Radiation Therapy in the Management of Epithelial Ovarian Cancer
Authors
INTRODUCTION
Contemporary strategies for the primary management of epithelial ovarian carcinoma usually involve surgical exploration, staging, and cytoreduction, most often followed by platinum- and paclitaxel-based adjuvant chemotherapy.1,2 Significant contributions in patient selection criteria, chemotherapy development, and surgical procedures have been made that fuel the continuous process of “fine-tuning” the management of this disease in an effort to alter what has been a frustratingly flat cure rate for more than 30 years. Radiation therapy, known since 1912 to induce long-term remission in certain patients with ovarian cancer, has largely been excluded from routine use in all but a few notable hospitals and clinical situations.3 This censorship has come from mounting investigator bias originating from a number of published heterogeneously controlled trials. Previous reviews on the subject have elegantly outlined the contribution of these works to patient and treatment selection.4,5,6,7,8,9,10,11 However, more recently, controlled trials in selected patients using standardized radiation techniques have demonstrated significant long-term survival rates rivaling those achieved through conventional chemotherapeutic strategies. The results of these trials warrant careful consideration of this treatment modality. In this chapter, the radiotherapy techniques for the treatment of ovarian cancer are reviewed and the various selected situations in which it has been investigated to arrive at evidence-based recommendations are discussed.
TECHNIQUES OF RADIATION THERAPY
Early in the evaluation of effective treatment strategies for ovarian cancer, it became clear that treatment based on the concept of regionalized therapy was inadequate in inducing long-term remissions. Analysis of data generated from two randomized, clinical trials of adjuvant therapy among patients with early-stage ovarian cancer helps to illuminate these shortcomings.12,13 These trials, one from the Princess Margaret Hospital in Toronto and the other from the Gynecologic Oncology Group (GOG) compared pelvic radiation with observation or single-agent chemotherapy (melphalan). Although limited, the first by lack of surgical staging and the second by patient exclusions (nearly one half of randomized patients), they provided critical information about the “at-risk” tissues in patients with ovarian cancer. Pelvic failures were reduced in those receiving radiotherapy. However, upper abdominal recurrence was a major contributor to overall recurrence, and survival was statistically similar among the three arms evaluated. This suggested that ovarian cancer is responsive to radiation, but to be effective, the treatment field needs to include the entire abdominopelvic cavity.
“Moving Strip” versus Open-Field Radiotherapy
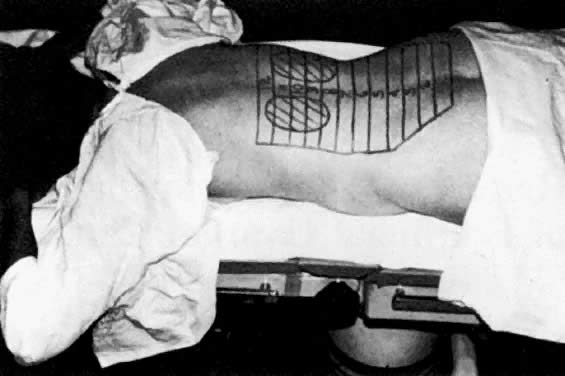
Two delivery techniques for abdominopelvic radiation therapy (APRT) have been developed and studied prospectively. The first developed was termed the “moving strip” technique. This technique, depicted in Figure 1, was developed in an era in which radiation therapy equipment could not adequately encompass the entire abdomen in a single portal. Therefore, the patient's abdomen was marked with 12 to 14 “strips,” each 2.5 cm in height, and treated for approximately 10 weeks.14 This hypofractionation technique delivered daily fractions of 225 to 300 cGy, accumulating in total abdomen doses of 2250 to 3000 cGy. Although the entire abdominopelvic cavity was treated, criticisms of this technique centered around the prolonged treatment time. Opponents have suggested that this delay could lead to reseeding of metastases and accelerated tumor repopulation. In addition, certain intra-abdominal contents (such as enteric viscera) were assured inhomogeneous treatment doses, given their movement within the treatment fields from day to day.
