Intra-amniotic and Postpartum Infections
Authors
INTRODUCTION
Intra-amniotic and postpartum infections are important causes of maternal and neonatal morbidity and mortality. Puerperal infections, frequently associated with intra-amniotic infection (IAI), are the fourth leading cause of maternal death in the United States, accounting for 13% of maternal deaths.1 IAI accounts for 10% to 40% of cases of febrile morbidity in the peripartum period and is associated with 20% to 40% of cases of early neonatal sepsis and pneumonia.2 Postpartum infections develop in 1% to 7% of women, accounting for more than 200,000 infections annually in the United States. Thus, intra-amniotic and postpartum infections remain significant public health problems.
INTRA-AMNIOTIC INFECTION
Intra-amniotic infection is an acute bacterial infection of the amniotic fluid and intrauterine contents during pregnancy. Prospective studies indicate that IAI occurs in 4% to 10% of all deliveries.3,4,5 Other terms used to describe IAI include amniotic fluid infection, amnionitis, and clinical chorioamnionitis. IAI is clinically diagnosed by maternal fever, uterine tenderness, leukocytosis, and fetal tachycardia and should be distinguished from histologic chorioamnionitis. Histologic chorioamnionitis is a histopathologic diagnosis characterized by inflammatory cell infiltration of the membranes. Histologic chorioamnionitis occurs more frequently than IAI, with a prevalence of 4% to 16% at term to greater than 60% in extremely preterm gestations, and is usually not associated with clinical signs or symptoms.6,7,8 Unfortunately, many authors have not distinguished between these two distinct entities, making comparisons between studies difficult.
IAI is an important cause of maternal and neonatal morbidity. IAI accounts for 10% to 40% of cases of febrile morbidity in the peripartum period and is associated with 20% to 40% of cases of early neonatal sepsis and pneumonia.2 Maternal bacteremia occurs in 2% to 6% of patients with IAI, and postpartum infectious morbidity increases with bacteremia. An increased risk of dysfunctional labor and cesarean delivery occurs among patients with IAI. A 75% incidence of dysfunctional labor and a 34% incidence of cesarean delivery is reported among patients who developed IAI while in labor.9 IAI also is associated with increased neonatal morbidity and mortality, particularly among preterm neonates. In general, a three- to four-fold increase occurs in perinatal mortality among low-birthweight neonates born to mothers with IAI;10,11 also increased is the incidence of respiratory distress syndrome, intraventricular hemorrhage, and neonatal sepsis.12 Recently, IAI has been implicated in neonatal periventricular leukomalacia and cerebral palsy; the risks of cerebral white matter damage and cerebral palsy are nine-fold greater in the setting of IAI.13,14 Finally, subclinical IAI has been found in at least 10% of women in preterm labor with intact fetal membranes, suggesting that IAI is an important, and potentially preventable, cause of prematurity.15
Pathogenesis
Intra-amniotic infection may result from an ascending infection of cervical or vaginal microbial flora, bacteremia, or iatrogenic causes. Most cases of IAI result from ascending infection after labor or rupture of membranes. Before labor or rupture of membranes, amniotic fluid is nearly always sterile. With the onset of labor or rupture of membranes, bacteria indigenous to the lower genital tract ascend into the amniotic cavity. Increasing duration of labor or rupture of membranes raises the rate of isolation of bacteria from amniotic fluid. In fact, all amniotic fluid samples taken from women with rupture of membranes of a duration of 6 hours or more contained microorganisms,16 and about 90% of those with a “high-virulence” microorganism develop an intrauterine infection.17
The second route of infection is hematogenous spread, across the placenta, from a bacteremic mother. A wide variety of microorganisms are involved, but the most notable infection occurs with Listeria monocytogenes. Transplacental passage of L. monocytogenes may occur in isolated cases or as an epidemic, usually after the ingestion of contaminated dairy products.18 Epidemics of food-borne listeriosis disproportionately affect perinatal cases and are associated with preterm birth, stillbirth, or abortion.
The third mechanism for IAI is the iatrogenic introduction of bacteria during an invasive procedure such as cervical cerclage, amniocentesis, cordocentesis, or intrauterine transfusion. The risk of iatrogenic IAI is small, ranging from less than 1% after amniocentesis, 0.5% to 1% after cordocentesis, and 1% to 2% after routine cervical cerclage.19 Emergency, or rescue, cerclage, performed after dilatation of the cervix has begun, has a much higher rate of IAI: 25% to 40%.20,21 This apparently high risk of IAI after emergency cerclage may represent an underlying subclinical intrauterine infection that precedes cerclage placement. In 33 women at 14 to 24 weeks of gestation with cervical dilatation of 2 cm or more, 7 (21%) had evidence of bacterial invasion of amniotic fluid by Gram stain before cerclage. Further, IAI was subsequently documented even in 50% of cerclage patients with negative amniotic fluid Gram stains.22 Two more recent studies that used a course of broad-spectrum antibiotics with emergency cerclage reduced the risk of IAI to 5% to 16%.23,24
Risk Factors
Many risk factors exist for IAI. The risk falls into three broad categories: prolonged labor or complicated pregnancies, abnormal vaginal flora, and preterm birth.
PROLONGED LABOR
Nulliparity, prolonged duration of ruptured membranes, prolonged duration of labor, multiple vaginal examinations, and internal fetal monitoring are consistently linked to IAI.3,4,25 One or more of these risk factors is present in most patients, and the strength of association is usually strong. Prelabor rupture of membranes with more than eight vaginal examinations increased the risk of IAI five-fold, and duration of labor of 12 hours or longer increased the risk of IAI four-fold compared with patients without these risk factors.25 In a stepwise logistic regression, duration of rupture of membranes and duration of internal fetal monitoring were the major risk factors for IAI, and patients with these two risks had the relative risk of IAI increase 19.7-fold.3 About 70% of patients who ultimately developed IAI met risk criteria several hours before the diagnosis was clinically evident.3 This is consistent with the view that clinical indicators of infection develop late in the time course of the infection, as discussed below. In addition, meconium-stained amniotic fluid, maternal colonization with group B streptococcus, prior history of an elective or spontaneous abortion, and prolonged second stage of labor are identified as risk factors for IAI.25,26,27
ABNORMAL VAGINAL FLORA
An association between bacterial vaginosis (BV) and IAI exists.28,29,30,31,32 The association between BV and IAI is based on: 1) epidemiologic studies showing an increased risk of IAI, with odds ratios of 1.5 to 6.8, for women with BV;28,29,30,31,32 2) the association between the presence of specific BV-associated microorganisms, or the absence of Lactobacillus sp. in the vagina and IAI;31,33 3) the recovery of BV-associated microorganisms from chorioamnion among women with histologic chorioamnionitis and preterm delivery;6 and 4) the recovery of BV-associated microorganisms from amniotic fluid of women with clinical IAI.30
The association of BV and IAI in the setting of preterm birth is particularly relevant, considering the prevalence of BV. BV is the most prevalent vaginal infection in sexually active women, occurring in approximately 20% of pregnant women.34 Anaerobes, frequently those associated with BV, were recovered from amniotic fluid significantly more often in women with IAI giving birth to low-birthweight neonates than in women with IAI at term.35 BV-associated microorganisms, including Mycoplasma hominis, Gardnerella vaginalis, and anaerobes, were recovered from the chorioamnion of 42% of women delivering at less than 37 weeks of gestation and from only 7% of women delivering at term (odds ratio 9.5).6 Thus, BV is associated both with IAI and with preterm birth.
PRETERM BIRTH
The incidences of IAI, microbial invasion of chorioamnion, and histologic chorioamnionitis are all strongly inversely correlated with gestational age. Histologic chorioamnionitis occurs in up to 60% to 90% of gestations ending between 20 and 24 weeks,7,8 and microbial infection of the chorioamnion occurs in up to 60% of patients with preterm delivery.6 The incidence of clinically apparent IAI also varies inversely with gestational age. In a literature review, the incidence of clinical IAI was 41% at gestational ages less than 27 weeks, 15% at gestational ages 27 to 37 weeks, and 2% at gestations of 38 weeks or greater.2 Bacteria indigenous to the lower genital tract also are recovered from the amniotic fluid of 10% to 20% of all women in preterm labor with intact fetal membranes and no clinical signs of IAI15 and of up to 67% of women in preterm labor with pregnancies ending at 23 to 24 weeks.36 Most patients with IAI deliver rapidly, and clinically apparent IAI develops in a few. These observations support the hypothesis that ascending, initially subclinical IAI precedes preterm labor and may be an important cause of extreme preterm deliveries.
Coitus has been suggested in epidemiologic studies to be a risk factor for chorioamnionitis.37 However, there is no direct evidence relating coitus with IAI, and a more recent study of 10,000 Israeli women found no increased risk of premature rupture of membranes, low birthweight, or perinatal death associated with coitus in the third trimester.38
Diagnosis
Ideally, an early diagnosis of IAI is important to allow timely treatment and intervention. Unfortunately, the early diagnosis is difficult because the clinical signs and symptoms of IAI occur late and are neither sensitive nor specific. In a nonhuman primate model, where experimental IAI was induced with group B streptococcus, fever and leukocytosis were present in only 50% of the primates at the onset of infection-induced preterm labor, which occurs 28 to 40 hours after experimental infection.39 Thus, to avoid a delay in diagnosis, a high index of suspicion is warranted, together with appropriate adjunctive laboratory tests. The clinical criteria commonly used to diagnose IAI include maternal fever (more than 37.8°C), and two or more of the following: maternal leukocytosis (15,000/mm3 or more), maternal or fetal tachycardia, uterine tenderness, or foul-smelling amniotic fluid (Table 1).10 Because low-grade maternal fever may arise from other sources, other nonobstetric causes of fever should be excluded.
Table 1. Criteria for the Clinical Diagnosis of Intra-amniotic Infection
Maternal fever 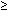 37.8°C
37.8°C
Two or more of the following:
Maternal leukocytosis 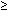 15,000/mm3
15,000/mm3
Uterine tenderness
Maternal tachycardia >100 beats per minute
Fetal tachycardia >160 beats per minute
Malodorous amniotic fluid
No other apparent source of infection.
