while those treated after evacuation of hydatidiform mole may have persistent mole, invasive mole, or, less commonly, choriocarcinoma. Several series have documented that a clinicopathologic diagnosis of choriocarcinoma imparts a worse prognosis for patients with metastatic GTT when compared with a clinicopathologic diagnosis of invasive mole.135, 137 Second, women with GTT after a nonmolar pregnancy often experience a delay in diagnosis that allows establishment of metastatic disease.137 Bagshawe reported a graded effect of antecedent pregnancy with patients who had spontaneous abortions and ectopic pregnancies having an intermediate prognosis between those with GTT following hydatidiform mole and term pregnancies.141 On the other hand, in our review of patients who presented for primary treatment of metastatic GTT, patients with an antecedent spontaneous abortion or ectopic pregnancy had a risk similar to that of patients with an antecedent term pregnancy.137 A prolonged interval from pregnancy termination to therapy was seen in the majority of women with nonmolar antecedent pregnancy.137 Other studies have also confirmed that GTT following a term pregnancy has a worse outcome than GTT following other types of pregnancy, and most patients often have other high-risk characteristics.148, 149 Because of the frequent association of antecedent term pregnancy with high-risk disease, we include GTT following term pregnancy in the poor prognosis group.
ABO blood type
Bagshawe documented a worse outcome among women with blood type B or AB, or maternal/paternal pairings of type O × A or A × O.141 However, Azab and associates did not confirm a prognostic effect for ABO blood type in their patient population.150 As mentioned earlier, because reliable information regarding paternal ABO blood type is lacking for many patients with GTT, several investigators have modified the WHO scoring system to exclude ABO blood type information.
Tumor size and number of metastases
In addition to hCG level, tumor burden should be reflected by the size of the largest tumor and number of metastatic sites or number of identifiable metastases. However, in GTT both the primary tumor and metastatic sites may be composed partially of hemorrhage or necrotic tissue. This is particularly true for uterine sites of persistent or invasive moles and metastatic sites of choriocarcinoma. Therefore tumor volume, as identified on examination or radiographic studies, may not correlate precisely with actual tumor burden. However, Bagshawe reported that increasing tumor size had an adverse effect on outcome.141 Several studies have also noted that increasing numbers of identifiable metastatic sites or increasing numbers of anatomic sites involved with metastases increase the risk for failure of therapy.137, 142, 144, 150
Prior chemotherapy
Failure of primary chemotherapy is, arguably, the single highest risk factor in patients with GTT. Hammond and colleagues reported a 14% salvage rate with multiagent chemotherapy for patients with high-risk disease who had failed primary chemotherapy versus 70% among those with high-risk disease who are treated initially with multiagent chemotherapy.133 Since this report, numerous other series have reported that failed previous chemotherapy is a high-risk factor.137, 138, 141, 142, 143, 144, 150, 146 This undoubtedly is caused by multiple factors, including inappropriate initial therapy, failure to initially diagnose high-risk sites of metastatic involvement, emergence of drug-resistant tumor because of drug-induced resistance or spontaneous mutations imparting resistance, alteration of host–tumor immunologic relationships, and development of metastatic foci that are relatively impervious to chemotherapy because of surrounding fibrosis. Unfortunately, inclusion of patients who have failed primary chemotherapy in several reports investigating prognostic factors undoubtedly confounds analysis because the majority of these patients have long duration of disease and other high-risk factors at the time they are treated with salvage therapy. Nonetheless, these patients are the most challenging group to treat, requiring intense therapy and often integrating components of chemotherapy, surgery, and radiation therapy in an attempt to optimize survival.
TREATMENT
Therapy for nonmetastatic tumors
Before effective chemotherapeutic regimens were widely available for GTT, surgical therapy for even nonmetastatic disease was generally unsatisfactory. Brewer and colleagues reported a poor survival rate when hysterectomy was used as the only treatment for choriocarcinoma.151 Since the introduction of methotrexate chemotherapy for GTT, most centers have reported almost 100% cure rates for patients with nonmetastatic disease using single-agent regimens. Therefore, we do not use the prognostic factors to assign treatment of patients without evidence of metastatic disease at the time of pretherapy metastatic survey.
Methotrexate and dactinomycin have been the principle agents used for treatment of nonmetastatic GTT; however, 5-fluorouracil and oral etoposide have been used with excellent results. Despite the high rate of success using primary chemotherapy, hysterectomy is still used in the treatment of selected patients with nonmetastatic GTT and should be considered in those who desire concurrent sterilization. Currently, many centers are investigating options of chemotherapy that would retain a high remission rate for this disease and reduce toxicity. Results of representative studies using chemotherapy for nonmetastatic GTT are reviewed in Table 5.
Table 5. Results of treatment for nonmetastatic gestational trophoblastic tumors
| Remission (%) | ||
Chemotherapy | No. of Patients | Primary | Overall |
Dactinomycin |
|
|
|
5 day |
|
|
|
Goldstein et al.152 | 12 | 84 | 100 |
Petrilli and Morrow153 | 13 | 77 |
|
Bolus |
|
|
|
Petrilli and Morrow153 | 5 | 80 |
|
Twiggs154 | 12 | 100 | 100 |
Petrilli et al.155 | 31 | 94 | 100 |
5-Fluorouracil |
|
|
|
Sung et al.156 | 69 | 93 |
|
Etoposide (VP-16), oral |
|
|
|
Wong et al.157* | 60 | 98 | 100 |
Methotrexate |
|
|
|
5 day, intramuscular |
|
|
|
Hammond et al.145 | 47 | 93 | 98 |
Smith et al.158 | 39 | 92 | 100 |
5 day, oral |
|
|
|
Barter et al.159 | 15 | 87 |
|
Methotrexate/folinic acid |
|
|
|
Berkowitz et al.160 | 163 | 90 | 100 |
Smith et al.158 | 29 | 72 | 100 |
Wong et al.157* | 68 | 76 | 100 |
Mutch et al.121 | 39 | 75 | 100 |
Bolis et al.161 | 51 | 88 | 100 |
Bagshawe et al.162* | 348 | 74 | 99.7 |
Weekly |
|
|
|
Homesley et al.163 | 63 | 81 | 99 |
* Includes patients with low-risk metastatic gestational trophoblastic tumors.
Methotrexate Methotrexate has been used in the treatment of GTT since the 1950s and has resulted in almost 100% cure rates in patients with nonmetastatic diseases. Methotrexate administered in a dose of 0.4 mg/kg intramuscularly for 5 days with cycles repeated every 12–14 days was used as primary therapy in 58 patients with nonmetastatic disease at NIH.133 Only four patients (7%) had tumors resistant to this first-line therapy, and three of these were salvaged with second-line therapy with dactinomycin. Toxicity from this regimen includes essentially universal alopecia, mucositis, significant neutropenia, and cutaneous toxicity in approximately 70%; gastrointestinal toxicity in approximately 50%; thrombocytopenia in approximately 40%; and symptomatic sterile inflammation of the pleura or peritoneum in approximately 30% of patients. There were no therapy-related deaths.134 Additional reports have documented excellent cure rates of nonmetastatic GTT using single-agent methotrexate, but alternative methotrexate regimens have also been designed to reduce toxicity.
Barter and associates reported that 5-day cycles of methotrexate given orally could be substituted for parenteral methotrexate.159 Thirteen (87%) of 15 patients who were treated exclusively with oral methotrexate achieved remission. Although their retrospective review revealed that this outpatient form of administration produced minimal toxicity,159 we have been reluctant to recommend oral methotrexate. Compliance may be unreliable, and erratic absorption of oral methotrexate might produce subtherapeutic or toxic levels in individual patients. Therefore, we prefer parenteral regimens for chemotherapy for women with GTT.
Bagshawe and Wilde first proposed methotrexate with folinic acid rescue as therapy for GTT.164 The rationale for folinic acid administration is to “rescue” the normal tissues from the dihydrofolate reductase block induced by methotrexate, allowing a higher dose of methotrexate to be administered with the rescue of normal tissues by folinic acid. Rotmensch and colleagues evaluated serial methotrexate levels during both single-agent daily methotrexate and alternating methotrexate-folinic acid therapy for patients with GTT.165 They observed that alternate-day dosing with methotrexate resulted in higher peak concentrations but also resulted in subtherapeutic and subtoxic trough levels more frequently than did conventional daily methotrexate.165 This finding alone might account for the observed therapeutic effect and diminished toxicity of this regimen compared with daily methotrexate without any need for postulating an effect of folinic acid rescue.
Various methotrexate–folinic acid schedules have been used. The New England Trophoblastic Disease Center has reported considerable experience with intramuscular methotrexate, 1 mg/kg, on days 1, 3, 5, and 7 alternately with intramuscular folinic acid, 0.1 mg/kg, on days 2, 4, 6, and 8.160 A single cycle of chemotherapy was given for nonmetastatic GTT, and hCG regression patterns were followed. If a patient had a progressive decrease in hCG levels and entered remission, no further therapy was given. If hCG levels plateaued for more than 3 weeks or became reelevated, patients were re-treated. Alternate therapy with dactinomycin was used for salvage. In the experience at this center, 147 women with nonmetastatic and 22 with low-risk metastatic GTT have been treated with this approach.160 One hundred twenty-one patients (82%) with nonmetastatic disease achieved remission with one cycle of chemotherapy, whereas the remainder required two or more cycles to achieve remission. Sixteen (10%) required alternative therapy. Patients with nonmetastatic disease were more likely to respond to this regimen than those with metastases. Likewise, patients with hCG levels greater than 50,000 mIU/ml responded less frequently than those with lower hCG levels. Toxicity was minimal, with significant myelotoxicity was observed in less than 6% and hepatic toxicity in 14% of patients.160 Increasing the methotrexate dose to 1.5 mg/kg alternatively with folinic acid, 0.15 mg/kg, did not improve the response rate but did increase both significant hepatic and hematologic toxicity.166
Methotrexate–folinic acid has also been administered as repetitive cycles until remission is achieved. Smith and associates158 and Mutch and co-workers121 used repeated courses of methotrexate, 1.0 mg/kg, on days 1, 3, 5, and 7, alternating with folinic acid, 0.1 mg/kg, given 24 hours after each dose of methotrexate. Wong and associates used the same schedule but administered folinic acid rescue 30 hours after each dose of methotrexate.167 In these reports, cycles were usually repeated at 14-day intervals until remission was achieved as determined by hCG measurements. Smith and associates reported a higher failure rate with the cyclical methotrexate–folinic acid regimen than with conventional 5-day methotrexate (27.5% vs. 7.7%).158 However, four (10%) of 39 patients treated with single-agent methotrexate had therapy changed to dactinomycin because of significant toxicity. All patients in their report who failed initial methotrexate–folinic acid or methotrexate therapy were salvaged using alternative single-agent chemotherapy.158 Mutch and colleagues, reporting from the same institution, confirmed a 26% failure rate for cyclical methotrexate–folinic acid as primary chemotherapy for nonmetastatic GTT.121 Eight of 10 patients who failed initial methotrexate–folinic acid chemotherapy had small pulmonary metastases detected by pulmonary CT scanning but not by conventional chest roentgenograms. This feature was identified to be a poor prognostic factor for the success of this regimen, but, again, all patients were salvaged with alternative treatment with dactinomycin.121
Wong and co-workers likewise compared their experience with single-agent methotrexate and methotrexate–folinic acid regimens in patients with nonmetastatic or low-risk metastatic GTT.167 They observed a lower failure rate for methotrexate–folinic acid than for single-agent methotrexate in similar groups of patients but noted significantly increased hepatic toxicity in those treated with the methotrexate–folinic acid regimen. Their protocol differed somewhat from that used by Smith and co-workers158 in that folinic acid was administered 30 hours after methotrexate rather than 24 hours. Additionally, they noted a high endemic rate of hepatitis in their patient population.