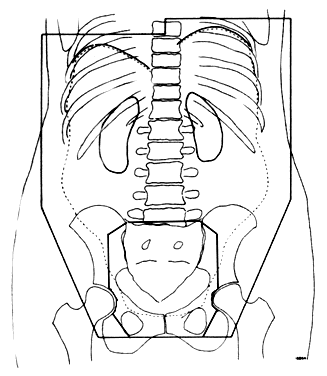
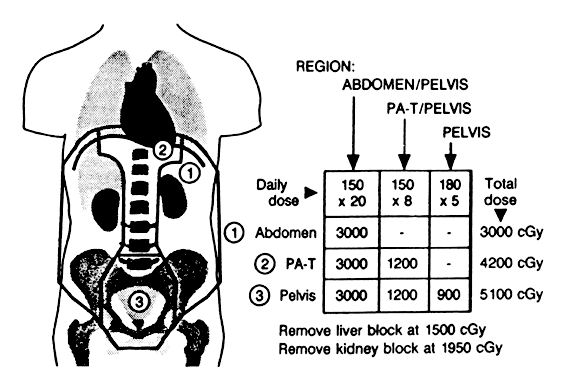
The subsequent development of more sophisticated radiotherapeutic machinery allowed clinicians to the explore “open-field” techniques as an alternative to the moving strip technique. Large treatment fields (up to 45 cm) were devised to encompass all “at-risk” tissues with standard fractionation and dosing schema. Thus, the entire treatment volume could be treated daily. In general, the treatment field extends superiorly to 1 to 3 cm above the diaphragm on full expiration and inferiorly to 2 cm below the obturator foramina. Laterally, the field goes beyond the peritoneal fat stripe. A typical treatment field is outlined in Figure 2. Dose prescribed to the upper abdomen varies by investigator but generally is about 25 Gy. The true pelvis often is boosted to 45 Gy. Several modifications to the open-field technique have been advocated, including methods of shielding vital organs (liver and kidneys), treating the hemiabdomen sequentially, T-shaped boost portals for the para-aortic nodal chains and medial diaphragms, and hyperfractionation schedules.15,16,17,18 An example of one such modification in technique is presented in Figure 3.
Stationary and radiosensitive organs such as the kidney, liver, spine, and portions of the gastrointestinal tract are encompassed with both radiation delivery techniques and present technical challenges because these organs often are proximate to the target volume. A review of these tissue tolerances and their associated injuries is listed in Table 1. Custom field blocking and delivery prescriptions must be made so that these tissue tolerances are not exceeded, yet no compromise is made in the target treatment volume. Observed toxicity from radiation therapy is discussed in a subsequent section.
TABLE 1. Tolerance Doses (TDs) in Whole Abdominal Radiation Therapy.
|
| TD 5% |
|
|
| Probability | Reference |
Organ | Injury | at 5 yrs | Volume |
Kidney | Renal failure | 15–23 Gy | Both kidneys |
Liver | Veno-occlusive disease | 30–35 Gy | Whole liver |
Spinal cord | Necrosis | 45 Gy | 40-cm length |
Gastrointestinal tract | Ulceration, obstruction, | 35–44 Gy | Whole abdomen |
| stricture |
|
|
The two techniques have been compared for efficacy and toxicity in two randomized, prospective clinical trials.19,20 In both trials, 5-year survival rates were equivalent, even through extensive subset analysis. In addition, early (acute) toxicity, theorized to be higher in the moving strip technique, was equal to that seen with the open-field technique. Reports of increased late effects were suggested for the moving strip technique in the Princess Margaret Hospital trial. Currently, most centers now consider open-field treatment to be the technique of choice as a matter of convenience, treatment time, technical ease, and a possible reduction in late (chronic) effects.
Although responses to measurable disease have been observed with whole abdominal radiation, the appropriate tumoricidal dose required to induce complete eradication is largely unknown and speculative; however, it is certainly related to implant size. In vitro models of solid tumors have suggested that macroscopic lesions (smaller than 2 cm) require 3500 to 5000 cGy to induce cell-kill 90% of the time, depending on tissue oxygenation.21 Clinically, this has been observed in a number of trials attempting to control advanced ovarian cancer with radiation therapy. Schray and associates observed that pelvic relapse occurred in only 2 of 26 (8%) patients with residual pelvic disease (<2 cm) at doses of 50 to 60 Gy, compared with 9 of 20 (45%) patients with larger ( 2 cm) tumors.22 In a recent trial of salvage therapy from the Stanford University School of Medicine, Cmelak and Kapp demonstrated 10-year disease-specific rates to be 40% for those with less than or equal to 1.5 cm residual disease versus 0% for those with residual disease greater than 1.5 cm (p = 0.0001).23 In comparison to macroscopic disease, radiation doses required to control microscopic disease are believed to be even lower. Trials comparing pelvic radiation alone to whole abdominal radiation for limited (stage I and II) disease have demonstrated better disease-specific survival figures for whole abdominal radiation therapy. In a series from Toronto, Dembo and associates argued that the improved 5-year survival was accounted for by a significant (30%) reduction in upper abdominal failures in the group receiving 2250 cGy to the whole abdomen.12,24 It has been from careful review of these outcomes that only patients whose abdominal disease is microscopic or small-volume macroscopic disease are recommended for treatment.