Gibbs RS, Castillo MA, Rodgers PJ: Management of acute chorioamnionitis. Am J Obstet Gynecol 136:709, 1980.
Because of the inconsistency of clinical features (Table 2), other adjunctive laboratory tests are used to aid in the diagnosis of IAI. These include measurement of maternal C-reactive protein, direct examination of amniotic fluid for leukocytes or bacteria by Gram stain, amniotic fluid culture, and measurement of amniotic fluid glucose concentrations, amniotic fluid leukocyte esterase, bacterial organic acids by gas-liquid chromatography, various amniotic fluid or vaginal cytokines (e.g, interleukins 2, 4, 6, granulocyte colony-stimulating factor, and tumor necrosis factor-alpha), matrix metalloproteinase-9, lactoferrin, and fetal activity (biophysical profile) by ultrasonography. Cytokine or other biochemical factor measurement is expensive and generally not clinically available. Further, these tests are no better than more readily available traditional tests such as amniotic fluid Gram stain and culture, amniotic fluid glucose concentrations, and detection of amniotic fluid leukocyte esterase. The efficiency of these tests is summarized in Table 3.40 Although all have reasonable sensitivity, specificity, and predictive value, none is sufficiently sensitive or specific to be used independently of clinical features in the diagnosis of IAI. Unfortunately, only a few studies of amniotic fluid markers for IAI have directly compared Gram stain, glucose, or leukocyte esterase in the same patients,41,42,43,44,45,46,47 and only one study has directly compared all three (Table 4).48 The range of the predictive values observed in these studies may depend, in part, on both the variable prevalence of IAI in the study population and on the cross-sectional nature of the studies, because amniotic fluid is obtained only at a single time during a temporally progressive infection. Animal models that allow longitudinal sampling of amniotic fluid after infection are helpful to delineate the natural progression of laboratory markers in infection.
Table 2. Reported Frequency of Clinical Criteria Among Patients With Intra-Amniotic Infection and Maternal Fever as an Index Criterion
Clinical Sign | Frequency (%) |
Intrapartum fever | 100* |
Maternal leukocytosis >15,000/mm3 | 70–90 |
Maternal tachycardia >100 bpm | 20–80 |
Fetal tachycardia >160 bpm | 40–70 |
Malodorous amniotic fluid | 5–22 |
Uterine tenderness | 2–25 |
*Intrapartum fever required by study for diagnosis of intra-amniotic infection.
Data are based on a summary of reported studies.
Table 3. Efficacy of Commonly Used Amniotic Fluid Diagnostic Tests To Detect Intra-amniotic Infection
|
| Reported Range (%) | |||
| Standard Used |
|
| Positive | Negative |
Test | for Diagnosis | Sensitivity | Specificity | Predictive Value | Predictive Value |
Gram stain | AF culture | 36–80 | 83–98 | 78–87 | 56–92 |
Glucose | AF culture | 63–87 | 92–99 | 78–87 | 56–92 |
Leukocyte esterase | AF culture | 19–83 | 85–100 | 42–100 | 67–78 |
Biophysical profile | Clinical intra-amniotic | 67–90 | 83–86 | 31–60 | 96–98 |
| infection |
|
|
|
|
* Adapted from data in Ohlsson A, Wang E: An analysis of antenatal tests to detect infection at preterm rupture of the membranes. Am J Obstet Gynecol 162:809, 1990.
Table 4. Comparison of Common Amniotic Fluid Diagnostic Tests to Diagnose Intra-amniotic Infection, Where Multiple Tests Were Performed in the Same Patients
|
|
|
|
|
|
|
| Positive Predictive | ||
|
| Sensitivity (%) | Specificity (%) | Value (%) | ||||||
Author | Patients | GS | Glu | LE | GS | Glu | LE | GS | Glu | LE |
Romero41 | 171 | 36 | - | 19 | 95 | - | 87 | 78 | - | 42 |
Hoskins42 | 42 | 62 | - | 91 | 81 | - | 95 |
|
|
|
Egley43 | 44 | 54 | - | 81 | 83 | - | 100 | 82 | - | 100 |
Romero44 | 168 | 65 | 87 | - | 99 | 92 | - | 61 | 63 | - |
Kiltz45 | 86 | 50 | 63 | - | 100 | 99 | - | 100 | 99 | - |
Gauthier46 | 204 | 30 | 79 | - | 97 | 94 | - | 87 | 87 | - |
Odibo47 | 88 | 26 | 28 | - | 95 | 95 | - | 88 | 88 | - |
Gauthier48 | 33 | 49 | 87 | 80 | 97 | 90 | 84 | 92 | 87 | 78 |
Gs, Gram Stain; Glu, glucose; LE, leukocyte esterase.
With the exception of maternal C-reactive protein and ultrasonography, these tests require amniotic fluid for analysis. Amniotic fluid may be obtained by aspiration through a transcervical intrauterine pressure catheter among women in labor with ruptured membranes. However, for women in preterm labor with intact membranes or for women with premature rupture of the membranes not in labor, an amniocentesis is required to obtain fluid, and the risks of amniocentesis must be weighed against the possibility of infection. With premature rupture of the membranes, amniocentesis is successful in only about half of patients. Thus, for many patients with clinically apparent IAI, amniotic fluid may not be available, or necessary. Amniocentesis may be clinically more useful among those in preterm labor with intact fetal membranes in whom infection is suspected.
Microbiology
Intra-amniotic infection is frequently polymicrobial, involving both facultative and anaerobic bacteria. In a case-control study of 52 patients with IAI and 52 matched controls, an average of 2.2 microbes were isolated from amniotic fluid of women with IAI.49 Among those with IAI, 48% had both facultative and anaerobic microorganisms isolated, 38% had only facultative organisms, and 8% had only anaerobes. Further, patients with IAI were more likely than controls to have higher concentrations of high-virulence microorganisms (e.g, anaerobes, group B streptococci, Escherichia coli) recovered from amniotic fluid. In the largest series, the most frequent microorganisms recovered from amniotic fluid of IAI included G. vaginalis, Bacteroides bivius, other gram-negative anaerobes, and the genital mycoplasmas M. hominis and Ureaplasma urealyticum (Table 5).35 In an important observation, although group B streptococci and E. coli were recovered from amniotic fluid in only about 20% of patients with IAI, these two microorganisms accounted for approximately two thirds of maternal or neonatal bacteremia.35 Bacteremia of the mother or neonate occurs in 25% of patients with group B streptococci recovered from amniotic fluid. Bacteremia develops in 33% of mother or neonate pairs when E. coli is recovered from amniotic fluid. Although it is frequently recovered from amniotic fluid, anaerobic maternal or neonatal bacteremia is rare. This may be interpreted to mean that anaerobes are locally active but not particularly invasive.50
Table 5. Microorganisms Recovered From Amniotic Fluid in 404 Women With Intra-amniotic Infection
|
| % Patients With |
|
| Total Isolates | Organisms | % Total Isolates |
Facultative |
|
|
|
Gardnerella vaginalis | 99 | 24.5 | 11.3 |
Group B streptococcus | 59 | 14.6 | 3.8 |
Escherichia coli | 33 | 8.2 | 3.8 |
Enterococci | 22 | 5.4 | 2.5 |
Anaerobic |
|
|
|
Bacteroides bivius | 119* | 29.5 | 13.6 |
Peptostreptococcus sp. | 38 | 9.4 | 4.3 |
Fusobacterium sp. | 22* | 5.4 | 2.5 |
Bacteroides fragilis | 14 | 3.5 | 1.6 |
Gram-negative anaerobe | 155* | 38.4 | 17.7 |
Genital Mycoplasma |
|
|
|
Ureaplasma urealyticum | 190 | 47.0 | 21.7 |
Mycoplasma hominis | 123 | 30.4 | 14.3 |
*Isolated significantly more frequently from amniotic fluid of women giving birth to low-birthweight neonates (<2500 g).
Adapted from Sperling RS, Newton E, Gibbs RS: Intraamniotic infection in low-birthweight infants. J Infect Dis 157:113, 1988.
In the series of 404 patients with IAI, pregnancies resulting in low-birthweight neonates (less than 2500 g) were more likely than pregnancies with normal-birthweight infants to have anaerobes(B. bivius, Fusobacterium sp, and gram-negative rods) in the amniotic fluid.35 In contrast, facultative microorganisms were recovered with equal frequency among preterm and term gestations.
M. hominis and U. urealyticum are recovered from amniotic fluid in 30% to 50% of women with IAI, making them the two most frequent isolates. However, they are frequently recovered in association with other virulent microorganisms, and their pathogenic potential is uncertain. M. hominis was recovered from the amniotic fluid of 35% of patients with IAI and only 8% of control patients.51 Bacteremia with M. hominis occurs in 2% of patients with both IAI and M. hominis in amniotic fluid.52 Thus, a pathologic potential of M. hominis clearly exists. In contrast, U. urealyticum was recovered from 50% of both patients with IAI and in noninfected control patients; neither U. urealyticum bacteremia nor a maternal serologic response occurred that would suggest infection.51,52 However, U. urealyticum is the most common microorganism recovered from amniotic fluid of women in preterm labor with intact fetal membranes,53,54 and the recovery of U. urealyticum from chorioamnion membranes is strongly associated with histologic chorioamnionitis.6,55 In an experimental nonhuman primate model of chorioamnionitis, intra-amniotic inoculation of U. urealyticum, in the absence of other pathogens, caused preterm delivery, IAI, histologic chorioamnionitis, and fetal pneumonitis and meningitis.56 Thus, U. urealyticum is a potential pathogen. The role of M. hominis and U. urealyticum in human intra-amniotic or intrauterine infection remains uncertain because most patients recover from infection despite antibiotic therapy that is not effective against either microorganism.
Both Chlamydia trachomatis and Neisseria gonorrhoeae have been associated with preterm labor and premature rupture of the membranes.28,57 However, neither is usually recovered from amniotic fluid among women with IAI, although C. trachomatis can grow in amniotic cell monolayers in vivo. In a prospective study of perinatal mortality complicated by maternal chlamydial infection, two of six fetal deaths in the chlamydial infection group were associated with histologic chorioamnionitis, in contrast to only one of eight in controls without chlamydial infection.58 Similarly, a higher rate of intrapartum fever was present among patients with than without antepartum cervical chlamydial infections.59 Thus, a role of C. trachomatis in acute IAI is suggested but not confirmed by intrapartum cultures, perhaps because C. trachomatis would be expected to grow poorly in the relatively cell-free environment of amniotic fluid.