Bolis and colleagues reported approximately an 89% complete remission rate among 51 women with nonmetastatic GTT who received a median two courses of methotrexate–folinic acid to achieve normal hCG values.161 Only three (50%) of six patients with low-risk metastatic disease achieved remission; however, all patients who failed the methotrexate–folinic acid regimen were salvaged with alternative therapy. Bolis and colleagues also found higher failure rates among women with pretreatment hCG levels greater than 10,000 mIU/ml, uterine enlargement suggestive of deep myometrial invasion, and a histologic diagnosis of choriocarcinoma.161
A large experience with methotrexate–folinic acid was reported by Bagshawe and co-workers.162 Between 1974 and 1986, 348 women with nonmetastatic and low-risk metastatic GTT received methotrexate–-folinic acid chemotherapy. Overall, 74% achieved remission with this regimen, while 20% required alternative therapy because of drug-resistance and 6% changed therapy because of drug-induced toxicity. The sustained remission rate for this group of women was 99.7%.162
Berkowitz and associates compared therapy with 300 mg/m2 methotrexate intravenous infusion over 12 hours followed by folinic acid “rescue” as primary therapy for 32 women with nonmetastatic GTT with their previous experience using the 8-day intramuscular methotrexate–folinic acid regimen.168 The primary complete remission rate of approximately 69% with the methotrexate infusion was significantly worse (p < 0.01) than their experience with the 8-day regimen toxicity. Toxicity, however, was minimal and all patients whose disease was resistant to the methotrexate infusion were salvaged with alternative therapy. Although this regimen had a higher failure rate than 8-day methotrexate–folinic acid, they believed that the shortened duration of chemotherapy and minimal toxicity offered advantages over many other regimens.168
Holmesley and co-workers evaluated a unique regimen using weekly intramuscular administration of methotrexate in women with nonmetastatic GTD.163 Initial therapy consisted of 30 mg/m2, with the dose progressively accelerated to 50 mg/m2 in responding patients. Eighty-two per cent of their patients achieved remission with a median of seven cycles of therapy. All nonresponders were salvaged with alternative single-agent therapy, and no significant toxicity was observed. The investigators concluded that this regimen represented efficacious therapy for nonmetastatic GTT that was minimally toxic and cost-effective. Unfortunately, all of the patients in this series were treated for GTT diagnosed after molar evacuation.163 Although we use this regimen for patients with postmolar nonmetastatic GTT, there is insufficient evidence of efficacy to recommend its use in treatment of women with nonmetastatic GTT arising after nonmolar gestations. Unfortunately, no prospective randomized trial has evaluated any of the methotrexate regimens in the treatment of women with nonmetastatic GTT.
Dactinomycin Following the reports that patients with metastatic GTT could achieve primary and secondary remissions with single-agent dactinomycin, Goldstein and colleagues reported on their experience with dactinomycin given intravenously in doses of 9–13 μg/kg/day for 5 days, recycled at 14-day intervals as primary therapy in 12 patients with nonmetastatic GTT.169 Two patients with the disease resistant to dactinomycin were salvaged with methotrexate therapy, and all patients eventually achieved sustained remission. The most common toxic effects included nausea and vomiting (reported in two thirds of patients), alopecia, and transient bone marrow depression.169 Petrilli and Morrow reported similar results with this regimen.153 There were no therapy-related deaths in either report. Because of the more frequent gastrointestinal toxicity, alopecia, and potential for extravasation injury, most investigators have reserved 5-day dactinomycin as salvage therapy for patients with nonmetastatic GTT after failure of initial methotrexate regimens.
Petrilli and Morrow first proposed using a single intravenous bolus of dactinomycin (40 μg/kg = 1.25 mg/m2) administered as a pulse every 2 weeks.153 Their rationale was that dactinomycin has a long half-life and that the total dose administered was equivalent to the more traditional 5-day courses. In comparative analysis, three of 13 patients failed 5-day dactinomycin versus one of five patients treated with the dactinomycin bolus.153 Toxicity was similar in both regimens. Twiggs subsequently treated 12 patients with this regimen and reported that all patients entered remission after an average of 4.8 cycles of chemotherapy.170 There was one episode of significant thrombocytopenia in his patient population. Likewise, a Gynecologic Oncology Group study reported on 31 patients treated with this regimen.155 Twenty-nine (94%) achieved remission after an average 4.4 cycles of chemotherapy. There was no grade 3 or 4 hematologic or hepatic toxicity and only a 10% incidence of grade 3 gastrointestinal toxicity, which responded to conventional antiemetics.155 Thus, bolus dactinomycin also represents cost-effective chemotherapy with an acceptable remission rate but slightly higher toxicity than weekly methotrexate.
5-Fluorouracil Sung and associates used 5-fluorouracil 28–30 mg/kg/day, as a slow intravenous infusion over 10 days to treat patients with metastatic and nonmetastatic GTT.156 Cycles were repeated at 24–31-day intervals. Sixty-four (93%) of 69 patients with nonmetastatic GTT entered sustained remission with 5-fluorouracil. Toxicity during the first cycle of 5-fluorouracil in 212 previously untreated patients included an 11.3% incidence of severe nausea and vomiting, 4.2% incidence of stomatitis, and 7.5% of hepatoxicity. Approximately 20% suffered diarrhea, with 15 patients developing pseudomembranous colitis. The incidence of severe hematologic toxicity was low, and significant toxicity was stated to be less than in the investigators' previous experience with bolus administration of 5-fluorouracil.156 Experience with 5-fluorouracil as therapy for GTT has been limited in reports from Western countries, but the drug must be considered an active agent in the treatment of GTT. The 10-day hospitalization required for the infusions and high rate of significant toxicity, however, do not make it an attractive agent for patients with nonmetastatic disease.
Etoposide Etoposide (VP-16) has been established as an active single agent in the therapy for GTT. Wong and colleagues used oral etoposide in doses of 200 mg/m2 for 5 days, recycled at 12–14-day intervals.157 Fifty-nine (98%) of 60 patients with nonmetastatic and low-risk metastatic GTT entered remission with the regimen. One patient was changed to methotrexate–folinic acid chemotherapy because of intolerable gastrointestinal toxicity. All patients developed alopecia, and 25% suffered mild to moderate myelosuppression, with only one episode of significant neutropenia less than 1000 cells/mm3. All patients reported some degree of nausea, but only five required antiemetic therapy.157 Because of the cost of oral etoposide and universal alopecia related to this drug, most investigators in the United States have preferred to use methotrexate or dactinomycin regimens as initial treatment for women with nonmetastatic GTT.
Surgical therapy Surgery alone was available as therapy for invasive mole or nonmetastatic choriocarcinoma before the availability of methotrexate. Brewer and colleagues reported only a 40% 2-year survival rate for women with nonmetastatic choriocarcinoma and 15% survival for those with metastatic choriocarcinoma who were treated with hysterectomy alone.151 In contrast, Wilson and associates locally excised intrauterine foci of invasive mole in five patients who wished to preservation of fertility.96 None of their patients received chemotherapy, and all subsequently entered sustained remission.
Since the advent of effective chemotherapy, surgical treatment of nonmetastatic GTT has assumed a lesser role but is still helpful in managing selected patients. Hammond and associates evaluated their experience with surgery integrated into the treatment of patients with GTT.139 One hundred thirty-nine patients with nonmetastatic GTT were evaluated in this report: 122 patients were treated with primary chemotherapy, whereas 17 were treated with chemotherapy and primary hysterectomy. Hysterectomy during the first cycle of chemotherapy significantly reduced the number of cycles of chemotherapy necessary to produce remission and shortened the total duration of therapy. One hundred and six (87%) of the 122 patients treated primarily with chemotherapy achieved remission without requiring additional forms of therapy, whereas 13 required surgery and three required pelvic infusion of chemotherapy. Therefore, 89% of patients with nonmetastatic GTT who desired preservation of fertility were able to retain child-bearing capacity.139
We have performed primary or secondary hysterectomy under the coverage of chemotherapy and have not observed unusual toxicity.139 Lewis and co-workers initially advocated the coverage of surgical extirpation of GTT coordinated with a chemotherapy “umbrella” and likewise noted no increase in wound complications or infection.171, 172 The rationale for this combined approach is that chemotherapeutic coverage destroys microscopic metastases that are undetected at the time of surgery and also prevents establishment of any trophoblastic elements that might be embolized intraoperatively.
We generally perform an abdominal hysterectomy with preservation of the adnexa unless there is evidence of adnexal metastases or the patient is in the perimenopausal age-group. This allows abdominal exploration to exclude the possibility of occult extrauterine metastases. However, we have performed standard vaginal hysterectomies in a few patients with nonmetastatic GTT without noting an increased rate of complications or failure of therapy.
Hysterectomy may remove foci GTT that can become relatively drug resistant on the basis of surrounding tissue fibrosis.139 Occasionally, patients with invasive mole are found to have diffuse disease in the endometrial cavity. In those treated with secondary hysterectomy, an intrauterine nodule of choriocarcinoma or invasive mole is often found to be encased in dense fibrosis that might be expected to reduce tissue perfusion and limit exposure of neoplastic tissues to chemotherapy.
General management Our general approach to the management of patients with nonmetastatic GTT is outlined in Table 6. We usually use either the weekly methotrexate or 8-day methotrexate–folinic acid regimens as initial chemotherapy and reserve dactinomycin for salvage therapy.
Table 6. Suggested management of gestational trophoblastic tumors
Nonmetastatic | |
Initial therapy | Single-agent methotrexate; primary hysterectomy if patient desires sterilization (only) |
Salvage therapy | Single-agent dactinomycin; combination chemotherapy, if needed; secondary hysterectomy |
Low-Risk/Good-Prognosis Metastatic | |
Initial therapy | Single-agent methotrexate; primary hysterectomy if patient desires sterilization (only) |
Salvage therapy | Single-agent dactinomycin; combination chemotherapy, if needed; secondary hysterectomy |
High-Risk/Poor-Prognosis Metastatic | |
Initial therapy | MAC or EMA/CO regimens; consider brain or liver irradiation for metastases |
Salvage therapy | Individualized combination chemotherapy; specific surgical extirpation as indicated |
MAC, methotrexate, dactinomycin, and chlorambucil or cyclophosphamide; EMA/CO, etoposide, methotrexate, dactinomycin/cyclophosphamide, vincristine.
Therapy for low-risk/good-prognosis metastatic tumors
The majority of patients with low-risk/good-prognosis metastatic GTT by the clinical classification criteria have low- or medium-risk WHO prognostic index scores. Dating from the 1965 report by Ross and associates, which reported a 95% complete remission rate in women with metastatic GTT having short duration of disease and low hCG levels,133 numerous investigators have reported excellent results using either methotrexate or dactinomycin as the initial therapy for patients with low-risk, good-prognosis metastatic GTT. In general, the remission rates for 5-day cycles of either agent are comparable and patients who fail initial therapy with one agent can be salvaged with the alternative single agent. In our experience, approximately one third of patients with low-risk metastatic GTT will require a change from 5-day methotrexate to dactinomycin; roughly equal numbers of patients are treated with alternative therapy because of toxicity or the development of drug-resistant disease, as evidenced by the development of new metastases or nonresponse of hCG levels to initial chemotherapy.
As noted earlier, patients with low-risk metastatic disease have been treated with methotrexate and folinic acid. Complete remission rates of 50–68% have been reported for patients with low-risk metastatic disease treated with this regimen.121, 160, 161, 162, 167 All patients with resistant disease were subsequently cured with 5-day dactinomycin or combination chemotherapy. Because of the slight lower remission rates with methotrexate–folinic acid regimens, we have continued to use 5-day methotrexate therapy for patients in this disease category.
Pulsed dactinomycin has also been used to treat a few patients with low-risk metastatic GTT. Petrilli and Morrow treated five patients with low-risk metastatic disease using the pulsed dactinomycin regimen described for patients with nonmetastatic GTT.153 All achieved remission. The major toxicity resulting from this schedule is gastrointestinal. Further experience is needed to confirm the efficacy of this schedule in the treatment of patients with low-risk metastatic GTT.
Both 5-fluorouracil infusional therapy156 and oral etoposide157 have been successfully used in the treatment of patients with low-risk metastatic GTT. However, since 5-fluorouracil requires a prolonged infusion given daily for 10 days and has significant mucosal and gastrointestinal toxicity and etoposide causes universal alopecia, these agents are unlikely to replace methotrexate or dactinomycin as initial therapy for patients with low-risk metastatic GTT.
Surgery In part because of the dismal results of treatment with surgical therapy of metastatic GTT, results of combined therapy were reported only sporadically in the 1970s. Hammond and co-workers reviewed a series of patients treated for GTT at our center and reported on hysterectomy applied to therapy for patients with good-prognosis metastatic GTT.139 Approximately 13% of 40 patients with good-prognosis metastatic disease treated initially with chemotherapy required hysterectomy to achieve sustained remission. Additionally, performance of primary hysterectomy in conjunction with chemotherapy reduced the time required to induce remission and limited chemotherapy requirements compared with treatment alone. Fifteen patients who underwent primary hysterectomy and chemotherapy required an average of only 3.8 cycles of chemotherapy versus 5.9 cycles among patients treated primarily with chemotherapy.139 Therefore, there is a rationale for combining primary hysterectomy with chemotherapy in patients with limited extrauterine metastasis who desire sterilization.