2 cm) tumors.22 In a recent trial of salvage therapy from the Stanford University School of Medicine, Cmelak and Kapp demonstrated 10-year disease-specific rates to be 40% for those with less than or equal to 1.5 cm residual disease versus 0% for those with residual disease greater than 1.5 cm (p = 0.0001).23 In comparison to macroscopic disease, radiation doses required to control microscopic disease are believed to be even lower. Trials comparing pelvic radiation alone to whole abdominal radiation for limited (stage I and II) disease have demonstrated better disease-specific survival figures for whole abdominal radiation therapy. In a series from Toronto, Dembo and associates argued that the improved 5-year survival was accounted for by a significant (30%) reduction in upper abdominal failures in the group receiving 2250 cGy to the whole abdomen.12,24 It has been from careful review of these outcomes that only patients whose abdominal disease is microscopic or small-volume macroscopic disease are recommended for treatment.
RADIATION IN PRIMARY OVARIAN CANCER MANAGEMENT
Radiation therapy has been used in a variety of ways in the primary treatment of ovarian carcinoma. Localized pelvic radiation, abdominopelvic radiation, and intraperitoneal radiocolloids all have been administered as sole modalities to patients with ovarian cancer. Varying success has been observed, but, as discussed previously, well-characterized parameters influence this success greatly. In reviewing the utility of primary irradiation as a sole treatment modality in ovarian cancer, it is useful to discuss its role, separately, in nonmetastatic (early stage) disease, where at-risk recurrence sites are treated directly, and in metastatic (advanced stage) disease, where cytoreduction is performed and known macroscopic residual disease is treated. It is from the observed activity in this latter situation that evidence-based recommendations can be made concerning treatment in the former.
Evidence of Effect
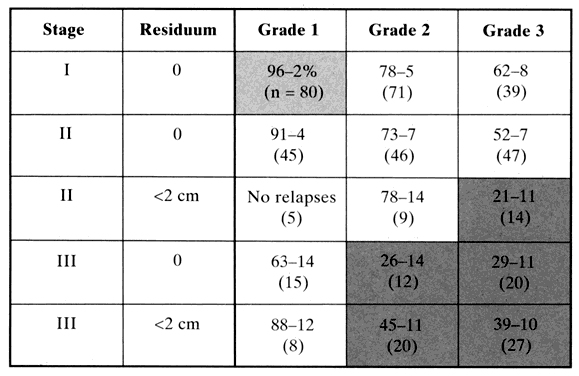
The first question to ask of this treatment modality is, “Is there conclusive evidence that radiation therapy induces tumor cell kill in patients with ovarian cancer?” To address this question, clinical trials should be reviewed in which patients with measurable or known residual disease after primary surgery were treated with radiation therapy alone in the adjuvant setting. As mentioned previously, analysis of treatment failures with localized external radiation require that the modality encompasses the entire peritoneal cavity. In addition, Dembo and Thomas have argued that data reflecting on the “cure potential” should include trials for which 10 or more years of follow-up are reported because less than 2% of first recurrences appear after 8 years of disease-free survival.6,8,25 Although data meeting these criteria are scarce, Table 2 presents five trials of patients with known macroscopic disease treated with primary abdominopelvic radiation for which at least 6-year disease-free survival data are reported. All patients were known to have residual disease and were stratified by volume. These data suggest that those patients with large residual disease (>2 cm) generally are incurable with this modality alone. However, in those patients in whom known small volume residual disease remains after initial debulking surgery, 42% to 62% of were disease-free 10 years later. These observations are comparable to those observed with standard chemotherapy in this subset of patients. On closer scrutiny of the data, one notes that the majority of these long-term survivors are the patients with stage II disease with residuum located solely in the pelvis. In these cases, higher boosting doses can be delivered to the pelvic disease to increase local control rates, and presumed microscopic disease is subjected to tolerable doses of abdominopelvic radiation. In addition, patients with stage III disease who had a residuum of less than 2 cm were significantly less likely to achieve a lasting response if the tumor grade was moderately or poorly differentiated. Further, because patients in these early trials were recruited before uniform surgical staging and aggressive cytoreductive efforts, it is unknown whether modern-day patients undergoing extensive debulking surgery to small volume disease would have an analogous observation. Nevertheless, investigators at the Princess Margaret Hospital have developed a useful risk model on the basis of stage, grade, and amount of residuum to define categories of patients destined to benefit most from abdominopelvic radiation.26Figure 4 presents these risk groups and 5-year disease-free survival rates. The validity of this model has been proved by at least three additional independent trials.27,28,29 Patients in the “intermediate risk” cohort make up approximately one third of primary ovarian cancer cases at presentation. It is clear from these data that long-term disease-free survival can be induced with primary adjuvant abdominopelvic radiation in selected patients.