Treatment
Three compelling principles guide the treatment of IAI. First, IAI is frequently a polymicrobial infection, with both facultative and anaerobic microorganisms, and broad-spectrum parenteral antibiotics are indicated. Second, although group B streptococci and E. coli account for only about 20% of isolates from IAI, these two pathogens are responsible for 60% to 70% of cases of neonatal sepsis and meningitis. Thus, antibiotics selected to treat IAI must have activity against these two pathogens. Finally, antibiotic therapy should be begun in the intrapartum period, as soon as the diagnosis is confirmed, because intrapartum antibiotics improve neonatal outcome.
A combination of parenteral ampicillin, 2 g intravenously every 6 hours, and an aminoglycoside such as gentamicin, 1.5 mg/kg intravenously every 8 hours, are most often used because of excellent activity against both group B streptococci and E. coli. However, these agents provide limited anaerobic coverage. Thus, clindamycin, 900 mg intravenously every 8 hours, should be added to this regimen for patients with IAI who undergo cesarean delivery to prevent postpartum endomyometritis. Because dysfunctional labor leads to cesarean delivery in 40% of patients with IAI, a high proportion of patients with IAI will ultimately require triple antibiotic therapy, with its associated expense and potential toxicity. For most infections, single-agent therapy with a broad-spectrum antibiotic is equally efficacious and more cost-effective. Recommended intravenous regimens include cefotetan (2 g every 12 hours), piperacillin or mezlocillin (4 g every 6 hours), or ampicillin/sulbactam (3 g every 6 hours) (Table 6). Because of the greater risk of postpartum endomyometritis after cesarean delivery, the chosen antibiotic regimen should be continued until the patient has been afebrile and symptoms have resolved for at least 24 hours. For patients delivering vaginally, the duration of antibiotic therapy is more arbitrary; most patients defervesce promptly, and more than one dose of antibiotics postpartum may be unnecessary.60
Table 6. Antibiotic Treatment of Intra-amniotic Infection
Ampicillin | 2 g IV every 6 hours |
+ |
|
Gentamicin | 1.5 mg/kg IV every 8 hours, |
| or 4.5 mg/kg IV every 24 hours |
If delivered by cesarean, add clindamycin 900 mg IV | |
every 8 hours |
|
Ampicillin/sulbactam | 3.0 g IV every 6 hours |
Piperacillin | 4.0 g IV every 6 hours |
Mezlocillin | 4.0 g IV every 6 hours |
Cefotetan | 2.0 g IV every 12 hours |
Antibiotic therapy should begin in the intrapartum period, as soon as the diagnosis of IAI is confirmed. Therapy should not be deferred until after delivery unless delivery is imminent. The benefits of intrapartum treatment clearly outweigh the potential disadvantage to the pediatrician to perform neonatal cultures without the presence of antibiotics. These antibiotics all rapidly penetrate into the fetal compartment and achieve therapeutic concentrations in cord blood after maternal intravenous administration.61 Recent studies comparing intrapartum with postpartum treatment consistently report a lower incidence of neonatal sepsis with intrapartum therapy.11,62,63 In the only randomized, prospective trial of intrapartum versus postpartum treatment, significant reductions in the rate of neonatal sepsis or pneumonia occurred in those receiving intrapartum treatment (0%) versus those with postpartum treatment (32%; p < 0.05).63 Intrapartum treatment led to a 2-day reduction in the average neonatal hospital length of stay.63 Further, maternal postpartum febrile morbidity also was reduced by intrapartum treatment.
Delivery is essential for adequate treatment, and prompt action to effect delivery should be begun at the time of diagnosis, but cesarean section should be reserved for usual obstetric indications. No advantage was attributed to cesarean delivery per se, and it increased maternal postpartum febrile morbidity. When cesarean delivery is necessary, extraperitoneal cesarean offers no benefit to reduce the risk of postpartum endomyometritis.64 Few data exist about the effect on neonatal infection of the interval between the diagnosis of infection and delivery. A critical time has not been identified beyond which maternal and neonatal complications increase. Most patients with IAI deliver within 5 to 7 hours of diagnosis. Several studies found no increase in neonatal infections for an interval of at least 12 hours among patients receiving intrapartum antibiotics.10,65,66 Whether intervals longer than 12 hours increase the risk of neonatal infections has not been determined. Continuous electronic fetal monitoring should be strongly considered. The combination of villous edema, hyperthermia, and fetal infection may contribute to fetal acidosis.2 Fetal tachycardia may be associated with maternal pyrexia but also is an independent predictor of fetal sepsis and pneumonia. Persistent fetal tachycardia may therefore portend a hemodynamically unstable neonate, and personnel skilled in neonatal resuscitation should be present at such deliveries.
Outcome
Significant maternal and perinatal morbidity may occur from IAI. However, with prompt diagnosis and antibiotic treatment, maternal and neonatal outcome may vastly improve.
MATERNAL OUTCOME
Dysfunctional labor and cesarean delivery are the most common adverse maternal outcomes associated with IAI; up to 75% of women with IAI have dysfunctional labor. The rate of cesarean delivery is increased approximately three-fold, to 35% to 40% with IAI, mainly as a result of dystocia.9,10,67 Women with IAI frequently have longer labors and slower rates of cervical dilatation, even at a high concentration of intravenous oxytocin.68 Complications associated with cesarean delivery, including blood loss, wound infection, and endomyometritis, are also increased among women with IAI.
NEONATAL OUTCOME
IAI increases the incidence of complications among term neonates despite the use of intrapartum antibiotics. Term neonates have increased risks of a low Apgar score, pneumonia, and sepsis, although most ultimately do well, and long-term neurologic deficits are rare.66,69
By contrast, preterm or low-birthweight neonates have a much higher frequency of morbidity and mortality from IAI. In addition to sepsis and pneumonia, preterm neonates also are at increased risk for death, respiratory distress syndrome, intraventricular hemorrhage, and neurologic deficits.12,35,70 A case-control study of 92 preterm neonates with IAI (cases) and 602 preterm controls found that a significant increase occurred among cases in the incidence of death (25% vs. 6%), respiratory distress syndrome (62% vs. 35%), intraventricular hemorrhage (56% vs. 22%), and sepsis (28% vs. 11%).12 More recently, a cohort analysis of 95 low-birthweight neonates born after IAI revealed an increased adjusted odds ratios for intraventricular hemorrhage (2.8, 95% confidence interval, 1.6 to 4.8), periventricular leukomalacia (3.4, 1.6 to 7.3), and early neonatal seizures (2.9, 1.2 to 6.8) when adjusted for preterm rupture of membranes, pregnancy-associated hypertension, cesarean birth, gestational age, and birth weight.70 These complications occurred more frequently in the low-birthweight population than among a cohort of term neonates born in a demographically similar population with IAI reported from the same institution (Table 7).69 Taken together, these data clearly show significant deleterious effects of IAI on the preterm or low-birthweight neonate.
Table 7. Multivariate Analysis of Neonatal Outcome in Term and Preterm Maternal Intra-amniotic Infection (IAI)
| Preterm IAI (n = 95) | Term IAI (n = 5144) |
Outcome | Odds Ratio (95% CI) | Odds Ratio (95% CI) |
Apgar, | 2.1 (1.3–3.3) | 1.3 (0.8–2.0) |
Umbilical artery pH, < 7.0 | 1.9 (0.5–6.9) | 1.2 (0.8–2.0) |
Sepsis | 2.7 (1.03–7.1) | 2.9 (2.1–4.1) |
Respiratory distress syndrome | 2.9 (1.5–5.5) |
|
Intraventricular hemorrhage, | 2.8 (1.6–4.8) |
|
grade 3 and 4 |
|
|
Periventricular leukomalacia | 3.4 (1.6–7.3) |
|
Early seizures | 2.9 (1.2–6.8) | 1.1 (0.6–2.0) |
Neonatal death | 1.7 (0.9–3.3) |
|
CI, confidence interval.
Adapted from Alexander JM, Mclntire DM, Leveno KJ et al: Chorioamnionitis and the prognosis for term infants. Obstet Gynecol 94:274, 1999, and Alexander JM, Gilstrap LC, Cox SM et al: Clinical Chorioamnionitis and the prognosis for very low birth weight infants. Obstet Gynecol 91:725, 1998.
INTRA-AMNIOTIC INFECTION, PRETERM BIRTH, AND CEREBRAL PALSY
Considerable evidence exists that IAI is an important cause of preterm birth and cerebral palsy.14,71 The prevalence of histologic chorioamnionitis is inversely related to gestational age and positively related to cerebral palsy.8,14 Bacteria indigenous to the lower genital tract are recovered from amniotic fluid of 10% to 20% of women in preterm labor with intact fetal membranes.15 The prevalence of amniotic fluid infection also is inversely related to gestational age, occurring in up to 67% of pregnancies ending at 23 to 24 weeks of gestation.36 Proinflammatory cytokines play a central role in the pathogenesis of infection-associated preterm delivery. The proinflammatory mediators (interleukin [IL]-1, IL-6, IL-8, and tumor necrosis factor [TNF]) are produced by maternal and fetal macrophages and decidual cells in response to a wide variety of bacteria or bacterial products. A role for selected cytokines in the onset of preterm labor is based on the following observations:15 1) elevated concentrations of IL-1, IL-6, and TNF and prostaglandins are found in the amniotic fluid of patients with IAI and preterm labor; 2) bacterial products stimulate the productions of IL-1, IL-6, and TNF by decidua; 3) these cytokines stimulate production of prostaglandins by amnion and decidua; and 4) intra-amniotic infusion of IL-1 induces preterm contractions and delivery in nonhuman primates, contractions that are inhibited by immunomodulators, including IL-10 (an anti-inflammatory cytokine) or dexamethasone.72,73
Recently, the role of intrauterine infections in the pathogenesis of periventricular leukomalacia and cerebral palsy has become a major focus of research, both in term and preterm infants.13,14 Periventricular leukomalacia, a cerebral lesion characterized by necrosis of the white matter near the lateral ventricles, is found in 7% to 26% of neonates with a birth weight of less than 1500 g, and 60% to 80% of these infants develop cerebral palsy.74,75 The role of infection, with release of proinflammatory cytokines, in the pathogenesis of periventricular leukomalacia and cerebral palsy was first hypothesized by Leviton in 1993.76 In this hypothesis, infection results in increased fetal concentrations of proinflammatory cytokines, which leads to not only synthesis of prostaglandins and preterm labor but also to damage to the cerebral white matter by one or more of the following mechanisms: hypotension and ischemia; intravascular coagulopathies; activation of platelet activating factor; or direct destruction of oligodendrocytes and toxic effects on myelin.