General management Our recommended approach to patients with low-risk/good-prognosis metastatic GTT is outlined in Table 6. We generally use 5-day intramuscular administration of methotrexate as the initial chemotherapeutic regimen.
Therapy for high-risk/poor-prognosis metastatic tumors
As previously noted, Hammond and co-workers first reported that patients with metastatic GTT who had one or more high-risk/poor-prognosis factors benefited from initial multiagent chemotherapy, rather than waiting for drug resistance to single-agent therapy to initial multiagent treatment.134 Subsequently, most American centers have treated patients with high-risk metastatic GTT with multiagent chemotherapy, based largely on the clinical classification system discussed previously.136, 137, 138, 142, 143, 144, 146 However, not all patients with this designation have high-risk disease by the WHO prognostic index score. Although the overall success for multiagent chemotherapy of patients with high-risk/poor-prognosis metastatic GTT ranges between 63% and 80% using methotrexate- and dactinomycin-based combinations,134, 136, 137, 138, 142, 143, 144, 146 several groups have reported that women with medium-risk disease by WHO prognosis index score are often cured with single-agent therapy and almost always cured with combination chemotherapy, compared with 40% to 50% among those with high WHO prognostic index scores.137, 142, 143, 144 Most of these series have analyzed patients treated with modifications of MAC combination chemotherapy; recent reports have focused on treatment of patients with regimens that employ etoposide with or without cisplatin. To date, no study has compared the efficacy and toxicity of etoposide- or cisplatin-containing regimens with MAC-based chemotherapy.
“Triple” MAC chemotherapy consisting of methotrexate, 0.3 mg/kg IM, dactinomycin, 8–10 μg/kg IV, and chlorambucil, 0.2 mg/kg PO (or cyclophosphamide, 250 mg IV), each given daily for 5 days has been the combination regimen most often reported in American centers.134 Cycles are repeated every 14–21 days and, in most American studies, patients with brain and liver metastases are given radiation therapy, as described subsequently. Using variations of this regimen, most investigators have reported sustained remission rates in 60–80% of patients with high-risk/poor-prognosis metastatic GTT.134, 136, 137, 138, 142, 143, 144, 146, 173, 174, 175 MAC chemotherapy frequently results in significant toxicity, particularly when recycled at intervals of less than 21 days.
In the mid 1970s, Bagshawe proposed a complex, alternating eight-drug schedule incorporating methotrexate, dactinomycin, cyclophosphamide, doxorubicin, melphalan, hydroxyurea, and vincristine (CHAMOMA) in women with high-risk metastatic GTT.176 Surwit and colleagues reported our initial experience with a modification of this regimen, commenting on the impression of reduced toxicity and high initial remission rates in women who had failed to respond to MAC chemotherapy.177 However, when Weed and associates reviewed a larger series of women treated with modified CHAMOMA, the primary remission rate dropped to less than 60% with a much higher incidence of toxicity.178 This undoubtedly reflected the preponderance of previously treated patients and longer follow-up in their series. Somewhat higher remission rates have been reported in other single-arm reports. When CHAMOMA was used as primary therapy for 50 patients with high-risk GTT, Wong and associates reported an 82% rate of sustained complete remission.179 In summarizing the Charing Cross experience in treating women with high-risk GTT, Newlands and associates reported a 75% remission rate among those who received CHAMOMA.180 However, a detailed analysis, including the primary remission rate, of these patients was not presented.
The Gynecologic Oncology Group subsequently reported the results of the only prospectively randomized trial of chemotherapy conducted for women with GTT.174 They compared a standard MAC combination regimen recycled at 21-day intervals to a modified CHAMOMA regimen in women with high-risk metastatic disease. Primary remissions were achieved in 73% of 22 patients treated with MAC versus 65% of 20 treated with CHAMOMA (p = not significant); however, the overall survival after salvage therapy was 96% for those treated initially with MAC versus 70% for those receiving CHAMOMA. The incidence of life-threatening hematologic and overall toxicity was higher in patients receiving CHAMOMA (p = 0.004 and 0.014, respectively). This study was terminated early because of the marked difference in toxicity between the two regimens. On the basis of the similar primary remission rate and only 9% incidence of life-threatening hematologic toxicity, it was concluded that the MAC regimen had a more favorable therapeutic index than the modified CHAMOMA regimen.174
Oral or intravenous etoposide is highly active in the therapy for GTT as primary156 or salvage therapy.176 A regimen using alternating cycles of etoposide-methotrexate-dactinomycin and cyclophosphamide-vincristine (EMA-CO, Table 7) was designed by the group at Charing Cross Hospital in London to incorporate etoposide into the initial therapy of women with high-risk GTT.180 Newlands and co-workers reported that survival of patients with high-risk metastatic GTT by WHO prognostic index score criteria was enhanced using this combination when compared with previous combinations used by their group.180 Bolis and associates also evaluated this regimen as therapy for 36 women with high-risk/poor-prognosis metastatic GTT.181 They reported an 86% primary complete remission rate, with 76% of their patients disease free but 12% alive with persistent hCG level elevations despite salvage therapy at completion of their study. Only 5.5% of patients experienced life-threatening hematologic toxicity.181
Table 7. EMA/CO chemotherapy for high-risk metastatic gestational trophoblastic tumors
Course A | |||
Day 1 | Dactinomycin | 500 μg | IV bolus |
| Etoposide | 100 mg/m2 | IV infusion over 30 minutes |
| Methotrexate* | 100 mg/m2 | IV bolus |
|
| 200 mg/m2 | IV infusion over 12 hours |
Day 2 | Dactinomycin | 500 μg | IV bolus |
| Etoposide | 100 mg/m2 | IV infusion over 30 minutes |
| Folinic acid | 15 mg | IM/PO q6th |
|
|
| Begin 12 hours after methotrexate infusion completed |
Course B | |||
Day 8† | Vincristine | 1 mg/m2 | IV bolus |
| Cyclophosphamide | 600 mg/m2 | IV infusion |
Day 15 | Recycle Course A |
|
|
* In patients with central nervous system metastases increase methotrexate to 1 g/m2 as 24-hour IV infusion. Increase folinic acid to 15 mg IM/PO q8h × 9 doses beginning 12 hours after methotrexate infusion completed.
† Patients with central nervous system metastases or with high-risk WHO prognostic index scores receive 12.5 mg methotrexate by intrathecal injection.
(Compiled from multiple sources180, 181, 182, 183)
One significant feature of this regimen is that the EMA portion can be given during a 2-day hospitalization, whereas the CO portion can be given on an outpatient basis. The Charing Cross group recommends administering intrathecal methotrexate with each CO portion of the cycle to prevent the development of brain metastases.180 This combination is attractive therapy for women with high-risk metastatic GTT because of the inclusion of etoposide and since it has a primary complete response rate that appears to be slightly better than that for MAC triple therapy. However, its efficacy has not been prospectively evaluated in randomized trials. It should also be noted that the incidence of life-threatening hematologic toxicity with EMA-CO reported by Bolis and associates181 was very similar to that reported for MAC by the Gynecologic Oncology Group study.174
Theodore and co-workers incorporated cisplatin and etoposide together as first-line therapy for patients with high-risk GTT using cisplatin 100 mg/m2 on day 1, with dactinomycin 300 μg/m2 and etoposide 100 mg/m2 delivered on days 1–3 and 14–16 of each 28-day cycle.184 All eight patients with high-risk GTT who received primary chemotherapy with this regimen responded. Despite the concerns of using cisplatin for front-line therapy for patients with GTT, toxicity was acceptable with no treatment-related deaths.
Central nervous system metastasis Patients with metastasis to the CNS have long been recognized to be at an increased risk for failure of primary therapy compared with patients who have metastatic disease limited to the lungs or vagina. Bagshawe assigned CNS involvement the highest level of risk among metastatic sites.141 Although the vast majority of patients with CNS involvement have lung and/or liver involvement, a few series have reported CNS metastases in patients who do not have evidence of active metastatic disease elsewhere.185
Based in part on the work by Brace,186 who reported a significant incidence of CNS control using whole-brain irradiation, we have incorporated whole-brain radiation therapy into the primary treatment of patients with brain metastasis. A dose of 3000 cGy is administered concurrently with MAC chemotherapy, delivered in 10 equal fractions. Overall survival for patients treated in this fashion is 50%, with survival of 70–89% in patients treated for primary brain metastases versus only 30% for those who receive salvage treatment for brain metastases.137, 187, 188, 189
In contrast, the group at Charing Cross Hospital recommends using primary chemotherapy with EMA-CO and reported a 72% survival among patients who received primary therapy for brain metastases using this regimen.183 They increase the dose of methotrexate to 1 g/m2 and administer intrathecal methotrexate during the CO portion of each cycle. They also advocate early surgical intervention for patients who have a lesion anatomically suitable for resection.
Patients with CNS metastases of GTT are at risk for neurologic decompensation caused by cerebral edema and acute hemorrhage into these highly vascular and often centrally necrotic lesions. We routinely administer dexamethasone throughout the course of whole-brain radiation therapy in an effort to minimize cerebral edema. Neurosurgical consultation is obtained early in the course of treatment, in the event that surgical decompression and extirpation is required. Surgical extirpation is generally reserved for patients who demonstrate neurologic decompensation or those who require salvage therapy for recurrent CNS metastases of GTT. Despite the fact that CNS metastasis of GTT constitutes a poor prognosis factor in this disease entity, it should be noted that the excellent survival of patients treated for primary brain metastases of GTT stands in sharp contrast to the grim outlook for patients with brain metastases from other sites of malignancy. Therefore, the diagnosis of GTT metastatic to the brain should be entertained in any woman of the reproductive age-group who presents with brain metastases from an unknown primary site or unexplained CNS hemorrhage.
Hepatic metastases Metastases to the liver are identified in 2–8% of patients presenting for primary therapy of metastatic GTT.137, 190 Involvement of the liver constitutes a poor prognostic factor, as evidenced by the 40–50% survival rates for patients with primary involvement at this site and dismal survival in patients who develop liver metastases while being treated for drug-resistant disease.137, 138, 191 The optimal management of hepatic metastases is unknown. Hepatic metastases tend to be highly vascular, and death is frequently caused by catastrophic intra-abdominal hemorrhage. In an effort to minimize this risk, we have used approximately 2000 cGy whole-liver radiation therapy in conjunction with combination chemotherapy.191 Toxicity has not prohibited the administration of chemotherapy using this approach. Barnard and associates reported that only one of 13 patients treated with combined therapy developed postirradiation hepatitis.191 Others have reported the use of selective hepatic embolization in an attempt to limit hemorrhage from hepatic metastases.192, 193 However, the experience is too small to draw strong conclusions regarding the most efficacious therapy for these high-risk patients.
Other high-risk metastases Metastatic GTT has been reported to involve virtually every organ in various studies from prechemotherapy literature. However, metastases to the kidneys, gastrointestinal tract, and other organs occur with a lower frequency than brain, vaginal, or pulmonary metastases in series of women who present for primary chemotherapy for GTT.137 Bagshawe considered renal and gastrointestinal metastases as intermediate in risk between pulmonary or vaginal metastases and brain metastases141 but did not give survival data to justify this ranking.
Gastrointestinal metastases may present as multiple submucosal metastases along the intestine, resulting in chronic gastrointestinal bleeding, or may result from direct invasion by pelvic disease. Unless there is significant gastrointestinal hemorrhage from a single focus of disease, these patients do not usually require surgery and can be treated successfully with chemotherapy alone. In our experience, all five patients with gastrointestinal or intra-abdominal metastases who presented for primary therapy were cured.137
Renal metastasis occurred in 1.3% of 154 women who presented for primary therapy and 14% of 42 referred for salvage therapy of metastatic GTT at our center.194 Only three of eight patients survived, all of whom presented for primary therapy with unilateral renal metastasis and limited systemic metastases involving the lungs only. The role of nephrectomy or radiation therapy in the treatment of patients with renal metastases is unclear. Six of our patients with renal metastases underwent nephrectomy and three survived. Nephrectomy should be considered in the treatment of patients with unilateral renal involvement and limited disease involving the lungs but may not contribute to survival in cases with extensive systemic metastases.194 Irradiation of both kidneys is unlikely to achieve significant disease control for patients with bilateral metastases given the low radiation tolerance of the kidneys; however, unilateral renal metastases might be treated with irradiation.