TABLE 2. Long-Term Outcomes From Trials of Patients with Known Macroscopic Residuum Stratified by Size
|
| Residuum | |
Study | End-Point | <2 cm | >2 cm |
Dembo24 | % 10 yr relapse-free | 38% | 6% |
Martinez15 | % 15 yr freedom from relapse | 50% | 14% |
Fuller92 | % 10 yr relapse-free | 62% | 0% |
Weiser93 | % 10-yr survival | 42% | 10% |
Goldberg29 | 6-yr survival fraction | 0.41 | — |
(Adapted from: Thomas G. Dembo A: Integrating radiation therapy into the management of ovarian cancer. Cancer 71:1710–8, 1993)
Chemotherapy versus Radiation
The second question to address is, “How do the results of APRT compare with those achieved with conventional chemotherapy?” Only prospective, randomized controlled trials can answer this question adequately. Currently, the only completed phase III trials in the literature comparing abdominopelvic radiation with chemotherapy have used single-agent non-platinum-containing regimens. In addition, attempts to complete phase III trials of abdominopelvic irradiation and platinum-containing standard regimens have been unsuccessful because of poor patient accrual; however, preliminary data have been published. Therefore, although a direct answer to this question cannot be made, it is useful to review available data to develop a sense of the capacity of each modality to incur lasting disease-specific survival.
Table 3 presents data from six randomized trials of abdominopelvic radiation and chemotherapy.12,13,28,30,31,32 In all but the trial from the Princess Margaret Hospital (PMH), 5-year survivorship was equivalent between the treatment arms. In the PMH trial of stage I, II, and III (no residual) ovarian cancer, patients were randomized either to whole abdominal radiotherapy or to single-agent chlorambucil (plus pelvic radiation). Review of the subgroup multivariate analysis reveals that the benefit of abdominopelvic radiation was significant (43% reduction in relapse, p = 0.009) only among patients with stages I to III whose tumors were adherent to surrounding structures.33 Two randomized trials comparing cisplatin to abdominopelvic radiation therapy were closed before final accrual was reached.9,30,31 In the first, 70 patients with stage I grade 3 to stage II ovarian cancer were randomized to 6 cycles of standard cisplatin/cyclophosphamide or abdominopelvic radiation (30 Gy). Eight patients (11%) randomized to the radiation arm received chemotherapy in protocol violation. Overall, 5-year survival rates were not significantly different between the treatment arms (chemotherapy: 71%, APRT: 53%, p = 0.16). In the second trial, patients with stage I to III ovarian cancer were randomized either to five courses of single-agent cisplatin or to APRT performed by the moving strip technique. Only 40 patients were recruited (15 stage III); no significant difference in survival was demonstrated. Currently, there are no trials in progress evaluating APRT versus taxine-based chemotherapy, but a prospective evaluation of APRT versus cisplatin chemotherapy is underway for patients with International Federation of Gynecology and Obstetrics (FIGO) stage IA/B grade 2 or 3; stage IC, and stage IIA/B through the European Organization for Research and Treatment of Cancer.4 Significant endpoints of this trial are survival and quality of life. However, because the survival rate in this subgroup of patients is 80% or better at 5 years, conclusions regarding these endpoints are years off.
TABLE 3. Randomized Trials of Whole Abdominal Radiation in Ovarian Cancer
|
|
|
| Outcome |
Study | N | Stages | Treatments | (5-yr Survival Rate) |
PMH12 | 147 | I-III | APRT | 58%* |
|
|
| Chlorambucil + pelvic RT | 41% |
NCIC32 | 257 | I-III | APRT | 61% |
|
|
| Melphalan + pelvic RT | 62% |
DACOVA28 | 118 | IB-II | APRT | 55%§ |
|
|
| Cyclophosphamide + pelvic RT | 63% |
MDACC13 | 149 | I-III | APRT | 71% |
|
|
| Melphalan | 72% |
NCI (Italy)30 | 70 | I Gr 3-II | APRT | 53% |
|
|
| Cisplatin + cyclophosphamide | 71% |
West Midlands31 | 40 | I-III | APRT | 58% |
|
|
| Cisplatin | 62% |
*p = 0.05.
§4-year survival rate.