This hypothesis is supported by human observational studies and animal experimental studies. The risk of cerebral white matter damage was 9.4-fold greater among preterm neonates with purulent amniotic fluid than those with nonpurulent fluid.13 Similarly, maternal intrapartum fever or chorioamnionitis was associated with a 9.3-fold increased risk of cerebral palsy among infants of normal birthweight born mostly at term.14 Studies subsequently reported elevated concentrations of amniotic fluid proinflammatory cytokines, including IL-6, IL-1β, and TNF-α, and elevated umbilical cord plasma concentrations of IL-6 among neonates with periventricular leukomalacia or cerebral palsy.77,78 Higher neonatal blood concentrations of interleukins 1, 6, 8, and TNF-α have also been reported among neonates with cerebral palsy.79 Finally, animal models using pregnant rabbits with experimental intrauterine infection showed brain white matter lesions characterized by increased karyorrhexis, rarefaction, disorganization of white matter, and increased apoptosis in the cerebral cortex.80
The findings of increased umbilical cord IL-6 and neonatal proinflammatory cytokine concentrations suggest a fetal origin to the destructive inflammatory response, consistent with a “fetal inflammatory response syndrome.” Increased expression of IL-6 and TNF-α within the brain lesions of infants dying with periventricular leukomalacia offers further support.81 A fetal inflammatory response to IAI suggests that intrapartum antibiotic treatment alone may be insufficient to prevent these neurologic sequelae, and that immunomodulator therapy may play a role in preventing periventricular leukomalacia.
Prevention
Premature rupture of the membranes (PROM), defined as rupture of the membranes before the onset of labor, occurs in 8% to 10% of all pregnancies; preterm PROM is responsible for one third of all preterm births. Because most IAIs result from ascending infections after rupture of membranes, strategies to prevent IAI are directed at either induction of labor to shorten the duration of PROM, or antibiotic treatment after PROM to prevent ascending infection.
IAI occurs in 10% to 50% and postpartum infection in 2% to 30% of women with preterm PROM. Neonatal sepsis occurs in 1% to 25% after preterm PROM. Approximately 75% of patients with preterm PROM deliver within 1 week of rupture.82 Despite differences in study design, patient inclusion criteria, adjunctive use of tocolysis and corticosteroids, and antibiotics used, many studies in the past decade have documented that antibiotic therapy after preterm PROM reduces the incidence of delivery within 7 days, intra-amniotic and postpartum infections, and neonatal sepsis and pneumonia. One recent meta-analysis of antibiotic treatment after preterm PROM included 13 trials, among them 6 placebo-controlled treatment trials.82 Women receiving antibiotics, compared with untreated women, had significant reductions in deliveries within 1 week (62% vs. 76%; odds ratio 0.5, 95% confidence interval 0.4 to 0.7), IAI (12% vs. 23%; odds ratio 0.5, confidence interval 0.3 to 0.6), and postpartum infection (8% vs. 12%; odds ratio 0.6, confidence interval 0.4 to 0.9).82 Fetal morbidity, including sepsis (5% vs. 9%; odds ratio 0.6, confidence interval 0.4 to 0.9), pneumonia (1% vs. 3%; odds ratio 0.3, confidence interval 0.1 to 0.9), and intraventricular hemorrhage (9% vs. 14%; odds ratio 0.7, confidence interval 0.5 to 0.9) also occurred less frequently after maternal antibiotic therapy. No significant differences were observed with treatment in overall neonatal survival, respiratory distress syndrome, or necrotizing enterocolitis (Table 8). Three recent randomized, placebo-controlled trials confirmed a significant prolongation of the latent period after rupture of membranes and before delivery, and significant reductions in neonatal sepsis, respiratory distress, neonatal oxygen dependency, or ventilator requirements with maternal antibiotic treatment.83,84,85 The incidence of maternal IAI also was reduced (10.5% with treatment vs. 32.4% without treatment, p < 0.05) in the one study that specifically addressed this outcome.85 A variety of antibiotic regimens were used, but most included broad-spectrum antibiotics such as erythromycin base and ampicillin or amoxicillin/clavulanate, initially administered intravenously, followed by oral therapy for a total of 7 to 10 days. The largest study of 4826 women with preterm PROM compared four groups of patients who received erythromycin, amoxicillin/clavulanate, or erythromycin plus amoxicillin/clavulanate, or placebo.84 Reductions in neonatal morbidity were observed only when erythromycin was administered; by contrast, amoxicillin/clavulanate alone was associated with an increase in the incidence of neonatal necrotizing enterocolitis (3.8% with treatment vs. 2.4% without, p < 0.005). The reasons for this small reported increase in the incidence of necrotizing enterocolitis remain speculative but point out the need for caution in interpreting data before widespread administration of antibiotics.
Table 8. Meta-analysis of Selected Maternal and Neonatal Outcomes With or Without Antibiotic Treatment After Preterm Premature Rupture of the Membranes
| With Antibiotic |
|
| Odds Ratio | |
Outcome | Treatment | Control | (95% CI) | ||
Maternal |
|
|
|
|
|
Delivery | 281/457 | (62%) | 349/460 | (76%) | 0.5 |
|
|
|
|
| (0.4–0.7) |
Chorioamnionitis | 74/639 | (12%) | 147/649 | (23%) | 0.5 |
|
|
|
|
| (0.3–0.6) |
Postpartum infection | 36/478 | (8%) | 55/479 | (12%) | 0.6 |
|
|
|
|
| (0.4–0.9) |
Neonatal |
|
|
|
|
|
Confirmed sepsis | 33/649 | (5%) | 56/647 | (9%) | 0.6 |
|
|
|
|
| (0.4–0.9) |
Pneumonia | 4/373 | (1%) | 12/369 | (3%) | 0.3 |
|
|
|
|
| (0.1–0.9) |
Respiratory distress | 217/577 | (38%) | 232/580 | (40%) | 0.9 |
|
|
|
|
| (0.7–1.2) |
Necrotizing enterocolitis | 34/619 | (6%) | 35/616 | (6%) | 0.9 |
|
|
|
|
| (0.6–1.6) |
Intraventricular hemorrhage | 58/619 | (9%) | 85/616 | (14%) | 0.7 |
|
|
|
|
| (0.5–0.9) |
Survival | 626/673 | (93%) | 626/684 | (92%) | 1.2 |
|
|
|
|
| (0.8–1.8) |
CI, confidence interval. Confidence intervals that include 1.0 reflect no significant difference between groups.
Adapted from Mercer BM, Arheart KL: Antimicrobial therapy in expectant management of preterm premature rupture of the membranes
POSTPARTUM INFECTION
Postpartum infections remain an important cause of maternal morbidity and mortality. A significant number of women (1% to 7%) develop postpartum infections.90 One of the most complete studies identified postpartum infections in 598 (5.9%) of 10,181 deliveries.91 Thus, approximately 200,000 postpartum infections occur among the 3.5 million women delivering annually in the United States.
However, an accurate assessment of the incidence of postpartum infection is difficult, largely because different studies have used different diagnostic criteria. Puerperal infectious morbidity was initially defined in the 1930s by the Joint Commission on Maternal Welfare as “a temperature of 100.4°F (38°C) or higher, the temperature to occur on any two of the first ten days postpartum exclusive of the first 24 hours.”92 Many women with postpartum infections do not meet this definition. Only one half to two thirds of patients from studies of endomyometritis fulfill this temperature criterion. Further, many parturients experience a low-grade fever (100.4°F or higher) that resolves spontaneously, especially after vaginal delivery.93 Finally, endometrial microbial cultures, which are necessary to confirm postpartum endomyometritis, the most common postpartum infection, are technically difficult to obtain and infrequently reported.
The most common cause of postpartum infection is endomyometritis, but infections of the breast, urinary tract, episiotomy, surgical wound, or respiratory tract may also occur.
Postpartum Endomyometritis
Postpartum endomyometritis (PPE) is the most common postpartum infection. PPE occurs after 2% to 5% of vaginal deliveries and 20% to 55% of cesarean deliveries.91,94 The diagnosis of PPE may be difficult to establish with certainty because of difficulty in obtaining reliable endometrial cultures. The diagnosis is therefore usually based on clinical signs and symptoms including fever, leukocytosis, lower abdominal pain, uterine tenderness, and foul-smelling lochia. Fever is usually defined as a temperature above 38°C for 4 consecutive hours at least 24 hours from delivery, or a temperature of 38.5°C or higher within the first 24 hours after delivery. However, the absence of fever does not preclude PPE. Approximately one third to one half of patients with clinical endomyometritis will not be febrile by these criteria.91,95 The diagnosis of PPE is based primarily on fever and uterine tenderness in the absence of other sources of infection. Abdominal tenderness, especially after a cesarean delivery, foul-smelling lochia, and leukocytosis are neither sufficiently sensitive nor specific to be helpful in establishing a diagnosis. Blood cultures and urinalysis and urine culture should be obtained. Microorganisms are recovered in 10% to 20% of blood cultures and provide valuable information about at least some of the causative microbes. Urine cultures are also positive in 15% of patients with PPE, and a urinary tract infection may masquerade as endomyometritis.96 Ultrasonography or computed tomography may be helpful to delineate a mass or abscess.
RISK FACTORS
Multiple risk factors for PPE have been identified that relate to intrapartum events, coexisting lower genital tract infections, general infectious risks, and intraoperative risks. The more important of these are discussed in greater detail below.