Drug-resistant disease Patients with high-risk metastatic GTT who have failed primary chemotherapy are extremely challenging to treat and have a very poor prognosis. The details of previous therapy must be reviewed completely to identify potentially active agents that have not been used. Surgical extirpation of drug-resistant foci of disease must be considered in patients with limited systemic metastases.
Before cisplatin and etoposide were available, salvage regimens for patients failing MAC-type regimens were rarely successful.139, 173 The CHAMOMA regimen was used as salvage therapy at our center through 1985, with an apparent improvement in results compared with previous single-agent and combination regimens.177, 178 Although the vinblastine-cisplatin-bleomycin regimen was reported by Azab and associates to produce high complete remission rates among their patients with drug-resistant GTT,195 others have observed few remissions using this regimen without including surgical resection.196, 197, 198 Additionally, toxicity from this regimen is often severe among heavily pretreated patients.198
Regimens containing etoposide, both with and without cisplatin, have definite activity in patients with drug-resistant GTT. The EMA/CO regimen180, 181 and EMA portion alternating with etoposide-cisplatin180 have been successfully used as salvage therapy. Wong and colleagues used an etoposide-methotrexate-bleomycin combination to produce remissions in eight of nine women who experienced failure on the CHAMOMA regimen.199 Others have successfully employed etoposide-cisplatin combinations for these extremely high-risk patients.183, 200
We generally employ EMA-CO for patients who have not been exposed to etoposide, and we have salvaged a few patients with etoposide-cisplatin regimens who have failed EMA-CO. Another regimen that should be considered is the etoposide-ifosfamide-mesna-cisplatin salvage regimen used in the treatment of patients with refractory testicular germ cell malignancies.201 We have noted anecdotal responses to this regimen in heavily pretreated patients. Therapy is often limited by accrued toxicity, and patients must be aggressively re-treated, even in the presence of life-threatening hematologic toxicity, in an effort to optimize the chance for survival.
Surgery Although the majority of women with high-risk metastatic GTT are not suitable candidates for surgical extirpation based on extensive metastases of their disease, surgery is incorporated in approximately 30% of women with poor-prognosis metastatic GTT.139 In addition to surgical procedures aimed at extirpating sites of disease, additional procedures are often needed to treat complications of malignancy such as hemorrhage or infection, thus stabilizing the patient to allow continuation of therapy. In particular, we frequently use tunnelled central venous catheters in these patients since they often require antibiotic therapy, support with blood products, and nutritional support202 during the course of treatment.
Hysterectomy incorporated into the primary therapy for women with poor-prognosis metastatic GTT does not appear to be as efficacious as for those with nonmetastatic or good-prognosis metastatic GTT.139 However, occasional patients with persistent disease confined to the uterus can be salvaged with a delayed hysterectomy. Before hysterectomy is performed, the patient must be carefully rescreened to rule out occult metastatic involvement and must be able to withstand a major surgical procedure. As in all extirpative procedures performed on patients with GTT, we perform surgery under the umbrella of systemic chemotherapy. Usually, we will administer single-agent therapy in an attempt to limit toxicity.
Thoracotomy with pulmonary wedge resection is the surgical procedure used most frequently for removal of distant drug-resistant metastases from GTT. Even though pulmonary resection can be performed safely under the coverage of chemotherapy, it is not necessary to resect pulmonary nodules in the majority of patients. Although those with persistent pulmonary nodules on chest roentgenography may be at an increased risk for recurrent GTT, radiographic evidence of tumor regression may lag far behind the hCG level response.203 Therefore, there is little justification for resection of pulmonary metastases that persist on chest roentgenography during chemotherapy with a satisfactory hCG level response or after the induction of hCG level remission.204
Many investigators have reported success with the resection of pulmonary nodules in highly selected women with drug-resistant disease.205, 206, 207, 208, 209 Before performing thoracotomy it is important to exclude the possibility of disease elsewhere. We perform computed tomographic evaluations of the brain, thorax, and abdomen, as well as simultaneous CSF and serum β-hCG levels to screen for occult extrapulmonary metastases. If hysterectomy has not been performed, active pelvic disease should be excluded radiographically before thoracotomy.
Prognostic factors for planned resection of pulmonary metastases of GTT have been reviewed by Tomoda and associates.208 These researchers found that the following criteria predicted a successful resection: (1) good surgical candidate; (2) primary malignancy controlled (uterus excised or no evidence of pelvic disease on angiography); (3) no evidence of other systemic metastases; (4) solitary pulmonary lesion; and (5) persistent hCG level of less than 1000 mIU/ml despite chronic chemotherapy. In their series, 14 of 15 (93%) patients who satisfied these criteria survived after pulmonary resection compared with none of the four patients who had one or more unfavorable factors.208 Other investigators have reported that a prompt hCG level remission after pulmonary resection predicts a favorable outcome.205, 206, 207, 208, 209
Several investigators have reported successful imaging of occult foci of choriocarcinoma using radioimmunoscintigraphy with iodine-131-labeled polyclonal210, 211, 212 or monoclonal213 anti-hCG antibodies or monoclonal antibodies directed against a human testicular germ cell cancer.214 Because many patients with persistent or recurrent disease may have sites of pulmonary scarring resulting from previous surgery or previous active metastases, these techniques are promising adjuncts to conventional imaging studies. Further investigations are needed to clarify the sensitivity and specificity of the various radioimmunoscintigraphy techniques in patients with GTT.
Coordination of therapy It should be stressed that the results cited for therapy for patients with high-risk/poor-prognosis metastatic GTT are from centers that specialize in the treatment of women with this disease. We believe strongly that all women with high-risk/poor-prognosis disease should be treated in consultation with a physician at a trophoblastic disease center or by a physician who is experienced in the management of patients with GTT who can coordinate all aspects of therapy. A general outline of our approach is given in Table 6.
MONITORING OF PATIENTS DURING THERAPY
Patients being treated with chemotherapy for GTT should be monitored at least weekly using sensitive assays for serum β-hCG. Rotmensch and co-workers have reported that women treated for nonmetastatic GTT have a log-linear clearance of serum hCG levels after initiation of treatment.165 In our experience, patients with low-risk metastatic GTT exhibit a similar pattern of hCG regression among patients who respond to methotrexate regimens. A 1-log reduction in hCG is observed between the first and second weeks of chemotherapy.215 In general, we will consider a change in regimens if there is less than 25% reduction in the hCG level or a rise following a single course of therapy. Chemotherapy should be continued beyond the first normal hCG level in an attempt to prevent the development of recurrent GTT. Remission is defined by three normal hCG levels over 14 days. After completion of chemotherapy, patients are monitored with serial hCG values at 2-week intervals for the first 3 months after completion of chemotherapy and at least monthly for the first year of surveillance. Although it is rare for a patient to develop recurrent disease more than 1 year after remission,216 we recommend following hCG values at 6-month intervals indefinitely.165 Late recurrences have been reported several years after completion of treatment.
RECURRENT DISEASE
Recurrent episodes of GTT are defined as reelevation of hCG levels or appearance of new metastases after induction of remission, without an intervening pregnancy. Recurrence is diagnosed in less than 5% of patients with nonmetastatic or low risk metastatic GTT but in approximately 20% of patients with high-risk disease.216 Approximately 80% of all recurrences are identified less than 12 months after completion of chemotherapy; therefore the risk of recurrence after the first year of normal hCG levels is less than 1% for an individual patient.182 Patients who have been treated for one episode of recurrent GTT are at an increased risk for a subsequent recurrence after induction of a secondary remission and must be monitored closely after completion of chemotherapy.215
It has long been recognized that normal hCG values can be present with a significant tumor burden. Bagshawe first recommended administering chemotherapy after normalization of hCG values in an attempt to reduce the risk of recurrence of GTT.141 We routinely administer one cycle of chemotherapy beyond the first normal hCG value in patients being treated for nonmetastatic GTT, one or two cycles for those being treated for good-prognosis/low-risk metastatic GTT, and three or more cycles for patients being treated for high-risk/poor-prognosis GTT. Patients who are being treated for recurrent GTT receive additional chemotherapy in proportion to the amount of treatment required to achieve hCG level remission. Several investigators have reported that similar strategies of maintenance chemotherapy appear to decrease the incidence of recurrent GTT when compared with historical experience.135, 216, 217
PLACENTAL SITE TROPHOBLASTIC TUMOR
Placental site trophoblastic tumors are composed of intermediate trophoblastic cells and deserve special consideration because they do not secrete hCG to the same extent as other forms of GTT.35, 36, 37, 38, 39, 40 These lesions run the gamut in clinical course from benign trophoblastic proliferations of the placental site that can be cured with uterine curettage to locally invasive neoplasms that will metastasize outside the uterus in less than 20% of patients.35, 36, 37, 38, 39, 40 Unlike other forms of GTT, placental site trophoblastic tumors are not sensitive to single-agent methotrexate or dactinomycin chemotherapy. Histologic identification of placental site trophoblastic tumor or radiographic identification of bulky disease in conjunction with a low hCG level should suggest the possibility of this lesion.35 Hysterectomy should be employed as primary therapy for cases of nonmetastatic placental site trophoblastic tumors, while combination chemotherapy should be employed for the treatment of metastatic disease. The optimal regimen has not been identified, but two apparent complete remissions have been reported in patients with metastatic placental site trophoblastic tumors who received EMA-CO chemotherapy.180
PREGNANCY AFTER TREATMENT OF GESTATIONAL TROPHOBLASTIC TUMORS
Gestational trophoblastic tumors characteristically develop in women in the prime reproductive age-group. The majority of these women can be cured using chemotherapy alone, thus preserving the potential for child-bearing capacity. During the first year after completion of therapy, pregnancy is deferred so that hCG surveillance is not disrupted by an intercurrent pregnancy. Several series of women treated with simple chemotherapy for nonmetastatic and low-risk metastatic GTT have reported an apparently normal reproductive capacity in women who desired child-bearing.139, 218, 219, 220 Although there is an apparent higher incidence of spontaneous abortions, this apparently higher abortion rate may be related to intense hCG surveillance in these patients and might not be different from that experienced by the general population.220 The congenital malformation rate appears to be comparable to that observed in the general population. Although placenta accreta may be increased in women with pregnancies after treatment of GTT,219 other obstetric complications do not appear to be increased.139, 220 The only other specific increased risk after treatment for GTT is the inherent increased risk of a repeat molar gestation.
Additional information is available from China, indicating that pregnancies after successful treatment of GTT with 5-fluorouracil or an antibiotic chemotherapeutic agent similar to dactinomycin (KSM) resulted in no increase in the incidence of pregnancy wastage, congenital anomalies, perinatal mortality, or multiple gestations.221 An increased incidence of placenta accreta and postpartum hemorrhage was noted, similar to studies in patients treated with methotrexate and dactinomycin. Cytogenetic studies performed on 94 children revealed no increase in chromosomal anomalies.221
Therefore, it appears that patients who have been successfully treated for GTT and desire preservation of fertility can be encouraged in this regard. The majority of pregnancies after treatment for GTT are uncomplicated, and there does not appear to be an increased risk of congenital malformations. These women should be counseled as to the increased risk for a repeat molar gestation and should undergo an ultrasound scan at 6–8 weeks of gestation to exclude the possibility of a second mole and to confirm fetal viability. A chest roentgenogram should be obtained with appropriate uterine shielding to exclude the possibility of occult metastatic disease. Products of conception or placenta should be examined histologically at the time of delivery to exclude choriocarcinoma. Six to 8 weeks after delivery, we recommend that an hCG value be obtained to exclude the possibility of recurrent GTT.