Combination Chemoradiation
At least one group has tried to combine chemotherapy and abdominopelvic radiation. In a novel treatment schema, Licter and associates at the National Cancer Institute devised a treatment plan to administer cyclophosphamide and hexamethylmelamine for 14 days in alternate 28-day cycles with cisplatin (20 mg/m2/day  5 days), intraperitoneal misonidazole (4 g in 2 L of fluid, days 1 and 4), and whole abdominal radiation (split field [150 cGy upper/200 cGy lower] daily for 5 days) to patients with advanced ovarian cancer.34 In this trial, 43% of the patients had residual disease greater than 5 cm in the abdomen. The entire cycle was repeated for 4 cycles. Complete clinical responses were observed in 50%, with pathologic complete response in 18%. Median survival was 15.2 months for the entire group but was significantly better for those patients with less than 2 cm residual disease after initial surgery (33 months vs. 13 months, p < 0.05). Toxicity was significant. One quarter of the cycles were associated with grade IV thrombocytopenia, and treatment delays from hematologic suppression were a median 7 days beginning with cycle 3. In all, the 4 cycles of prescribed chemotherapy required a median of 6.1 months (range, 5.5–7.5 months) to administer. Retrospective comparison to an earlier nonplatinum combination chemotherapy protocol conducted by this group with similar patients demonstrated no significant improvement in durable response with this regimen. Currently, trials of combination chemotherapy and radiation include predominately radiation administered as a consolidation program to those patients with either no visible disease or those secondarily cytoreduced to small-volume disease.
5 days), intraperitoneal misonidazole (4 g in 2 L of fluid, days 1 and 4), and whole abdominal radiation (split field [150 cGy upper/200 cGy lower] daily for 5 days) to patients with advanced ovarian cancer.34 In this trial, 43% of the patients had residual disease greater than 5 cm in the abdomen. The entire cycle was repeated for 4 cycles. Complete clinical responses were observed in 50%, with pathologic complete response in 18%. Median survival was 15.2 months for the entire group but was significantly better for those patients with less than 2 cm residual disease after initial surgery (33 months vs. 13 months, p < 0.05). Toxicity was significant. One quarter of the cycles were associated with grade IV thrombocytopenia, and treatment delays from hematologic suppression were a median 7 days beginning with cycle 3. In all, the 4 cycles of prescribed chemotherapy required a median of 6.1 months (range, 5.5–7.5 months) to administer. Retrospective comparison to an earlier nonplatinum combination chemotherapy protocol conducted by this group with similar patients demonstrated no significant improvement in durable response with this regimen. Currently, trials of combination chemotherapy and radiation include predominately radiation administered as a consolidation program to those patients with either no visible disease or those secondarily cytoreduced to small-volume disease.
Whole Abdominal Radiotherapy as Consolidation Therapy
The rationale for the use of abdominopelvic radiation in patients with no detectable residual disease after primary chemotherapy is based on the consistent observation that 40% to 60% of patients achieving a pathologic complete response after chemotherapy ultimately recur and succumb to their disease.35,36,37,38 Although this observation has been attributed to a host of factors, unrecognized microscopic residual disease has been implicated most often. A number of consolidation regimens aimed at the treatment of microscopic residual disease have been proposed and include additional intravenous chemotherapy, intraperitoneal chemotherapy, intraperitoneal radiocolloids, intraperitoneal immunoconjugates, hormones, and high-dose chemotherapy (bone marrow transplant/peripheral stem cell transplant).39,40,41,42,43,44,45 Fuks and colleagues, recognizing the cytotoxic effect of radiation on small-volume (microscopic) disease, suggested in 1982 that abdominopelvic radiation would be a reasonable choice to affect this recurrence rate, if given after primary induction chemotherapy.46 Since then, more than 30 reports have appeared in the literature examining the role of APRT after induction chemotherapy. These have been chronicled by Thomas and Mychalczak and associates.5,47 Unfortunately, in the platinum era, there are very few prospective randomized, controlled trials of APRT addressing recurrence and survival in the consolidation setting. Most trials have attempted to induce secondary remission in those patients with either grossly progressive disease or those in whom debulking efforts have left minimal residual disease. The result of these trials are discussed in a subsequent section.