Route of Delivery
Cesarean section is the major predisposing risk factor for endomyometritis. Women who deliver by cesarean have a higher rate of pelvic infections than do women who deliver vaginally. PPE rates rarely exceed 3% after a vaginal delivery, but the rates are commonly 30% to 50% after cesarean delivery.97 Women with PPE who deliver by cesarean also have increased severity of disease, infectious complications, and mortality rates compared with those who deliver vaginally.92
Causes of the increased incidence and severity of PPE after cesarean section have not been systematically studied, but three mechanisms are suggested. First, bacteria in surgically devitalized tissue may accelerate the spread of infection into the myometrium and parametrium.16 Second, the uterine incision could expose the myometrium and lymphatics to direct bacterial invasion. Finally, direct contamination of the peritoneum by bacteria present in amniotic fluid occurs at cesarean section.
Labor
For patients undergoing cesarean delivery, the most important determinant for infection is the length of labor. Puerperal infectious morbidity is three times higher in patients in labor than those not in labor before cesarean.98 This association was confirmed with a discriminate analysis; by eliminating other potential confounding variables, the most significant event for postpartum febrile morbidity was the duration of labor.99
Rupture of Membranes
Some but not all studies have reported an association between the duration of rupture of membranes and PPE. Bacteria colonize the amniotic fluid after rupture of membranes, and it is reasonable to expect that rupture of membranes may be a risk factor for PPE. All amniotic fluid samples taken from women with rupture of membranes of 6 hours or more in duration appear to contain microorganisms, and 95% of those with microorganisms developed endometritis.16 Despite these reports, not all investigators confirmed an association between rupture of membranes and endomyometritis, when adjusted for the independent effect of labor. The uncertain role of rupture of membranes as an independent risk factor has been underscored by a finding that duration of labor, and not duration of rupture of membranes, correlated with postpartum infection.99 Thus, the role of ROM is unclear in PPE, independent of labor.
Socioeconomic Status
Regardless of race, indigent patients have higher puerperal infection rates than nonindigent patients. The causes are unclear but may be related to differences in vaginal microbial flora, nutrition, or other unidentified factors.
Vaginal Examinations and Internal Fetal Monitoring
Few data support a direct role for either vaginal examinations or internal fetal monitoring in PPE. In a stepwise discriminate analysis, the number of vaginal examinations was correlated more closely with the duration of labor than with the presence or absence of infection.99 Likewise, internal fetal monitoring may increase the risk of PPE very slightly among patients delivered by cesarean, but its role is small compared with other factors.100
Lower Genital Tract Infections
BV has recently been recognized as an important independent risk factor for PPE among patients delivered either by cesarean or vaginally. Bacterial species associated with BV, including G. vaginalis, anaerobes, and M. hominis, are among the most frequent isolates from endometrial cultures of patients with PPE.96 In a prospective study, patients with BV had a 5.8-fold higher risk of PPE after cesarean section than patients with normal vaginal flora, after adjusting for maternal age, duration of labor, and duration of rupture of membranes.29 The increased risk occurred despite the routine use of antibiotic prophylaxis with cephalexin. It is likely that cephalexin prophylaxis is inadequate to inhibit the greatly increased concentration of anaerobes and other bacteria present with BV. The impact of prophylaxis in patients with BV who undergo cesarean section requires further study. Even among patients delivering vaginally, BV is associated with an increased risk of PPE. The presence of BV-associated microorganisms (primarily anaerobes, G. vaginalis, and M. hominis) resulted in a 14-fold increased risk of PPE among a cohort of women delivering vaginally.101
Other Factors
Anemia was associated with puerperal infections in some studies. Rather than being a cause of infection, anemia may simply reflect poor nutritional status, young age, or lower socioeconomic status. Obesity has not been a consistent risk factor for PPE, but it is a risk factor for wound infection. General anesthesia could be a risk factor for PPE, but because general anesthesia is frequently associated with emergent surgeries or severe maternal illness, its independent role in PPE is not clear.
MICROBIOLOGY
PPE usually results from a mixed, polymicrobial infection with both facultative and anaerobic microorganisms. On average, two or three species can be recovered from the endometrium; 80% of patients with early-onset infection have two or more microorganisms.96 Most cases of PPE among women who deliver by cesarean occur within the first 48 hours postpartum, whereas women who deliver vaginally develop PPE later, from 3 days to 6 weeks after delivery. Facultative and anaerobic bacteria and genital mycoplasmas are usually recovered from women with early-onset PPE, whereas C. trachomatis and genital mycoplasmas are recovered from women with late-onset PPE.59,102
The role of specific microorganisms in early PPE is difficult to identify, in part because cervical cultures or endometrial samples obtained by transcervical sampling are contaminated by cervicovaginal flora. Protected sampling devices, such as a triple-lumen sampling device, reduce cervicovaginal microbial contamination.103 Triple-lumen endometrial cultures yielded a mean of 3.3 organisms per patient in 150 women with early PPE (Table 9).96 Two or more organisms were recovered from the endometrium in 81% of patients, emphasizing the polymicrobial nature of PPE. Interrelationships between groups of microorganisms were also identified in this study. In general, four large and overlapping groups of organisms were present in the endometrium: U. urealyticum; M. hominis; BV-related organisms, including G. vaginalis and selected anaerobes; and miscellaneous facultative and anaerobic microorganisms. The largest bacterial group included 91 (61%) patients with BV-related organisms recovered from the endometrium. Anaerobes accounted for 25% of the endometrial isolates and 29% of 52 blood isolates (see Table 9) and together were the most frequent organisms recovered. Facultative gram-positive cocci, including group B streptococci and enterococci, accounted for 23% of endometrial and 21% of blood isolates. Facultative gram-negative rods accounted for 17% of blood and 25% of blood isolates. The most common gram-negative rod recovered from either the endometrium or blood was G. vaginalis. E. coli was recovered from the endometrium of only 12 patients and from the blood of only 2 patients.
Table 9. Microorganisms Recovered from Endometrial and Blood Among 150 Women With Early-Onset Postpartum Endomyometritis
| Total Isolates |
|
| Endometrium (n = 477) | Blood (n = 52) |
Facultative | 216 (45%) | 25 (46%) |
Gram-positive cocci | 108 | 11 |
Group B streptococci | 12 | 4 |
Enterococcus sp. | 18 | 1 |
Other streptococci | 71 | 6 |
Gram-negative rods | 81 | 13 |
Gardnerella vaginalis | 57 | 10 |
Escherichia coli | 12 | 3 |
Anaerobes | 117 (25%) | 15 (29%) |
Peptostreptococcus sp. | 65 | 3 |
Bacteroides sp. | 44 | 3 |
Genital Mycoplasma | 144 (30%) | 10 (19%) |
Mycoplasma hominis | 36 | 4 |
Ureaplasma urealyticum | 107 | 6 |
Adapted from Watts DH, Eschenbach DA, Kenny GE: Early postpartum endometritis. Obstet Gynecol 73:52, 1989.
M. hominis and U. urealyticum have also been implicated in postpartum fever.104 Genital mycoplasmas accounted for 144 (30%) of the 477 total endometrial isolates and 10 (19%) of the 52 blood isolates.96 In 11% of cases of PPE, only genital mycoplasmas were recovered from the endometrium.96 Thus, it is likely that genital mycoplasmas play a small but significant role in PPE.
C. trachomatis does not appear to be an important pathogen in early-onset PPE. However, it may be responsible for 25% of cases of endometritis occurring 48 hours to 6 weeks postpartum among patients who delivered vaginally.59,105
THERAPY
Ideally, the choice of antibiotic therapy depends on the responsible microorganisms. However, endometrial cultures are difficult to obtain, and results are usually not available for several days. With the recognition that PPE is usually a polymicrobial infection with a high chance of anaerobes, empiric antibiotic therapy should be started with a broad-spectrum antibiotic with anaerobic activity. Patients can usually be clinically classified into mild-to-moderate endomyometritis or severe endomyometritis characterized by frankly purulent discharge, marked peritoneal tenderness, or systemic signs of sepsis.
Patients with mild-to-moderate infection should be treated with parenteral extended-spectrum cephalosporins or penicillins (Table 10). For mild-to-moderate infection, more than 50 clinical trials have compared various cephalosporins or penicillins.106 All regimens result in clinical improvement in 85% to 90% of patients. Parenteral therapy should be continued until the patient has been afebrile, and symptoms resolved, for 24 hours, and then therapy should be discontinued. Oral therapy after parenteral therapy is unnecessary and does not reduce delayed febrile morbidity.107 Patients who do not respond to therapy within 48 to 72 hours should be scrutinized for wound infection, pelvic abscess, septic pelvic thrombophlebitis, inadequate antibiotic dosage, or resistant microorganisms. In particular, infection with Enterococcus sp should be suspected if the patient has been treated with cephalosporins or other antibiotics ineffective against enterococci.
Table 10. Antibiotic Treatment of Postpartum Endomyometritis
Mild/Moderate Infection |
|
Cefotetan | 2.0 g IV every 12 hours |
Cefoxitin | 2.0 g IV every 6 hours |
Ampicillin/sulbactam | 3.0 g IV every 6 hours |
Piperacillin | 4.0 g IV every 6 hours |
Severe Infection |
|
Gentamicin | 4.5-5.0 mg IV once daily |
+ |
|
Clindamycin | 900 mg IV every 8 hours |
Gentamicin | 4.5-5.0 mg IV once daily |
+ |
|
Clindamycin | 900 mg IV every 8 hours |
+ |
|
Ampicillin | 2 g IV every 6 hours |
Imipenem/cilastatin | 500 mg IV every 6 hours |
Meropenem | 1 g IV every 8 hours |
Patients with severe infection should be treated with antibiotic regimens highly active against both gram-negative enteric and anaerobic bacteria. Initial therapy should consist of intravenous gentamicin (4.5 to 5.0 mg/kg every 24 hours) and clindamycin (900 mg every 8 hours) or a carbapenem such as imipenem/cilastatin or meropenem. It has now been established that once-daily administration of gentamicin in the treatment of endomyometritis is as efficacious, less toxic, and more cost-effective than traditional administration of gentamicin every 8 hours.108,109
PROPHYLAXIS
Short-course antibiotic prophylaxis reduces by two thirds to three quarters the incidence of both PPE and wound infection among women undergoing either elective or nonelective cesarean delivery.110,111,112 More than 50 trials of antibiotic prophylaxis after cesarean section have been reported, using a wide variety of antibiotic regimens, and all have reported equivalent findings. Therefore, the preferred antibiotic is a first-generation cephalosporin, such as cefazolin (1 g), cephalothin (1 g), or ampicillin (1 to 2 g) given intravenously after cord clamping. Newer extended-spectrum cephalosporins or penicillins are no more effective and are more expensive. Single-dose prophylaxis is sufficient; in several studies, it has been shown to have similar efficacy to that of three-dose prophylaxis and is less likely to result in the emergence of resistant microorganisms or adverse drug reactions.