REFERENCES
Brewer JI, Gerbie AB, Dolkart ER et al: Chemotherapy in trophoblastic disease. Am J Obstet Gynecol 90: 566, 1964 |
|
Hammond CB, Borchert L, Tyrey L et al: Treatment of metastatic trophoblastic disease: Good and poor prognosis. Am J Obstet Gynecol 115: 451, 1973 |
|
Smith EB, Weed JC, Tyrey L et al: Treatment of nonmetastatic gestational trophoblastic disease: Results of methotrexate alone versus methotrexate-folinic acid. Am J Obstet Gynecol 144: 88, 1982 |
|
Hammond CB, Hertz R, Ross GT et al: Primary chemotherapy for nonmetastatic gestational trophoblastic neoplasms. Am J Obstet Gynecol 98: 71, 1967 |
|
Hammond CB, Parker RT: Diagnosis and treatment of trophoblastic disease. Obstet Gynecol 35: 132, 1970 |
|
Hertz R, Lewis J, Lipsett MB: Five years' experience with chemotherapy of metastatic choriocarcinoma and related trophoblastic tumors in women. Am J Obstet Gynecol 82: 631, 1961 |
|
Berkowitz RS, Goldstein DP: Methotrexate with citrovorum factor rescue for nonmetastatic gestational trophoblastic neoplasia. Obstet Gynecol 54: 725, 1979 |
|
Lewis J, Gore H, Hertig AT et al: Treatment of trophoblastic neoplasms: With rationale for the use of adjunctive chemotherapy at the time of indicated operation. Am J Obstet Gynecol 96: 710, 1966 |
|
Ross GT, Goldstein DP, Hertz R et al: Sequential use of methotrexate and actinomycin-D in treatment of metastatic choriocarcinoma and related trophoblastic diseases in women. Am J Obstet Gynecol 93: 223, 1965 |
|
Goldstein DP, Saracco P, Osathanondh R et al: Methotrexate with citrovorum factor rescue for gestational trophoblastic neoplasms. Obstet Gynecol 51: 93, 1978 |
|
Maroulis GB, Hammond CB, Johnsrude IS et al: Arteriography and infusional chemotherapy in localized trophoblastic disease. Obstet Gynecol 45: 397, 1975 |
|
Bagshawe KD: Chemotherapy regimens for high-risk trophoblastic disease. J Reprod Med 29: 813, 1984 |
|
Hammond CB, Weed JC, Currie JL: The role of surgery in the current therapy of gestational trophoblastic disease. Am J Obstet Gynecol 136: 844, 1980 |
|
Ober WB, Fass RO: The early history of choriocarcinoma. J Hist Med Allied Sci 16: 49, 1961 |
|
Hertig AT, Mansell H: Tumors of the female sex organs: I. Hydatidiform mole and choriocarcinoma. In: Atlas of Tumor Pathology. Washington, DC, Armed Forces Institute of Pathology, 1956 |
|
Park WW: Choriocarcinoma: A general review with analysis of 516 cases. Arch Pathol 49: 73, 1950 |
|
Hertig AT, Sheldon WH: Hydatidiform mole: A pathological correlation of 200 cases. Am J Obstet Gynecol 53: 1, 1947 |
|
Curry SL, Hammond CB, Tyrey L et al: Hydatidiform mole: Diagnosis, management and long-term follow-up of 347 patients. Obstet Gynecol 45: 1, 1975 |
|
Brewer JI, Rinehart JJ, Dunbar R: Choriocarcinoma. Am J Obstet Gynecol 81: 574, 1961 |
|
Bulmer JW, Johnson PM: Antigen expression by trophoblast populations in human placenta and their possible immunobiologic relevance. Placenta 6: 127, 1985 |
|
Szulman AE: The A, B, and H blood-group antigens in human placenta. N Engl J Med 286: 1028, 1972 |
|
Pijnenborg R, Bland JM, Robertson WB et al: The pattern of interstitial trophoblast invasion of the myometrium in early human pregnancy. Placenta 2: 303, 1981 |
|
Covone AE, Johnson PM, Mutton D et al: Trophoblast cells in peripheral blood from pregnant women. Lancet 2: 841, 1984 |
|
Szulman AE: Trophoblastic disease: Clinical pathology of hydatidiform moles. Obstet Gynecol Clin North Am 15: 433, 1988 |
|
Szulman AE, Surti U: The syndromes of hydatidiform moles: I. Cytogenetic and morphologic correlations. Am J Obstet Gynecol 131: 665, 1978 |
|
Szulman AE, Surti U: The syndromes of hydatidiform moles: II. Morphologic evolution of the complete and partial mole. Am J Obstet Gynecol 132: 20, 1978 |
|
Szulman AE, Surti U: The clinicopathologic profile of the partial hydatidiform mole. Obstet Gynecol 59: 597, 1982 |
|
Hertig AT, Sheldon WH: Hydatidiform mole: A pathologicoclinical correlation of 200 cases. Am J Obstet Gynecol 53: 1, 1947 |
|
Lurain J, Brewer JI: Invasive mole. Semin Oncol 9: 174, 1982 |
|
Jacobs PA, Szulman AE, Funkhouser J et al: Human triploidy: Relationship between paternal original of the additional haploid complement and development of partial hydatidiform mole. Ann Hum Genet 46: 223, 1982 |
|
Kajii T, Ohama K: Androgenetic origin of hydatidiform mole. Nature 268: 633, 1977 |
|
Surti U, Szulman AE, O'Brien S: Dispermic origin and clinical outcome of three complete hydatidiform moles with 46, XY karyotype. Am J Obstet Gynecol 144: 84, 1982 |
|
Fisher RA, Lawler SD: Heterozygous complete moles: Do they have a worse prognosis than homozygous complete moles? Lancet 2: 51, 1984 |
|
Lawler SD, Fisher RA, Pickthall VJ et al: Genetic studies on hydatidiform moles. I. The origin of partial moles. Cancer Genet Cytogenet 5: 309, 1982 |
|
Mazur MT, Kurman RJ: Choriocarcinoma and placental site tumor. In Szulman AE, Buchsbaum HJ (eds): Gestational Trophoblastic Disease, p 45. New York, Springer-Verlag, 1987 |
|
Twiggs LB, Okagaki T, Phillips GC et al: Trophoblastic pseudotumor: Evidence of malignant disease potential. Gynecol Oncol 12: 238, 1981 |
|
Gloor E, Dialdas J, Hurlimann J et al: Placental site trophoblastic tumor (trophoblastic pseudotumor) of the uterus with metastases and fatal outcome. Am J Surg Pathol 7: 483, 1983 |
|
Hopkins M, Nunez C, Murphey JR et al: Malignant placental site trophoblastic tumor. Obstet Gynecol 66: 95S, 1985 |
|
Finkler NS, Berkowitz RS, Driscoll SG et al: Clinical experience with placental site trophoblastic tumors at the New England Trophoblastic Disease Center. Obstet Gynecol 71: 854, 1988 |
|
Lathrop JC, Lauchlan S, Nayak R et al: Clinical characteristics of placental site trophoblastic tumor (PSTT). Gynecol Oncol 31: 32, 1988 |
|
Buckley J: Epidemiology of gestational trophoblastic diseases. In Szulman AE, Buchsbaum HJ (eds): Gestational Trophoblastic Disease, p 8. New York, Springer-Verlag, 1987 |
|
Chattopadhyay SK, Sengupta BS, al-Ghreimi M et al: Epidemiologic study of gestational trophoblastic diseases in Saudi Arabia. Surg Gynecol Obstet 167: 393, 1988 |
|
McCorriston CC: Racial incidence of hydatidiform mole. Am J Obstet Gynecol 101: 377, 1968 |
|
Matsuura J, Chin D, Jacobs PA et al: Complete hydatidiform mole in Hawaii: An epidemiological study. Genet Epidemiol 1: 271, 1984 |
|
Bagshawe KD, Dent J, Webb J: Hydatidiform mole in England and Wales 1973-1983. Lancet 2: 673, 1986 |
|
Mazzanti P, LaVecchia C, Parazzini F et al: Frequency of hydatidiform mole in Lombardy, Italy. Gynecol Oncol 24: 337, 1986 |
|
Parazzini F, LaVecchia C, Pampallona S: Parental age and risk of complete and partial hydatidiform mole. Br J Obstet Gynaecol 93: 582, 1986 |
|
Acaia B, Parazzini F, LaVecchia C et al: Increased frequency of complete hydatidiform mole in women with repeated abortion. Gynecol Oncol 31: 310, 1988 |
|
Brinton LA, Wu BZ, Wang W et al: Gestational trophoblastic disease: A case-control study from the People's Republic of China. Am J Obstet Gynecol 161: 121, 1989 |
|
Atrash HK, Hogue CJ, Grimes DA: Epidemiology of hydatidiform mole during early gestation. Am J Obstet Gynecol 154: 906, 1986 |
|
Berkowitz RS, Cramer DW, Brenstein MR et al: Risk factors for complete molar pregnancy from a case-control study. Am J Obstet Gynecol 152: 1016, 1985 |
|
Parazzini F, LaVecchia C, Mangili G et al: Dietary factors and risk of trophoblastic disease. Am J Obstet Gynecol 158: 93, 1988 |
|
Park WW, Lees JC: Choriocarcinoma: A general review, with analysis of 516 cases. Arch Pathol 49: 73, 1950 |
|
McAna JF: Gestational choriocarcinoma in New York State. Diss Abstr Int 48: 694, 1987 |
|
Brinton LA, Braken MB, Connelly RR: Choriocarcinoma incidence in the United States. Am J Epidemiol 123: 1094, 1986 |
|
Bandy LC, Clarke-Pearson DL, Hammond CB: Malignant potential of gestational trophoblastic disease at the extremes of reproductive life. Obstet Gynecol 138: 6, 1984 |
|
Buckley JD, Henderson BE, Morrow CP et al: Case-control study of gestational choriocarcinoma. Cancer Res 48: 1004, 1988 |
|
Tyrey L: Human chorionic gonadotropin assays and their uses. Obstet Gynecol Clin North Am 15: 457, 1988 |
|
Morgan FJ, Birken S, Canfield RE: The amino acid sequence of human chorionic gonadotropin: The &b.alpha; subunit and &b.beta; subunit. J Biol Chem 250: 5247, 1975 |
|
Birken S: Chemistry of human choriogonadotropin. Ann Endocrinol (Paris) 45: 297, 1984 |
|
Nurreddin A, Atassi MZ: Differentiation of the contribution of the two subunits of lutropin to its in vivo activity. Biochim Biophys Acta 533: 257, 1978 |