The likelihood of benefit from consolidation therapy, as discussed with primary therapy, appears to be related to the amount of tumor residuum at the time of treatment. Thomas, collecting data from 28 diverse, mostly single-armed studies, reported that among 473 patients in whom tumor residuum could be determined, overall survival correlated with size of this residual disease.6 Although survival was defined differently among the trials, approximately 76% of patients with no residuum at second look were disease free at 6 to 10 years compared with 49% of patients with microscopic disease or residual disease smaller than 5 mm after second look. Patients with macroscopic disease did not benefit from the treatment. Comparative trials with historical controls also have been reported. In a trial from Toronto, 44 stage II and III optimally cytoreduced patients completing 6 cycles of platinum-based chemotherapy were treated with open-field, consolidation APRT.48 This cohort was compared with 48 patients treated with APRT as primary postsurgical treatment and were matched for age, stage, and residual disease. At 5 years' follow-up, disease-free survival was nearly double (22% vs. 43%, p = 0.03) for patients receiving consolidation therapy. Median survival also was increased significantly (2.4 vs. 5.7 years) for this cohort. There are five prospective randomized, clinical trials in which at least preliminary data are available from patients undergoing primary platinum-based postsurgical chemotherapy and who were offered either APRT or either additional chemotherapy or observation.49,50,51,52,53 In a preliminary report from the Swedish Norwegian Ovarian Cancer Study, 172 patients received initial chemotherapy (cisplatin and doxorubicin  4 cycles), second-look laparotomy. On the basis of tumor residuum (none or microscopic) 98 patients were randomized to one of three treatment arms: continued chemotherapy, APRT, or observation. Those with macroscopic residual (74 patients) were randomized either to APRT or to further chemotherapy. In this latter stratification, no difference in survival was demonstrated. In the former, however, a significant improvement in disease-free survival was noted for abdominal pelvic radiation therapy, although follow-up is premature. In a recently updated study from Ben-Baruch and colleagues, 37 patients were randomized either to APRT (n = 19) after second-look laparotomy or to 3 courses of intraperitoneal cisplatin with thiosulfate (n = 18).54 In this trial, no significant difference was observed between the treatment arms initially; however, in this updated report with now 5-years of minimum follow-up, a nonsignificant trend to poorer survival was seen in the APRT arm (39% vs. 68%, p = 0.07). In addition, these authors report that survival after secondary recurrence was reduced significantly in those patients receiving APRT as their initial salvage regimen (0% vs. 41% at 5 years, p = 0.03).54 In contrast, Pickel and associates recently presented data from 64 patients randomized to either APRT or observation after a negative, postchemotherapy clinical assessment.53 In this trial, patients with FIGO stage IC to IV were enrolled after complete surgical staging or maximal cytoreduction. All patients underwent 6 cycles of platinum-based chemotherapy (carboplatin, epirubicin, prednimustine). If clinically determined (radiologically and CA 125) to have no residual disease, they were randomized to either APRT (30 Gy whole abdomen, 50.6 Gy pelvis, 42 Gy paraortic) or observation. Of the 64 patients randomized, 58 had stage III or IV disease at presentation. Progression-free survival at 10 years was significantly higher for those patients undergoing APRT (50% vs. 30%, p = 0.012). In addition, overall survival at 10 years was significantly higher (62% vs. 38%, p = 0.029) in the APRT arm. Subset analysis of the stage III/IV patients was comparable. In this study, however, only 14 of 32 (44%) patients received APRT without delay.
4 cycles), second-look laparotomy. On the basis of tumor residuum (none or microscopic) 98 patients were randomized to one of three treatment arms: continued chemotherapy, APRT, or observation. Those with macroscopic residual (74 patients) were randomized either to APRT or to further chemotherapy. In this latter stratification, no difference in survival was demonstrated. In the former, however, a significant improvement in disease-free survival was noted for abdominal pelvic radiation therapy, although follow-up is premature. In a recently updated study from Ben-Baruch and colleagues, 37 patients were randomized either to APRT (n = 19) after second-look laparotomy or to 3 courses of intraperitoneal cisplatin with thiosulfate (n = 18).54 In this trial, no significant difference was observed between the treatment arms initially; however, in this updated report with now 5-years of minimum follow-up, a nonsignificant trend to poorer survival was seen in the APRT arm (39% vs. 68%, p = 0.07). In addition, these authors report that survival after secondary recurrence was reduced significantly in those patients receiving APRT as their initial salvage regimen (0% vs. 41% at 5 years, p = 0.03).54 In contrast, Pickel and associates recently presented data from 64 patients randomized to either APRT or observation after a negative, postchemotherapy clinical assessment.53 In this trial, patients with FIGO stage IC to IV were enrolled after complete surgical staging or maximal cytoreduction. All patients underwent 6 cycles of platinum-based chemotherapy (carboplatin, epirubicin, prednimustine). If clinically determined (radiologically and CA 125) to have no residual disease, they were randomized to either APRT (30 Gy whole abdomen, 50.6 Gy pelvis, 42 Gy paraortic) or observation. Of the 64 patients randomized, 58 had stage III or IV disease at presentation. Progression-free survival at 10 years was significantly higher for those patients undergoing APRT (50% vs. 30%, p = 0.012). In addition, overall survival at 10 years was significantly higher (62% vs. 38%, p = 0.029) in the APRT arm. Subset analysis of the stage III/IV patients was comparable. In this study, however, only 14 of 32 (44%) patients received APRT without delay.