Despite antibiotic prophylaxis, approximately 10% of patients subsequently develop PPE. Reasons for failure of antibiotic prophylaxis include pre-existing myometrial colonization with microorganisms113 or endometrial colonization with Enterococcus faecalis, group B streptococci, facultative gram-negative rods, and BV-associated microbes.114,115 Thus, antibiotics effective against these pathogens should be used to treat patients who fail to respond to prophylaxis.
Prophylaxis does not decrease the incidence of serious postpartum infectious morbidity (e.g, pelvic abscess, septic pelvic thrombophlebitis, or rapidly progressive soft tissue infections). Finally, simple adherence to strict aseptic technique may significantly reduce the rate of PPE without exposure to antibiotics and should be emphasized.116
Septic Pelvic Thrombophlebitis
Septic pelvic thrombophlebitis (SPT) is an unusual cause of puerperal infectious morbidity. Typically, SPT involves the ovarian veins and results from a combination of venous stasis, hypercoagulability associated with pregnancy, and vascular endothelial damage. The reported incidence of SPT is approximately 1 in 2000 deliveries,117 but it may occur in as many as 1% to 2% of patients with postcesarean PPE.118
SPT may present in two distinct clinical forms. The acute ovarian vein thrombophlebitis form is characterized by the acute onset of lower abdominal pain in the first 2 to 3 days postpartum. Patients are usually febrile and appear acutely ill. Gastrointestinal symptoms including nausea, vomiting, and distention may be present. The most striking physical finding is a tender tubular mass on abdominal or pelvic examination; this is present in half of patients. However, because of the abrupt onset and the severity of associated symptoms, many patients undergo laparotomy for presumed appendicitis. The second, less distinct clinical form is characterized by persistently high spiking fevers several days postpartum. Most patients received antibiotic therapy for PPE and otherwise do not appear toxic, often appearing less ill than suggested by their high persistent fever. Physical findings are minimal, and there is no evidence of a pelvic abscess or wound infection.
A wide variety of microorganisms are associated with SPT. Anaerobic bacteria including peptococci, peptostreptococci, and Bacteroides sp. are usually implicated, although facultative streptococci have also been recovered.
Traditionally, the diagnosis of SPT has been one of exclusion and defervescence after the initiation of anticoagulant therapy with intravenous heparin. Pelvic computed tomography also can be used to exclude the diagnosis of pelvic abscess and to diagnose SPT with greater than 90% accuracy; it has largely replaced heparinization as a diagnostic test. Traditionally, heparin therapy has been used as both a diagnostic test and treatment. If defervescence occurred within 48 to 72 hours, the diagnosis was confirmed and heparin was continued for a total of 10 days. Prolonged anticoagulation was not recommended in the absence of documented pulmonary emboli. However, the mechanism of action of heparin is unknown, and its use in the treatment of SPT has recently been questioned. In a recent series of patients with SPT randomized to continued antibiotic therapy alone or antibiotic therapy with heparin, there was no difference in the time required to become afebrile (134 hours with heparin vs. 140 hours without), the duration of the hospital stay, or long-term infectious or embolic sequelae.119 Thus, the role of heparinization in the treatment of SPT should be re-evaluated and possibly abandoned.
Urinary Tract Infection
Urinary tract infection (UTI) is a common cause of postpartum fever. The diagnosis of UTI is based on clinical signs and symptoms, including fever, dysuria, frequency, and costovertebral angle tenderness and urine culture and urinalysis. Because urine specimens from puerperal patients are frequently contaminated with lochia, care must be taken in obtaining a midvoid specimen. The diagnosis of bacteriuria is defined as the presence of 100,000 or more colony-forming units per milliliter of a single microorganism. However, recent evidence among nonpregnant women suggests that a bacterial colony count of 100 or more bacteria per milliliter is more specific and sensitive in the diagnosis of UTI.120 The presence of leukocytes in urine (pyuria) suggests infection but is not diagnostic because bladder inflammation from the trauma associated with birth may lead to sterile pyuria. Rapid alternative screening methods for pyuria and bacteriuria are available, including detection of leukocyte esterase, indicative of pyuria, within urine, and the detection of urinary nitrites, indicating bacteriuria. When compared with quantitative culture, these rapid diagnostic tests have poor specificity and sensitivity and should not be used as the only criteria to diagnose UTI in the postpartum patient.
The microorganisms generally responsible for UTI are representative of the fecal and genital flora. E. coli accounts for 80% to 90% of all acute UTIs. Additional gram-negative microorganisms causing urinary infection include Klebsiella sp., Proteus sp., Enterobacter sp., and Pseudomonas sp. Gram-positive bacteria that may cause UTI include group B streptococci, Staphylococcus saprophyticus, and Enterococcus sp.
The febrile postpartum patient with UTI should be initially treated with parenteral antibiotics. Because of the high prevalence of ampicillin-resistant microorganisms, intravenous therapy with a first-, second-, or third-generation cephalosporin should be used as initial therapy. If septic shock occurs or if a resistant microorganism is suspected, an aminoglycoside or aztreonam should be added. Patients with pyelonephritis who fail to respond to therapy after 72 hours should be evaluated for urinary tract obstruction or other pathology.
Mastitis
Most breast infections occur among women who are breast-feeding their neonates. Two types of infection have been identified: an epidemic infection usually attributed to Staphylococcus aureus, and an endemic form that occurs with a frequency of 1% to 5% of lactating women. S. aureus is also the most frequently recovered microorganism from the endemic form, but other gram-positive cocci have also been reported in endemic mastitis. Gram-negative microorganisms are rarely isolated from breast infection.
Most infections begin during the second or third week postpartum and are characterized by localized pain, erythema, and induration.121 Fever may develop rapidly. Patients who report breast pain and fever should be immediately examined because of an increased risk of breast abscess if therapy is delayed. Because of the frequency of penicillase-producing strains of S. aureus, penicillinase-resistant antibiotics such as cloxacillin, dicloxacillin, or cephalosporins are preferred for the treatment of mastitis. In most cases, therapy may be given orally. Regular and complete drainage of the breast is important to prevent abscess formation. In the absence of an abscess, breast-feeding may be continued. However, with breast abscess, a breast pump should be used and the milk discarded until the abscess has cleared.
Anesthesia Complications
Infections resulting from regional spinal or epidural anesthesia are extremely rare. A large population-based study of complications of regional anesthesia in Finland found only six bacterial infections resulting from 720,000 spinal or epidural regional blocks, an incidence of 0.08 per 10,000.122 Nevertheless, the sequelae of spinal or epidural abscess can be severe, with nearly half of such patients left with persistent neurologic deficits.123 S. aureus is usually the causative microorganism, but Staphylococcus epidermidis may also cause infection. Treatment should include surgical debridement and antibiotics with activity against S. aureus. With general anesthesia, atelectasis and pulmonary infections may masquerade as a puerperal infection. Infections of the pudendal nerve tract or psoas space have been reported after transvaginal pudendal nerve blocks. These infections are frequently associated with E. coli and are characterized by fever, severe hip pain, and limited hip motion. Therapy is directed toward early recognition, broad-spectrum antibiotics, and surgical drainage if an abscess is suspected.
Surgical Therapy
In puerperal infections, 1% to 2% of patients require surgical intervention. Curettage of retained placental fragments may be necessary. Drainage of a pelvic abscess may require percutaneous aspiration or, infrequently, laparotomy. Surgical debridement of rapidly progressive soft tissue infections may be life-saving. Most puerperal infections readily respond to antibiotics. However, surgery must be considered primary therapy with severe sepsis indicated by septic shock, adult respiratory distress syndrome, disseminated intravascular coagulation, and an increasing area of cellulitis or clinical deterioration in a clinically ill patient. These details are discussed elsewhere in this volume.