|
Keutmann HT, Charlesworth MC, Mason KA et al: A receptor-binding region in human choriogonadotropin/lutropin beta subunit. Proc Natl Acad Sci USA 84: 2038, 1987 |
|
Vaitukaitis JL, Braunstein GD, Ross GT: A radioimmunoassay which specifically measures human chorionic gonadotropin in the presence of human luteinizing hormone. Am J Obstet Gynecol 113: 751, 1972 |
|
Vaitukaitis JL: Radioimmunoassay of human choriogonadotropin. Clin Chem 31: 1749, 1985 |
|
Hussa RO, Rinke ML, Schweitzer PG: Discordant human chorionic gonadotropin results: Causes and solutions. Obstet Gynecol 65: 211, 1985 |
|
Pettersson K, Siitari H, Hemmila I et al: Time-resolved fluoroimmunoassay of human choriogonadotropin. Clin Chem 29: 60, 1983 |
|
Lougren T, Hemmila I, Pettersson K et al: Time-resolved fluorometry in immunoassays. In Collins WP (ed): Alternative Immunoassays, p 205, New York, John Wiley & Sons, 1985 |
|
Nishimura R, Ashitaka Y, Tojo S: The clinical evaluation of the simultaneous measurements of human chorionic gonadotropin (hCG) and its alpha subunit in sera of patients with trophoblastic diseases. Endocrinol Jpn 26: 575, 1979 |
|
Quigley MM, Tyrey L, Hammond CB: Utility of assay of alpha subunit of human chorionic gonadotropin in management of gestational trophoblastic malignancies. Am J Obstet Gynecol 138: 545, 1980 |
|
Bagshawe KD: Clinical applications of hCG. In Patillo RA, Hussa RO (eds): Human Trophoblast Neoplasms, p 313. New York, Plenum Press, 1984 |
|
Kohorn EI, Caldwell BV, Cortes JM: Alpha-subunit in gestational trophoblastic disease. Placenta 3: 231, 1981 |
|
Khazaeli MB, Buchina ES, Patillo RA et al: Radioimmunoassay of free beta-subunit of human chorionic gonadotropin in diagnosis of high-risk and low-risk gestational trophoblastic disease. Am J Obstet Gynecol 160: 444, 1989 |
|
Berkowitz R, Ozturk M, Goldstein DP et al: Human chorionic gonadotropin and free subunits serum levels in patients with partial and complete hydatidiform moles. Obstet Gynecol 74: 212, 1989 |
|
Berkowitz RS, Goldstein MR, Bernstein MR et al: Natural history of partial molar pregnancy. Obstet Gynecol 66: 677, 1986 |
|
Berkowitz RS, Goldstein DP, DuBeshter B et al: Management of complete molar pregnancy. J Reprod Med 32: 634, 1987 |
|
Morrow CP, Kletzky OA, DiSaia PJ et al: Clinical and laboratory correlates of molar pregnancy and trophoblastic disease. Am J Obstet Gynecol 128: 424, 1977 |
|
Curry SL, Hammond CG, Tyrey L et al: Hydatidiform mole: Diagnosis, management and long-term follow up of 347 patients. Obstet Gynecol 45: 1, 1975 |
|
Montz FJ, Schlaerth JB, Morrow CP: The natural history of theca lutein cysts. Obstet Gynecol 72: 247, 1988 |
|
Amir SM, Osathanoudh R, Berkowitz RS et al: Human chorionic gonadotropin and thyroid function in patients with hydatidiform mole. Am J Obstet Gynecol 150: 723, 1984 |
|
Delfs E: Chorionic gonadotropin determinations with hydatidiform mole and choriocarcinoma. Ann NY Acad Sci 80: 125, 1957 |
|
Brewer JI, Torok EE, Webster A et al: Hydatidiform mole: A follow-up regimen for identification of invasive mole and choriocarcinoma and for selection of patients for treatment. Am J Obstet Gynecol 101: 557, 1968 |
|
Goldstein DP: The chemotherapy of gestational trophoblastic disease: Principles of clinical management. JAMA 220: 209, 1972 |
|
Bagshawe KD, Wilson H, Dublon P et al: Follow-up after hydatidiform mole: Studies using radioimmunoassays for urinary human chorionic gonadotropin (hCG). J Obstet Gynecol Br Commonw 80: 461, 1973 |
|
Hatch KD, Shingleton HM, Austin JM Sr: Southern Regional Trophoblastic Disease Center, 1972-1977. South Med J 71: 1334, 1978 |
|
Schlaerth JB, Morrow CP, Kletzky OA et al: Prognostic characteristics of serum human chorionic gonadotropin titer regression following molar pregnancy. Obstet Gynecol 58: 478, 1981 |
|
Kohorn EI: Hydatidiform mole and gestational trophoblastic disease in southern Connecticut. Obstet Gynecol 59: 78, 1982 |
|
Lurain JR, Brewer JI, Torok EE et al: Natural history of hydatidiform mole after primary evacuation. Am J Obstet Gynecol 145: 591, 1983 |
|
Bahar AM, el-Ashnei MS, Senthilsel van A: Hydatidiform mole in the elderly: Hysterectomy or evacuation. Int J Obstet Gynecol 29: 233, 1989 |
|
Schlaerth JB, Morrow CP, Montz FJ et al: Initial management of hydatidiform mole. Am J Obstet Gynecol 158: 1299, 1988 |
|
Tow WSH: The place of hysterotomy in the treatment of hydatidiform mole. Aust N Z J Obstet Gynaecol 7: 97, 1967 |
|
Twiggs LB, Morrow DP, Schlaerth JB: Acute pulmonary complications of molar pregnancy. Am J Obstet Gynecol 135: 189, 1979 |
|
Kohorn EI, McGinn RC, Bernard J et al: Pulmonary embolism of trophoblastic tissue in molar pregnancy. Obstet Gynecol 51: 16, 1978 |
|
Kohorn EI: Clinical management and the neoplastic sequelae of trophoblastic embolization associated with hydatidiform mole. Obstet Gynecol Surv 42: 484, 1987 |
|
Hankins GD, Wendel GD, Snyder RR et al: Trophoblastic embolization during molar evacuation: Central hemodynamic observations. Obstet Gynecol 69: 368, 1987 |
|
Cotton DB, Bernstein SG, Reed JA et al: Hemodynamic observations in evacuation of molar pregnancy. Am J Obstet Gynecol 138: 6, 1980 |
|
Wilson RB, Beecham CT, Symmonds RE: Conservative surgical management of chorioadenoma destruens. Obstet Gynecol 26: 814, 1965 |
|
Kanazawa K, Sasagawa M, Suzuki T et al: Clinical evaluation of focal excision of myometrial lesion for treatment of invasive hydatidiform mole. Acta Obstet Gynecol Scand 67: 487, 1988 |
|
Fisher RA, Sheppard DM, Lawler SD: Twin pregnancy with complete hydatidiform mole (46, XX) and fetus (46, XY): Genetic origin proved by analysis of chromosome polymorphisms. Br Med J 284: 1218, 1982 |
|
Vejerslev LO, Dueholm M, Nielsen FH: Hydatidiform mole: Cytogenetic marker analysis in twin gestation. Am J Obstet Gynecol 155: 614, 1986 |
|
Waxman M, Bern B, Zilkha SS et al: Concurrence of hydatiform mole with a viable baby: A twin gestation. Am J Diagn Gynecol Obstet 155: 614, 1986 |
|
Parazzini F, Mangili G, Belloni C et al: The problem of identification of prognostic factors for persistent trophoblastic disease. Gynecol Oncol 30: 57, 1988 |
|
Khazaeli MB, Hedayat MM, Hatch KD et al: Radioimmunoassay of free beta-subunit of human chorionic gonadotropin as a prognostic test for persistent trophoblastic disease in molar pregnancy. Am J Obstet Gynecol 155: 320, 1986 |
|
Hemming JD, Quirke P, Womack C et al: Diagnosis of molar pregnancy and persistent trophoblastic disease by flow cytometry. J Clin Pathol 40: 615, 1987 |
|
Martin DA, Sutton GP, Ulbright TM et al: DNA content as a prognostic index in gestational trophoblastic neoplasia. Gynecol Oncol 34: 385, 1989 |
|
Ratnam SS, Teoh ES, Dawood MY: Methotrexate for prophylaxis of choriocarcinoma. Am J Obstet Gynecol 111: 1021, 1971 |
|
Kim DS, Moon H, Kim KT: Effects of prophylactic chemotherapy for persistent trophoblastic disease in patients with complete hydatidiform mole. Obstet Gynecol 67: 690, 1986 |
|
Kashimura Y, Kashimura M, Sugimori H et al: Prophylactic chemotherapy for hydatidiform moles. Five to 15 years follow up. Cancer 58: 624, 1986 |
|
Stone M, Dent J, Kardena A et al: Relationship of oral contraception to development of trophoblastic tumor after evacuation of a hydatidiform mole. Br J Obstet Gynecol 83: 913, 1976 |
|
Berkowitz RS, Goldstein DP, Marean AR et al: Oral contraceptives and postmolar trophoblastic disease. Obstet Gynecol 58: 474, 1981 |
|
Ho Yuen B, Burch P: Relationship of oral contraceptives and the intrauterine contraceptive devices to the regression of concentrations of the beta subunit of human chorionic gonadotropin and invasive complications after molar pregnancy. Am J Obstet Gynecol 145: 214, 1983 |
|
Morrow CP, Nakamura R. Schlaerth J et al: The influence of oral contraceptives on the postmolar human chorionic regression curve. Am J Obstet Gynecol 151: 906, 1985 |
|
Curry SL, Schlaerth JB, Kohorn EI et al: Hormonal contraception and trophoblastic sequelae after hydatidiform mole (a Gynecologic Oncology Group Study). Am J Obstet Gynecol 160: 805, 1989 |
|
Acosta-Sison H: Is chorioadenoma destruens so benign that it cannot threaten the life of the patient? Phillipine Med Assoc J 35: 80, 1959 |
|
Berkowitz RS, Goldstein DP, Bernstein MR et al: Subsequent pregnancy outcomes in patients with molar pregnancies and gestational trophoblastic tumors. J Reprod Med 32: 680, 1987 |
|
Lurain JR, Sand PK, Carson SA et al: Pregnancy outcome subsequent to consecutive hydatidiform moles. Am J Obstet Gynecol 142: 1060, 1982 |
|