Toxicity of treatment in this setting is significant and, as discussed subsequently, is related to several factors inherent to patients' identification. Although the presented data are far from conclusive on the topic of consolidation APRT after chemotherapy, patients most likely to benefit are those with microscopic or no residual disease at the time of reassessment. Whether this treatment offers improved survival and quality of life is the focus of ongoing clinical trials, and until this information is known, APRT in this setting should be considered experimental.
Salvage Abdominopelvic Radiation Therapy in Epithelial Ovarian Cancer
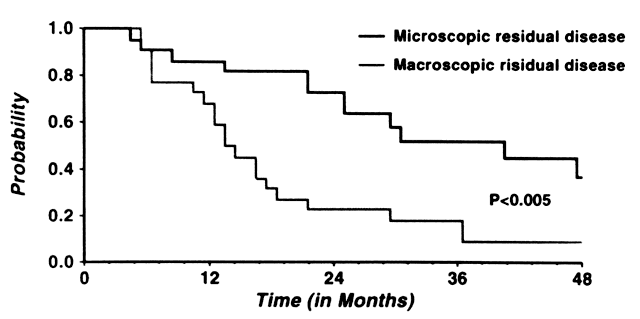
For patients with macroscopic disease found at second-look laparotomy, at reassessment for progressive disease while on therapy, or after a remission, little benefit has been seen for the use of whole abdominal radiation. More than two dozen studies using a variety of techniques have addressed this issue, and disappointing results have been reported. Table 4 presents data from nine trials reporting outcomes of patients with macroscopic residual disease at either second-look laparotomy or reassessment laparotomy.55,56,57,58,59,60,61,62,63 It is easily appreciated from this heterogeneous group of patients that durable progression-free survival is rare. In a recent trial from Rush-Presbyterian-St. Luke's Medical Center, Reddy and colleagues studied the outcome of abdominopelvic radiation in 44 patients failing one (80%) or two (20%) chemotherapy regimens for advanced or recurrent ovarian cancer.64 One half of these patients had macroscopic disease (>1 cm) after a debulking effort. Survival at 48 months was 9% in this group compared with 37% for those with microscopic residual disease (p < 0.005, Fig. 5). Only 5% of those patients with macroscopic residual disease were disease free at 48 months. Disappointingly, progression-free survival at 4 years was no different than overall survival (22% vs. 23%), suggesting that patients rapidly succumbed to their disease shortly after progression. Significant benefit was seen for those patients with pelvic-only residual disease. In patients with pelvic-only disease, 56% were disease free at 48 months compared with 0% for those with upper abdominal disease (p < 0.005). Recurrence after APRT occurred within 24 months in 90% of patients. Review of the patterns of failure demonstrated that upper abdominal control was critical to induction of long-term disease-free survival. In the 31 patients with recurrence after APRT, 28 (90%) had abdominal disease as a component of their failure. Major late toxicity was encountered in nine (21%) patients. In all, 11 complications were seen, including 8 related to bowel injury; five patients required surgical intervention. These authors64 and others have concluded that whole abdominal radiation is unlikely to benefit those patients with macroscopic disease, and only those patients with localized microscopic pelvic disease stand to gain from this treatment. Schray and colleagues performed a multivariate analysis looking at parameters to identify individuals most likely to benefit from APRT therapy in a salvage setting.22 They found prechemotherapy residuum, preradiotherapy residuum, and grade to be predictive of response to APRT. When all three characteristics were favorable, the likelihood of 5-year disease-free survival was 69%; with two favorable characteristics, the likelihood was 53%; with one, the likelihood was 29%; and if no favorable characteristics were present, the likelihood of disease-free survival was 0%. These data, along with that presented previously, supports the contention that if APRT is used, only in the most favorable of subgroups is a benefit seen. However, there are little data to support that these observed responses are any different than those that may be seen with conventional therapy. Among patients with macroscopic disease, there is no proven role for APRT.