REFERENCES
Berg CJ, Atrash HK, Koonin LM, Tucker M: Pregnancy-related mortality in the United States. Obstet Gynecol 88: 161, 1996 |
|
Newton ER: Chorioamnionitis and intraamniotic infection. Clin Obstet Gynecol 36: 795, 1993 |
|
Newton ER, Prihoda TJ, Gibbs RS: Logistic regression analysis of risk factors for intra-amniotic infection. Obstet Gynecol 73: 571, 1989 |
|
Soper DE, Mayhall CG, Dalton HP: Risk factors for intraamniotic infection: a prospective epidemiologic study. Am J Obstet Gynecol 161: 562, 1989 |
|
Lopez-Zeno JA, Peaceman AM, Adashek JA, Socol ML: A controlled trial of a program for the active management of labor. N Engl J Med 326: 450, 1992 |
|
Hillier SL, Martius J, Krohn M et al: A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med 319: 972, 1988 |
|
Mueller-Heubach E, Rubinstein D, Schwarz SS: Histologic chorioamnionitis and preterm delivery in different patient populations. Obstet Gynecol 75: 622, 1990 |
|
Russell P: Inflammatory lesions of the human placenta: 1. Clinical significance of acute chorioamnionitis. Am J Diagn Gynecol Obstet 1: 127, 1979 |
|
Duff P, Sanders R, Gibbs RS: The course of labor in term pregnancies with chorioamnionitis. Am J Obstet Gynecol 147: 391, 1983 |
|
Gibbs RS, Castillo MA, Rodgers PJ: Management of acute chorioamnionitis. Am J Obstet Gynecol 136: 709, 1980 |
|
Gilstrap LC III, Leveno KJ, Cox SM et al: Intrapartum treatment of acute chorioamnionitis: Impact on neonatal sepsis. Am J Obstet Gynecol 159: 579, 1988 |
|
Morales WJ: The effect of chorioamnionitis on the developmental outcome of preterm infants at one year. Obstet Gynecol 70: 183, 1987 |
|
Bejar R, Wozniak P, Allard M, et al: Antenatal origin of neurologic damage in newborn infants. I. Preterm infants. Am J Obstet Gynecol 159: 357, 1988 |
|
Grether JK, Nelson KB: Maternal infection and cerebral palsy in infants of normal birth weight. JAMA 278: 207, 1997 |
|
Romero R, Avila C, Brekus CA, Morotti R: The role of systemic and intrauterine infection in preterm parturition. Ann NY Acad Sci 622: 355, 1991 |
|
Gilstrap LC III, Cunningham FG: The bacterial pathogenesis of infection following cesarean section. Obstet Gynecol 53: 545, 1979 |
|
Blanco JD, Gibbs RS, Castaneda YS, St. Clair PJ: Correlation of quantitative amniotic fluid cultures with endometritis after cesarean section. Am J Obstet Gynecol 143: 897, 1982 |
|
Linnan MJ, Mascola L, Lou XD et al: Epidemic listeriosis associated with Mexican-style cheese. N Engl J Med 319: 823, 1988 |
|
Aarnoudse JG, Huisjes HJ: Complications of cerclage. Acta Obstet Gynecol Scand 58: 255, 1979 |
|
Charles D, Edwards WR: Infectious complications of cervical cerclage. Am J Obstet Gynecol 141: 1065, 1981 |
|
Treadwell MC, Bronsteen RA, Bottoms SF: Prognostic factors and complication rates for cervical cerclage: a review of 482 cases. Am J Obstet Gynecol 165: 555, 1991 |
|
Romero R, Gonzalez R, Sepulveda W et al: Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: Prevalence and clinical significance. Am J Obstet Gynecol 167: 1086, 1992 |
|
Rust OA, Atlas RO, Jones KJ et al: A randomized trial of cerclage versus no cerclage among patients with ultrasonographically detected second-trimester preterm dilatation of the internal os. Am J Obstet Gynecol 183: 830, 2000 |
|
Novy MJ, Gupta A, Wothe DD et al: Cervical cerclage in the second trimester of pregnancy: A historical cohort study. Am J Obstet Gynecol 184: 1447, 2001 |
|
Seaward PG, Hannah ME, Myhr TL et al: International Multicentre Term Prelabor Rupture of Membranes Study: Evaluation of predictors of clinical chorioamnionitis and postpartum fever in patients with prelabor rupture of membranes at term. Am J Obstet Gynecol 177: 1024, 1997 |
|
Krohn MA, Hitti J: Characteristics of women with clinical intra-amniotic infection who deliver preterm compared with term. Am J Epidemiol 147: 111, 1998 |
|
Krohn MA, Germain M, Muhlemann K, Hickok D: Prior pregnancy outcome and the risk of intraamniotic infection in the following pregnancy. Am J Obstet Gynecol 178: 381, 1998 |
|
Gravett MG, Nelson HP, DeRouen T et al: Independent associations of bacterial vaginosis and Chlamydia trachomatis infection with adverse pregnancy outcome. JAMA 256: 1899, 1986 |
|
Watts DH, Krohn MA, Hillier SL, Eschenbach DA: Bacterial vaginosis as a risk factor for post-cesarean endometritis. Obstet Gynecol 75: 52, 1990 |
|
Silver HM, Sperling RS, St. Clair PJ, Gibbs RS: Evidence relating bacterial vaginosis to intraamniotic infection. Am J Obstet Gynecol 161: 808, 1989 |
|
Krohn MA, Hillier SL, Nugent RP et al: The genital flora of women with intraamniotic infection. Vaginal Infection and Prematurity Study Group. J Infect Dis 171: 1475, 1995 |
|
Newton ER, Piper J, Peairs W: Bacterial vaginosis and intraamniotic infection. Am J Obstet Gynecol 176: 672, 1997 |
|
Hitti J, Hillier SL, Agnew KJ et al: Vaginal indicators of amniotic fluid infection in preterm labor. Obstet Gynecol 97: 211, 2001 |
|
Martius J, Eschenbach DA: The role of bacterial vaginosis as a cause of amniotic fluid infection, chorioamnionitis and prematurity-a review. Arch Gynecol Obstet 247: 1, 1990 |
|
Sperling RS, Newton E, Gibbs RS: Intraamniotic infection in low-birth-weight infants. J Infect Dis 157: 113, 1988 |
|
Watts DH, Krohn MA, Hillier SL, Eschenbach DA: The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol 79: 351, 1992 |
|
Naeye RL, Ross S: Coitus and chorioamnionitis: A prospective study. Early Human Devel 6: 91, 1982 |
|
Mills JL, Harlap S, Harley EE: Should coitus late in pregnancy be discouraged? Lancet 2: 136, 1981 |
|
Gravett MG, Witkin SS, Haluska GJ et al: An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol 171: 1660, 1994 |
|
Ohlsson A, Wang E: An analysis of antenatal tests to detect infection at preterm rupture of the membranes. Am J Obstet Gynecol 162: 809, 1990 |
|
Romero R, Emamian M, Wan M et al: The value of the leukocyte esterase test in diagnosing intra-amniotic infection. Am J Perinatol 5: 64, 1988 |
|
Hoskins IA, Johnson TR, Winkel CA: Leukocyte esterase activity in human amniotic fluid for the rapid detection of chorioamnionitis. Am J Obstet Gynecol 157: 730, 1987 |
|
Egley CC, Katz VL, Herbert WN: Leukocyte esterase: A simple bedside test for the detection of bacterial colonization of amniotic fluid. Am J Obstet Gynecol 159: 120, 1988 |
|
Romero R, Jimenez C, Lohda AK et al: Amniotic fluid glucose concentration: A rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol 163: 968, 1990 |
|
Kiltz RJ, Burke MS, Porreco RP: Amniotic fluid glucose concentration as a marker for intra-amniotic infection. Obstet Gynecol 78: 619, 1991 |
|
Gauthier DW, Meyer WJ, Bieniarz A: Correlation of amniotic fluid glucose concentration and intraamniotic infection in patients with preterm labor or premature rupture of membranes. Am J Obstet Gynecol 165: 1105, 1991 |
|
Odibo AO, Rodis JF, Sanders MM et al: Relationship of amniotic fluid markers of intra-amniotic infection with histopathology in cases of preterm labor with intact membranes. J Perinatol 19: 407, 1999 |
|
Gauthier DW, Meyer WJ: Comparison of gram stain, leukocyte esterase activity amniotic fluid glucose concentration in predicting amniotic fluid culture results in preterm premature rupture of membranes. Am J Obstet Gynecol 167: 1092, 1992 |
|
Gibbs RS, Blanco JD, St. Clair PJ, Castaneda YS: Quantitative bacteriology of amniotic fluid from women with clinical intraamniotoic infection at term. J Infect Dis 145: 1, 1982 |
|
Gibbs RS, Duff P: Progress in pathogenesis and management of clinical intraamniotic infection. Am J Obstet Gynecol 164: 1317, 1991 |
|
Blanco JD, Gibbs RS, Malherbe H et al: A controlled study of genital mycoplasmas in amniotic fluid from patients with intra-amniotic infection. J Infect Dis 147: 650, 1983 |
|
Gibbs RS, Cassell GH, Davis JK, St. Clair PJ: Further studies on genital mycoplasmas in intra-amniotic infection: blood cultures and serologic response. Am J Obstet Gynecol 154: 717, 1986 |
|
Gravett MG, Hummel D, Eschenbach DA, Holmes KK: Preterm labor associated with subclinical amniotic fluid infection and with bacterial vaginosis. Obstet Gynecol 67: 229, 1986 |
|
Yoon BH, Romero R, Park JS et al: Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol 179: 1254, 1998 |
|
Hillier SL, Krohn MA, Kiviat NB et al: Microbiologic causes and neonatal outcomes associated with chorioamnion infection. Am J Obstet Gynecol 165: 955, 1991 |
|
Novy MJ, Duffy L, Axthelm MK et al: Experimental primate model for Ureaplasma chorioamnionitis and preterm labor. Society for Gynecologic Investigation 2001. |
|
Edwards LE, Barrada MI, Hamann AA, Hakanson EY: Gonorrhea in pregnancy. Am J Obstet Gynecol 132: 637, 1978 |
|
Martin DH, Koutsky L, Eschenbach DA et al: Prematurity and perinatal mortality in pregnancies complicated by maternal Chlamydia trachomatis infections. JAMA 247: 1585, 1982 |
|
Wager G, Martin DH, Koutsky L et al: Puerperal infectious morbidity: Relationship to route of delivery and to antepartum Chlamydia trachomatis infection. Am J Obstet Gynecol 138: 1028, 1980 |
|
Chapman SJ, Owen J: Randomized trial of single-dose versus multiple-dose cefotetan for the postpartum treatment of intrapartum chorioamnionitis. Am J Obstet Gynecol 177: 831, 1997 |
|
Gilstrap LC III, Bawdon RE, Burris J: Antibiotic concentration in maternal blood, cord blood, and placental membranes in chorioamnionitis. Obstet Gynecol 72: 124, 1988 |
|