Matalon M, Modau B: Epidemiologic aspects of hydatidiform mole in Israel. Am J Obstet Gynecol 112: 107, 1972 |
|
Sand PK, Lurain JR, Brewer JI: Repeat gestational trophoblastic disease. Obstet Gynecol 63: 140, 1984 |
|
Hammond CB, Hertz R. Ross GT et al: Diagnostic problems of choriocarcinoma and related trophoblastic neoplasms. Obstet Gynecol 29: 224, 1967 |
|
Magrath IT, Golding PR, Bagshawe KD: Medical presentations of choriocarcinoma. Br Med J 2: 633, 1971 |
|
Sung HC, Wu PC, Ho TH: Treatment of choriocarcinoma and chorioadenoma destruens with 6-mercaptopurine and surgery. Chin Med J 82: 24, 1963 |
|
Mutch DG, Soper JT, Baker ME et al: Role of computed axial tomography of the chest in staging patients with nonmetastatic gestational trophoblastic disease. Obstet Gynecol 68: 348, 1986 |
|
Hricak H, Demas BE, Braga CA et al: Gestational trophoblastic neoplasm of the uterus: MR assessment. Radiology 161: 11, 1986 |
|
Powell MC, Buckley J, Worthington BS: Magnetic resonance imaging and hydatidiform mole. J Radiol 59: 561, 1986 |
|
Maroulis GB, Hammond CB, Johnsrude IS et al: Arteriography and infusional chemotherapy in localized trophoblastic disease. Obstet Gynecol 45: 397, 1975 |
|
Bagshawe KD, Harlan S: Immunodiagnosis and monitoring of gonadotropin producing metastases in the central nervous sytem. Cancer 38: 112, 1976 |
|
Goldstein DP, Berkowitz RS: Non-metastatic and lowrisk metastatic gestational trophoblastic neoplasms. Semin Oncol 9: 191, 1982 |
|
Berkowitz RS, Goldstein DP, Driscoll SG et al: Pretreatment curettage: A predictor of chemotherapy response in gestational trophoblastic neoplasia. Gynecol Oncol 10: 39, 1980 |
|
Schlaerth JB, Morrow CP, Rodriguez M: Diagnostic and therapeutic curettage in gestational trophoblastic disease. Am J Obstet Gynecol 162: 1465, 1990 |
|
Lao TT, Lee FH, Yeung SS: Repeat curettage after evacuation of hydatidiform mole: An appraisal. Acta Obstet Gynecol Scand 66: 305, 1987 |
|
Flam F, Lundstrom V: The value of endometrial curettage in the follow-up of hydatidiform mole. Acta Obstet Gynecol Scand 67: 649, 1988 |
|
Hunter V, Raymond E, Christensen C et al: Efficacy of the metastatic survey in the staging of gestational trophoblastic disease. Cancer 65: 1647, 1990 |
|
Pettersson F, Kolstad P, Ludwig H et al: Annual Report on the Results of Treatment in Gynecologic Cancer, vol 19. Stockholm, International Federation of Gynecology and Obstetrics, 1985 |
|
Ross GT, Goldstein DP, Hertz R et al: Sequential use of methotrexate and actinomycin D in the treatment of metastatic choriocarcinomas and related trophoblastic tumors in women. Am J Obstet Gynecol 93: 223, 1965 |
|
Hammond CB, Borchert LG, Tyrey L et al: Treatment of metastatic trophoblastic disease: Good and poor prognosis. Am J Obstet Gynecol 115: 4, 1973 |
|
Lurain JR, Brewer JI, Torok EE et al: Gestational trophoblastic disease: Treatment results at the Brewer Trophoblastic Disease Center. Obstet Gynecol 60: 354, 1982 |
|
Lewis JL Jr: Treatment of metastatic trophoblastic neoplasms. Am J Obstet Gynecol 136: 163, 1979 |
|
Soper JT, Clarke-Pearson DL, Hammond CB: Metastatic gestational trophoblastic disease: Prognostic factors in previously untreated patients. Obstet Gynecol 71: 338, 1988 |
|
Surwit EA, Hammond CB: Treatment of metastatic trophoblastic disease with poor prognosis. Obstet Gynecol 55: 565, 1980 |
|
Hammond CB, Weed JC Jr, Currie JL: The role of operation in the current therapy of gestational trophoblastic disease. Am J Obstet Gynecol 136: 844, 1980 |
|
World Health Organization Scientific Group on Gestational Trophoblastic Diseases, Technical Report Series No. 692. Geneva, World Health Organization, 1983 |
|
Bagshawe KD: Risk and prognostic factors in trophoblastic neoplasia. Cancer 38: 1373, 1976 |
|
DuBeshter B, Berkowitz RS, Goldstein DP et al: Metastatic gestational trophoblastic disease: Experience at the New England Trophoblastic Disease Center, 1965 to 1985. Obstet Gynecol 69: 630, 1987 |
|
DuBeshter B, Berkowitz RS, Goldstein DP et al: Analysis of failure in high-risk metastatic gestational trophoblastic disease. Gynecol Oncol 29: 199, 1988 |
|
Gordon AN, Gershenson DM, Copeland LJ et al: Highrisk metastatic gestational trophoblastic disease: Further stratification into two clinical entities. Gynecol Oncol 34: 54, 1989 |
|
Hammond CB, Hertz R. Ross GT et al: Primary chemotherapy for nonmetastatic gestational trophoblastic neoplasms. Am J Obstet Gynecol 98: 71, 1967 |
|
Gordon AN, Gershenson DM, Copeland LJ et al: High-risk metastatic gestational trophoblastic disease. Obstet Gynecol 65: 550, 1985 |
|
Sung H, Wu PC, Wang YE et al: A proposal on the staging of malignant trophoblastic neoplasms based on study of process of development of the disease. In Petillo RA, Hussa RO (eds): Human Trophoblastic Neoplasm, p 327. New York, Plenum Press, 1984 |
|
Miller JM Jr: Surwit EA, Hammond CB: Choriocarcinoma following term pregnancy. Obstet Gynecol 53: 207, 1979 |
|
Olive DL, Lurain JR, Brewer JI: Choriocarcinoma associated with term gestation. Am J Obstet Gynecol 148: 711, 1984 |
|
Azab MB, Pejovic MH, Theodore C et al: Prognostic factors in gestational trophoblastic tumors: A multivariate analysis. Cancer 62: 585, 1988 |
|
Brewer JI, Smith RT, Pratt GB: Choriocarcinoma: Absolute survival rates of 122 patients treated by hysterectomy. Am J Obstet Gynecol 85: 84, 1963 |
|
Goldstein DP, Winig P, Shirley RL: Actinomycin D as initial therapy of gestational trophoblastic disease: A reevaluation. Obstet Gynecol 45: 341, 1975 |
|
Petrilli ES, Morrow CP: Actinomycin D toxicity in the treatment of trophoblastic disease: A comparison of the five-day course to single-dose administration. Gynecol Oncol 9: 18, 1980 |
|
Twiggs LB: Pulse actinomycin D scheduling in nonmetastatic gestational trophoblastic disease: Cost-effective chemotherapy. Gynecol Oncol 16: 190, 1983 |
|
Petrilli ES, Twiggs LB, Blessing JA et al: Single-dose actinomycin D treatment for nonmetastatic gestational trophoblastic disease: A prospective phase II trial of the Gynecologic Oncology Group. Cancer 60: 2173, 1987 |
|
Sung H, Wu P, Yan H: Reevaluation of 5-fluorouracil as a single therapeutic agent for gestational trophoblastic neoplasms. Am J Obstet Gynecol 150: 69, 1984 |
|
Wong MA, Choo YC, Ma HK: Primary oral etoposide therapy in gestational trophoblastic disease: An update. Cancer 58: 14, 1986 |
|
Smith EB, Weed JC Jr, Tyrey L et al: Treatment of nonmetastatic gestational trophoblastic disease: Results of methotrexate alone versus methotrexate-folinic acid. Am J Obstet Gynecol 144: 88, 1982 |
|
Barter JF, Soong SJ, Hatch KD et al: Treatment of nonmetastatic gestational trophoblastic disease with oral methotrexate. Am J Obstet Gynecol 157: 1166, 1987 |
|
Berkowitz RS, Goldstein DP, Bernstein MR: Ten year's experience with methotrexate and folinic acid as primary therapy for gestational trophoblastic disease. Gynecol Oncol 23: 111, 1986 |
|
Bolis G, Colombo N, Epis A et al: Methotrexate with citrovorum factor in low-risk gestational trophoblastic tumor. Tumori 73: 309, 1987 |
|
Bagshawe KD, Dent J, Newlands ES et al: The role of low-dose methotrexate and folinic acid in gestational trophoblastic tumors (GTT). Br J Obstet Gynecol 96: 795, 1989 |
|
Holmesley HD, Blessing JA, Rettenmaier M et al: Weekly intramuscular methotrexate for nonmetastatic gestational trophoblastic disease. Obstet Gynecol 72: 413, 1988 |
|
Bagshawe KD, Wilde CE: Infusion therapy for pelvic tumours. Br J Obstet Gynaecol 71: 565, 1964 |
|
Rotmensch J, Rosenshein N, Donehower R et al: Plasma methotrexate levels in patients with gestational trophoblastic regimens. Am J Obstet Gynecol 148: 730, 1984 |
|
Berkowitz RS, Goldstein DP: Methotrexate with citrovorum factor rescue for nonmetastatic gestational trophoblastic neoplasms. Obstet Gynecol 54: 725, 1979 |
|
Wong MA, Choo YC, Ma HK: Methotrexate with citrovorum factor rescue in gestational trophoblastic disease. Am J Obstet Gynecol 152: 59, 1985 |
|
Berkowitz RS, Goldstein DP, Bernstein MR: Methotrexate infusion and folinic acid in the primary therapy of nonmetastatic gestational trophoblastic tumors. Gynecol Oncol 36: 56, 1990 |
|
Goldstein DP, Winig P, Shirley RL: Actinomycin D as initial therapy of gestational trophoblastic disease: A reevaluation. Obstet Gynecol 45: 341, 1975 |
|
Twiggs LB: Pulse actinomycin D scheduling in nonmetastatic gestational trophoblastic disease: Cost-effective chemotherapy. Gynecol Oncol 16: 190, 1983 |
|
Lewis J Jr, Ketcham AS, Hertz R: Surgical intervention during chemotherapy of gestational trophoblastic neoplasms. Cancer 19: 1517, 1966 |
|
Lewis J Jr, Gore H, Hertig AT et al: Treatment of trophoblastic disease with rationale for the use of adjuvant chemotherapy at time of indicated operation. Am J Obstet Gynecol 96: 710, 1966 |
|
Lurain JR, Brewer JI: Treatment of high-risk gestational trophoblastic disease with methotrexate, actinomycin D and cyclophosphamide chemotherapy. Obstet Gynecol 65: 830, 1985 |
|
Curry SL, Blessing JA, DiSaia PJ et al: A prospective, randomized comparison of methotrexate, dactinomycin and chlorambucil versus methotrexate, dactinomycin, cyclophosphamide, doxorubicin, melphalan, hydroxyurea, and vincristine in “poor prognosis” metastatic gestational trophoblastic disease: A Gynecologic Oncology Group study. Obstet Gynecol 73: 357, 1989 |
|
Berkowitz RS, Goldstein DP, Bernstein MR: Modified triple chemotherapy in the management of high-risk metastatic gestational trophoblastic tumors. Gynecol Oncol 19: 173, 1984 |
|
Bagshawe KD: Treatment of trophoblastic tumours. Ann Acad Med (Singapore) 5: 273, 1976 |
|
Surwit EA, Succiu TN, Schmidt HJ et al: A new combination chemotherapy for resistant trophoblastic disease. Gynecol Oncol 8: 110, 1978 |
|
Weed JC Jr, Barnard DE, Currie JL et al: Chemotherapy with modified Bagshawe protocol for poor prognosis metastatic trophoblastic disease. Obstet Gynecol 59: 377, 1982 |
|
Wong LC, Choo YC, Ma HK: Modified Bagshawe's regimen in high-risk gestational trophoblastic disease. Gynecol Oncol 23: 87, 1986 |
|
Newlands ES, Bagshawe KD, Begent RHJ et al: Developments in chemotherapy for medium- and high-risk patients with gestational trophoblastic tumours (19791984). Br J Obstet Gynaecol 93: 63, 1986 |
|
Bolis G, Bonazzi C, Landori F et al: EMA/CO regimen in high-risk gestational trophoblastic tumor (GTT). Gynecol Oncol 31: 439, 1988 |
|
Newlands ES: Preliminary experience with high-dose cisplatin and epipodophyllin derivative VP16-213 in resistant malignant teratomas and choriocarcinomas. Curr Chemotherapy 2: 1315, 1978 |
|
Rustin GJ, Newlands ES, Begent RH et al: Weekly alternating etoposide, methotrexate and actinomycin/vincristine and cyclophosphamide chemotherapy for the treatment of CNS metastases of choriocarcinoma. J Clin Oncol 7: 900, 1989 |
|
Theodore C, Azab M, Droz JP et al: Treatment of highrisk gestational trophoblastic disease with chemotherapy combinations containing cisplatin and etoposide. Cancer 64: 1824, 1989 |
|
Athanassiou A, Begent RHJ, Newlands ES et al: Central nervous system metastases of choriocarcinoma: 23 years' experience at Charing Cross Hospital. Cancer 52: 1728, 1983 |
|
Brace KC: The role of irradiation in the treatment of metastatic trophoblastic disease. Radiology 91: 540, 1968 |
|
Weed JC, Hammond CB: Cerebral metastatic choriocarcinoma: Intensive therapy and prognosis. Obstet Gynecol 55: 89, 1980 |
|
Weed JC, Woodward KT, Hammond CB: Choriocarcinoma metastatic to the brain: Therapy and prognosis. Semin Oncol 9: 208, 1982 |
|
Yordan EL Jr, Schlaerth J. Gaddis O et al: Radiation therapy in the management of gestational choriocarcinoma metastatic to the central nervous sytem. Obstet Gynecol 69: 627, 1987 |
|
Wong LC, Choo YC, Ma HK: Hepatic metastases in gestational trophoblastic disease. Obstet Gynecol 67: 107, 1986 |
|
Barnard DE, Woodward KT, Yancy SG et al: Hepatic metastases of choriocarcinoma: A report of 15 patients. Gynecol Oncol 25: 73, 1986 |
|
Heaton GE, Matthews TH, Christopherson WH: Malignant trophoblastic tumors with massive hemorrhage presenting as liver primary. A report of two cases. Am J Surg Pathol 10: 342, 1986 |
|
Grumbine FC, Rosenschein NB, Brewerton MD et al: Management of liver metastasis from gestational trophoblastic neoplasia. Am J Obstet Gynecol 137: 959, 1980 |
|
Soper JT, Mutch DG, Chin N et al: Renal metastases of gestational trophoblastic disease: A report of eight cases. Obstet Gynecol 72: 796, 1988 |
|
Azab M, Droz JP, Theodore C et al: Cisplatin, vinblastine and bleomycin in combination in the treatment of resistant high-risk gestational trophoblastic tumors. Cancer 64: 1829, 1989 |
|
Hammond CB, Soper JT: Poor prognosis metastatic gestational trophoblastic neoplasia. Clin Obstet Gynecol 27: 228, 1984 |
|
Gordon AN, Kavanaugh JJ, Gershenson DM et al: Cisplatin, vinblastine, and bleomycin combination therapy in resistant gestational trophoblastic disease. Cancer 58: 1407, 1986 |
|
DuBeshter B, Berkowitz RS, Goldstein DP et al: Vinblastine, cisplatin and bleomycin as salvage therapy for refractory high-risk metastatic gestational trophoblastic disease. J Reprod Med 34: 189, 1989 |
|
Wong LC, Choo YC, Ma HK: Etoposide, methotrexate and bleomycin in drug-resistant gestational trophoblastic disease. Gynecol Oncol 24: 51, 1986 |
|
Willemse PH, Aalders JG, Bouma J et al: Chemotherapy of resistant gestational trophoblastic neoplasia treated successfully with cisplatin, etoposide and bleomycin. Obstet Gynecol 71: 438, 1988 |
|
Loehrer PJ Sr, Einhorn LH, Williams SD: VP-16 plus ifosfamide plus cisplatin as salvage therapy in refractory germ cell cancer. J Clin Oncol 4: 528, 1986 |
|
Bandy LC, Chin N, Soper JT et al: Total parental nutrition in poor prognosis gestational trophoblastic disease. Gynecol Oncol 28: 305, 1987 |
|
Liftshitz H, Barber CE, Hammond CB: The pulmonary metastases of choriocarcinoma. Obstet Gynecol 49: 412, 1977 |
|
Wong LC, Ma HK: Persistent chest opacity in trophoblastic disease: Is thoracotomy justified? Aust N Z J Obstet Gynecol 23: 237, 1983 |
|
Shirley RL, Goldstein DP, Collins JJ Jr: The role of thoracotomy in management of patients with chest metastases from gestational trophoblastic disease. J Thorac Cardiovasc Surg 63: 545, 1972 |
|
Edwards JL, Makey AR, Bagshawe KD: The role of thoracotomy in the management of pulmonary metastases of gestational choriocarcinoma. Clin Oncol 1: 329, 1975 |
|
Sink JD, Hammond CB, Young WG: Pulmonary resection in the management of metastases from choriocarcinoma. J Thorac Cardiovasc Surg 81: 830, 1978 |
|
Tomoda Y, Arii Y, Kaseki S et al: Surgical indications for resection in pulmonary metastasis of choriocarcinoma. Cancer 46: 2723, 1980 |
|
Xu LT, Suu CF, Wong YE et al: Resection of pulmonary metastatic choriocarcinoma in 43 drug-resistant patients. Ann Thorac Surg 39: 257, 1985 |
|
Quinones J, Mizejewski G, Beierwaltes WH: Choriocarcinoma scanning using radiolabelled antibody to chorionic gonadotropin. J Nucl Med 12: 69, 1971 |
|
Begent RHJ, Searle F, Stanway G et al: Radioimmunolocalization of tumors by external scintigraphy after administration of 131 I antibody to human chorionic gonadotrophin: Preliminary communication. J R Soc Med 73: 624, 1980 |
|
Hatch KD, Mann WJ, Boots LR et al: Localization of choriocarcinoma by 131 I-hCG antibody. Gynecol Oncol 10: 253, 1980 |
|
Patillo RA: Trophoblastic neoplasms: Occult disease. Obstet Gynecol Clin North Am 15: 577, 1988 |
|
Wahl R, Khazaeli M, Lo Buglio A et al: Radioimmunoscintigraphic detection of occult gestational choriocarcinoma. Am J Obstet Gynecol 156: 108, 1987 |
|
Soper JT, Dodge R, Tyrey L et al: Human chorionic gonadotropin regression patterns in patients receiving methotrexate for metastatic gestational trophoblastic disease. Submitted for publication, 1992 |
|
Mutch DG, Soper JT, Babcock CS et al: Recurrent gestational trophoblastic disease: Experience of the Southeastern Regional Trophoblastic Disease Center. Cancer 66: 978, 1990 |
|
Surwit EA, Hammond CB: Recurrent gestational trophoblastic disease. Gynecol Oncol 12: 177, 1981 |
|
Van Thiel DH, Ross GT, Lipsett MB: Pregnancies after chemotherapy of trophoblastic neoplasms. Science 169: 1326, 1970 |
|
Van Thiel DH, Grodin JM, Ross GT: Partial placenta accreta in pregnancies following chemotherapy for gestational trophoblastic neoplasia. Am J Obstet Gynecol 112: 54, 1972 |
|
Berkowitz RS, Goldstein DP, Bernstein MR: Management of nonmetastatic trophoblastic tumors. J Reprod Med 26: 219, 1981 |
|
Song H, Wu P, Wong Y et al: Pregnancy outcomes after successful chemotherapy for choriocarcinoma and invasive mole: Long-term follow up. Am J Obstet Gynecol 158: 538, 1988 |


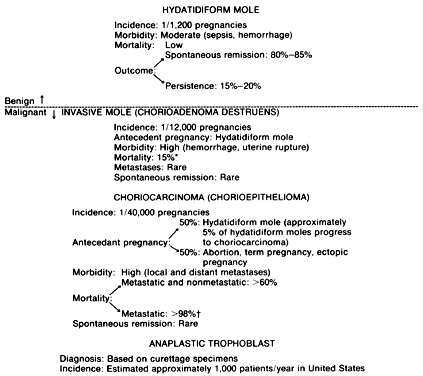
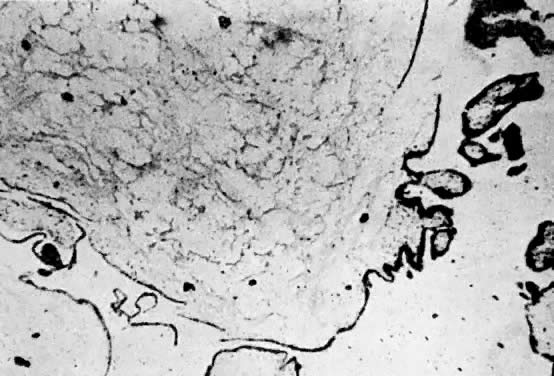
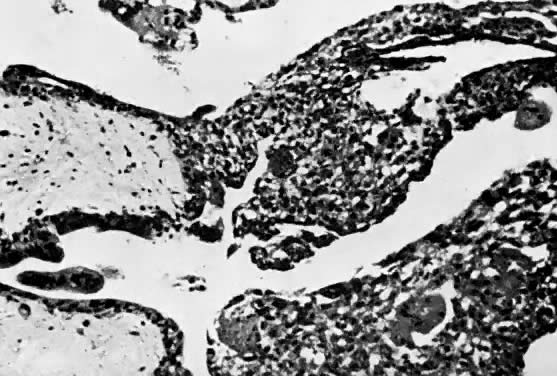
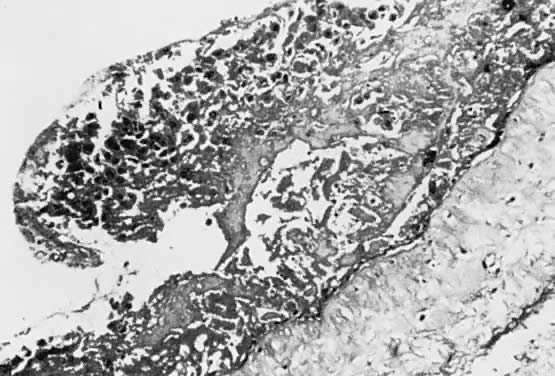
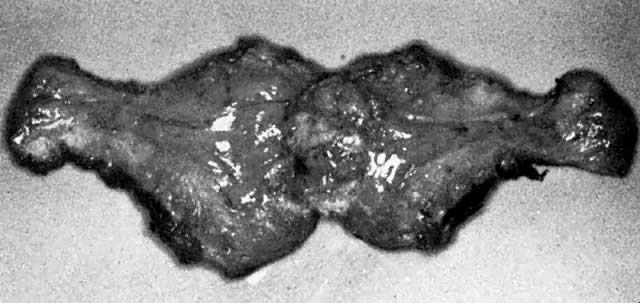
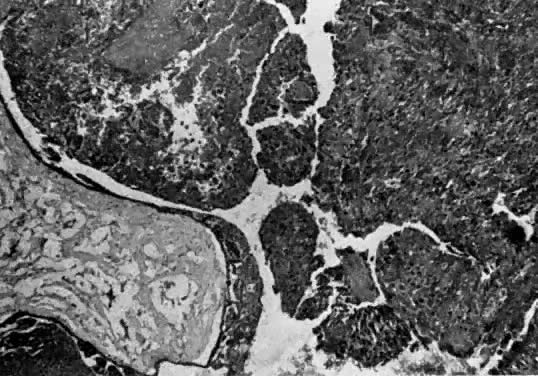
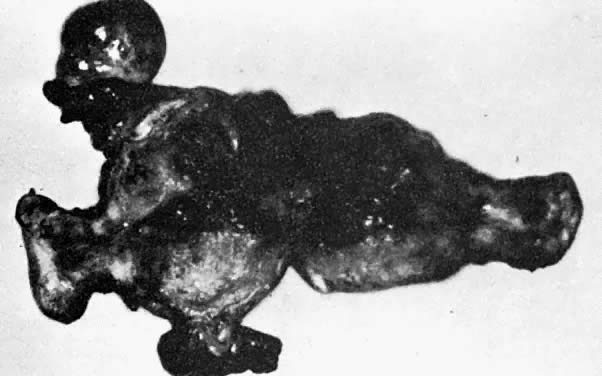
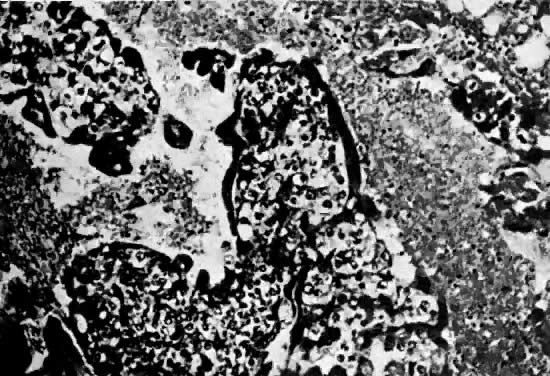
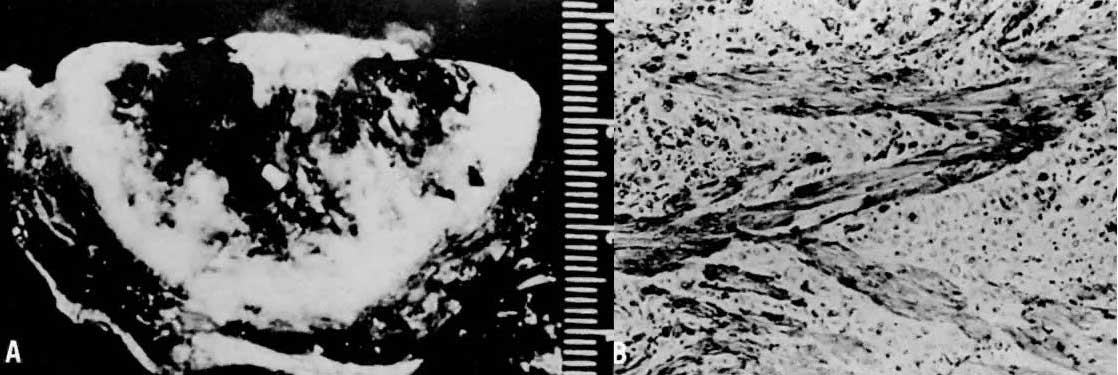
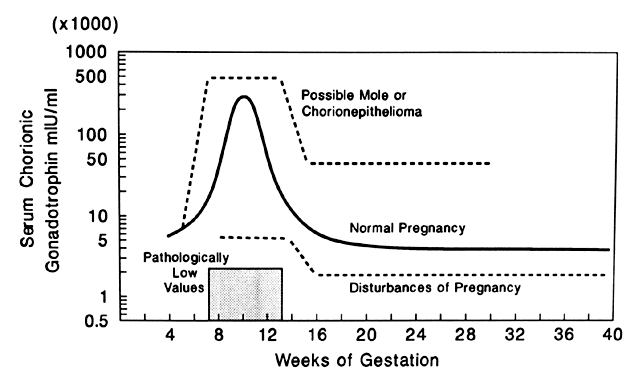
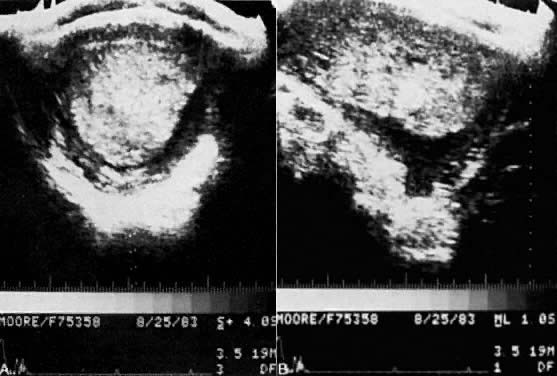
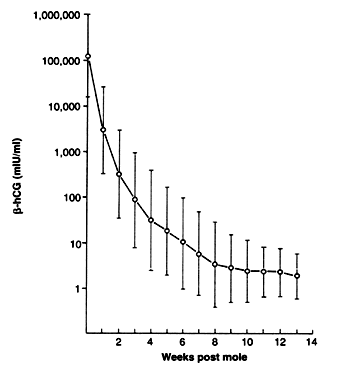
 35
35 4 doses
4 doses