Sperling RS, Ramamurthy RS, Gibbs RS: A comparison of intrapartum versus immediate postpartum treatment of intraamniotic infection. Obstet Gynecol 70: 861, 1987 |
|
Gibbs RS, Dinsmoor MJ, Newton ER, Ramamurthy RS: A randomized trial of intrapartum versus immediate postpartum treatment of women with intra-amniotic infection. Obstet Gynecol 72: 823, 1988 |
|
Wallace RL, Eglinton GS, Yonekura ML, Wallace TM: Extraperitoneal cesarean section: a surgical form of infection prophylaxis? Am J Obstet Gynecol 148: 172, 1984 |
|
Garite TJ, Freeman RK: Chorioamnionitis in the preterm gestation. Obstet Gynecol 59: 539, 1982 |
|
Hauth JC, Gilstrap LC, Hankins GDV, Connor KD: Term maternal and neonatal complications of acute chorioamnionitis. Obstet Gynecol 66: 59, 1985 |
|
Koh KS, Chan FH, Monfared AH et al: The changing perinatal and maternal outcome in chorioamnionitis. Obstet Gynecol 53: 730, 1979 |
|
Silver RK, Gibbs RS, Castillo M: Effect of amniotic fluid bacteria on the course of labor in nulliparous women at term. Obstet Gynecol 68: 587, 1986 |
|
Alexander JM, McIntire DM, Leveno KJ: Chorioamnionitis and the prognosis for term infants. Obstet Gynecol 94: 274, 1999 |
|
Alexander JM, Gilstrap LC, Cox SM et al: Clinical chorioamnionitis and the prognosis for very low birth weight infants. Obstet Gynecol 91: 725, 1998 |
|
Gibbs RS, Romero R, Hillier SL et al: A review of premature birth and subclinical infection. Am J Obstet Gynecol 166: 1515, 1992 |
|
Baggia S, Gravett MG, Witkin SS et al: Interleukin-1 beta intra-amniotic infusion induces tumor necrosis factor-alpha, prostaglandin production, and preterm contractions in pregnant rhesus monkeys. J Soc Gynecol Invest 3: 121, 1996 |
|
Sadowsky DW, Gravett MG, Witkin SS et al: Does interleukin-10 block interleukin-1-beta-induced preterm labor in rhesus monkeys? Society for Gynecologic Investigation Annual Scientific Meeting, Atlanta, GA, 1999 |
|
Trounce JQ, Rutter N, Levene MI: Periventricular leucomalacia and intraventricular haemorrhage in the preterm neonate. Arch Dis Child 61: 1196, 1986 |
|
Levene MI, Wigglesworth JS, Dubowitz V: Hemorrhagic periventricular leukomalacia in the neonate: A real-time ultrasound study. Pediatrics 71: 794, 1983 |
|
Leviton A: Preterm birth and cerebral palsy: Is tumor necrosis factor the missing link? Dev Med Child Neurol 35: 553, 1993 |
|
Yoon BH, Jun JK, Romero R et al: Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1 beta tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol 177: 19, 1997 |
|
Yoon BH, Romero R, Yang SH et al: Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol 174: 1433, 1996 |
|
Nelson KB, Dambrosia JM, Grether JK, Phillips TM: Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol 44: 665, 1998 |
|
Yoon BH, Kim CJ, Romero R et al: Experimentally induced intrauterine infection causes fetal brain white matter lesions in rabbits. Am J Obstet Gynecol 177: 797, 1997 |
|
Yoon BH, Romero R, Kim CJ et al: High expression of tumor necrosis factor-alpha and interleukin-6 in periventricular leukomalacia. Am J Obstet Gynecol 177: 406, 1997 |
|
Mercer BM, Arheart KL: Antimicrobial therapy in expectant management of preterm premature rupture of the membranes. Lancet 346: 1271, 1995 |
|
Mercer BM, Miodovnik M, Thurnau GR et al: Antibiotic therapy for reduction of infant morbidity after preterm premature rupture of the membranes. A randomized controlled trial. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. JAMA 278: 989, 1997 |
|
Kenyon SL, Taylor DJ, Tarnow-Mordi W: Broad-spectrum antibiotics for preterm, prelabour rupture of fetal membranes: The ORACLE I randomised trial. Lancet 357: 979, 2001 |
|
Lovett SM, Weiss JD, Diogo MJ et al: A prospective, double-blind, randomized, controlled clinical trial of ampicillin-sulbactam for preterm premature rupture of membranes in women receiving antenatal corticosteroid therapy. Am J Obstet Gynecol 176: 1030, 1997 |
|
Kappy KA, Cetrulo CL, Knuppel RA et al: Premature rupture of the membranes at term. A comparison of induced and spontaneous labors. J Reprod.Med 27: 29, 1982 |
|
Duff P, Huff RW, Gibbs RS: Management of premature rupture of membranes and unfavorable cervix in term pregnancy. Obstet Gynecol 63: 697, 1984 |
|
Hannah ME, Ohlsson A, Farine D et al: Induction of labor compared with expectant management for prelabor rupture of the membranes at term. TERMPROM Study Group. N Engl J Med 334: 1005, 1996 |
|
Centers for Disease Control and Prevention: Prevention of perinatal group B streptococcal disease: A public health perspective. MMWR 45 RR-7:1, 1996 |
|
Gibbs RS, Weinstein AJ: Puerperal infection in the antibiotic era. Am J Obstet Gynecol 124: 769, 1976 |
|
Sweet RL, Ledger WJ: Puerperal infectious morbidity: A two-year review. Am J Obstet Gynecol 117: 1093, 1973 |
|
Eschenbach DA, Wager G: Puerperal infections. Clin Obstet Gynecol 23: 1003, 1980 |
|
Filer R, Monif GRG: The significance of temperature during the first 24 hours postpartum. Obstet Gynecol 53: 358, 1979 |
|
Humphrey MD: Post-partum infection: A survey. Med J Australia 2: 657, 1972 |
|
Ledger WJ, Reite AM, Headington JT: A system for infectious disease surveillance on an obstetrical service. Obstet Gynecol 37: 769, 1971 |
|
Watts DH, Eschenbach DA, Kenny GE: Early postpartum endometritis: The role of bacteria, genital mycoplasmas, and Chlamydia trachomatis. Obstet Gynecol 73: 52, 1989 |
|
Cunningham FG, Hauth JC, Strong JD et al: Infectious morbidity following cesarean section: Comparison of two treatment regimens. Obstet Gynecol 52: 656, 1978 |
|
Sohlberg OS, Goodlin RC: The impact of prior labor on cesarean section morbidity. Pac Med Surg 75: 54, 1967 |
|
D'Angelo LJ, Sokol RJ: Time-related peripartum determinants of postpartum morbidity. Obstet Gynecol 55: 319, 1980 |
|
Haverkamp AD, Orleans M, Langendoerfer S et al: A controlled trial of the differential effects of intrapartum fetal monitoring. Am J Obstet Gynecol 134: 399, 1979 |
|
Newton ER, Prihoda TJ, Gibbs RS: A clinical and microbiologic analysis of risk factors for puerperal endometritis. Obstet Gynecol 75: 402, 1990 |
|
Hoyme UB, Kiviat N, Eschenbach DA: The microbiology and treatment of late postpartum endometritis. Obstet Gynecol 68: 21, 1986 |
|
Eschenbach DA, Rosene K, Tompkins LS et al: Endometrial cultures obtained by a triple-lumen method from afebrile and febrile postpartum women. J Infect Dis 153: 1038, 1986 |
|
Lamey RJ, Eschenbach DA, Mitchell SH et al: Isolation of mycoplasmas and bacteria from the blood of postpartum women. Am J Obstet Gynecol 143: 104, 1982 |
|
Hoyme UB, Kiviat N, Eschenbach DA: Microbiology and treatment of late postpartum endometritis. Obstet Gynecol 68: 226, 1986 |
|
French LM, Smaill FM: Antibiotic regimens for endometritis after delivery. Cochrane Database Syst Rev CD001067, 2000 |
|
Hager WD, Pascuzzi M, Vernon M: Efficacy of oral antibiotics following parenteral antibiotics for serious infections in obstetrics and gynecology. Obstet Gynecol 73: 326, 1989 |
|
Del Priore G, Jackson-Stone M, Shim EK et al: A comparison of once-daily and 8-hour gentamicin dosing in the treatment of postpartum endometritis. Obstet Gynecol 87: 994, 1996 |
|
Sunyecz JA, Wiesenfeld HC, Heine RP: The pharmacokinetics of once-daily dosing with gentamicin in women with postpartum endometritis. Infect Dis Obstet Gynecol 6: 160, 1998 |
|
Hopkins L, Smaill F: Antibiotic prophylaxis regimens and drugs for cesarean section. Cochrane Database Syst Rev CD001136, 2000 |
|
Chelmow D, Ruehli MS, Huang E: Prophylactic use of antibiotics for nonlaboring patients undergoing cesarean delivery with intact membranes: A meta-analysis. Am J Obstet Gynecol 184: 656, 2001 |
|
Smaill F, Hofmeyr GJ: Antibiotic prophylaxis for cesarean section. Cochrane Database Syst Rev CD000933, 2000 |
|
Gonik B, Shannon RL, Shawar R et al: Why patients fail antibiotic prophylaxis at cesarean delivery: Histologic evidence for incipient infection. Obstet Gynecol 79: 179, 1992 |
|
Watts DH, Hillier SL, Eschenbach DA: Upper genital tract isolates at delivery as predictors of post-cesarean infections among women receiving antibiotic prophylaxis. Obstet Gynecol 77: 287, 1991 |
|
Newton ER, Wallace PA: Effects of prophylactic antibiotics on endometrial flora in women with postcesarean endometritis. Obstet Gynecol 92: 262, 1998 |
|
Iffy L, Kaminetzky HA, Maidman JE et al: Control of perinatal infection by traditional preventive measures. Obstet Gynecol 54: 403, 1979 |
|
Josey WE, Staggers SR: Heparin therapy in septic pelvic vein thrombophlebitis: A study of 46 cases. Am J Obstet Gynecol 120: 228, 1974 |
|
Gibbs RS, Jones PM, Wilder CJ: Antibiotic therapy of endometritis following cesarean section. Obstet Gynecol 52: 31, 1978 |
|
Brown CE, Stettler RW, Twickler D, Cunningham FG: Puerperal septic pelvic thrombophlebitis: incidence and response to heparin therapy. Am J Obstet Gynecol 181: 143, 1999 |
|
Stamm WE, Counts GW, Running KR et al: Diagnosis of coliform infection in acutely dysuric women. N Engl J Med 307: 463, 1982 |
|
Deveraux WP: Acute puerperal mastitis. Obstet Gynecol 108: 78, 1970 |
|
Aromaa A, Lahdensuu M, Cozanitis DA: Severe complications associated with epidural and spinal anaesthesias in Finland 1987-1993. A study based on patient insurance claims. Acta Anaesthesiol Scand 41: 445, 1997 |
|
Kindler CH, Seeberger MD, Staender SE: Epidural abscess complicating epidural anesthesia and analgesia. An analysis of the literature. Acta Anaesthesiol Scand 42: 614, 1998 |


 10–14 mg/dL
10–14 mg/dL