TABLE 4. Advanced or Recurrent Ovarian Cancer Patients with Macroscopic Disease Treated with Abdominopelvic Radiation Therapy
|
| Patients |
Author | n | Surviving Disease-Free |
Haisworth et al55 | 17 | 0/1 |
Hacker et al57 | 40 | 0/18 |
Peters et al58 | 22 | 0/6 |
Solomon et al59 | 18 | 0/6 |
Green et al60 | 23 | 0/3 |
Kersh et al61 | 21 | 1/4 |
Kong et al62 | 23 | 1/9 |
Fuks et al63 | 19 | 0/6 |
Reddy et al64 | 44 | 2/20 |
Total | 227 | 4/73 (5.5%) |
Mechanisms of Radioresistance
Although the data reported here are disappointing given their initial optimism, recent understanding of the mechanisms of cross-resistance between chemotherapy and radiation may help to explain some of these observations. It has been appreciated clinically that, under certain conditions, patients receiving platinum or platinum analogues and other alkylating agents, such as cyclophosphamide, readily develop pleotrophic drug resistance.65,66,67,68,69 The molecular and biologic mechanisms for this observed and inducible resistance are many and are incompletely understood. One well-described resistance model in ovarian cancer involves the ability of the cell to neutralize and metabolize intracellular electrophilic conjugates that may have otherwise induced apoptosis through DNA strand breaks and cisplatin-adduct formation. The enzyme most closely linked to this observation is the tripeptide glutathione (GSH). It has been demonstrated that intracellular binding of glutathione S-transferase with cisplatin leads to less toxic intermediates and reduced pharmacologic function.66 This has been corroborated by demonstrating resistance in the presence of high levels of GSH and return of cisplatin sensitivity in the presence of buthionine sulfoximine (BSO), a synthetic amino acid that inhibits gamma-glutamylcysteine synthetase, causing GSH depletion.66 The ability of cells to repair DNA damage induced by cisplatin and other alkylators is inversely proportional to GSH activity. This mechanism also appears to be crucial to cellular survival after strand breaks induced by ionizing radiation. De Pooter and colleagues evaluated the cross-resistance characteristics in a study of induced resistant ovarian cancer cell lines.67 The study was unique because cellular resistance was induced and studied in a cross-over design. These investigators demonstrated that cisplatin-resistant cell lines were 1.7 to 2.0 times more resistant to ionizing radiation (1.5 Gy) than controls, and this variance could not be accounted for by differences in growth kinetics or DNA content. Additionally, Oshita and colleagues studying six drug-resistant (cisplatin-resistant) lung cancer cell lines demonstrated one, PC-9/CDDP, that also was cross-resistant to irradiation in a growth inhibition assay.68 Measurement of intracellular glutathione content in all cell lines demonstrated only the PC-9/CDDP line to have significantly increased levels compared with parental cell lines. Radiosensitivity was restored with BSO administration.
These data notwithstanding, it has been suggested that the role of GSH in clinical radioresistance may be relatively insignificant. A more likely explanation for cross-resistance is increased cellular efficiency in mechanisms of DNA repair. Behrens and colleagues demonstrated that enhanced repair of intrastrand cross-linked adducts was the principle mechanism for cisplatin resistance in one cell line.69 Hamilton and associates subsequently found that administration of aphidicolin (an inhibitor of repair DNA polymerases α and δ) could overcome resistance in this same human ovarian cell line.70 Lines of evidence such as these have led to clinical application with adjuvant pharmacologic agents in an attempt to augment resistance reversal. The mechanisms of cross-radioresistance probably are also linked to local and systemic tumor factors.
RADIOACTIVE CHROMIC PHOSPHATE (32P) AND OVARIAN CANCER
Intraperitoneal radiocolloids have been used in the treatment of ovarian cancer since the early 1950s.71 These agents were popular and advocated by investigators because of their pharmacologic advantages of intraperitoneal administration, safe but significant local radioactivity, and ability to treat all peritoneal surfaces. Several agents were studied (e.g., 198Au), but radioactive phosphorus (32P) became the most commonly used because of its profile as a pure beta emitter, improved tumor penetration, and less hazard to treating personnel. 32P is administered most often intraperitoneally at a dose of 10 to 20 mCi mixed in 1 to 2 L of saline. Most treatment algorithms require body positional changes every 15 to 30 minutes for up to 6 hours. Before instillation of the active 32P, a technetium99 scan usually is performed in conjunction with catheter placement to determine whether distribution of the colloid in the abdominal cavity is satisfactory. A review of this agent's activity as a primary adjuvant and in the consolidation setting is discussed subsequently.
Radioactive Chromic Phosphate in Primary Therapy
Radioactive chromic phosphate has been evaluated in randomized, prospective trials predominately in early-stage, high-risk ovarian cancer or in the adjuvant setting after negative second-look laparotomy or reassessment. Several investigators have studied this therapy in combination with other modalities, such as chemotherapy (nonplatinum) or pelvic radiation, but because of significant complications, they have concluded that this agent is best considered singly.72 In a review of 95 patients using 32P for ovarian cancer, Tharp and Hornback demonstrated that the 5-year chronic complication rates (predominately gastrointestinal) were 44% when adjunctive pelvic or whole abdominal radiation also was given compared with 17% (5% if minor complaints excluded) if used alone (p = 0.04).73 Klaassen and colleagues from the National Cancer Institute of Canada (NCIC), similarly abandoned further development of a protocol for high-risk early-stage ovarian cancer in which 32P was administered with pelvic radiotherapy.74 In this trial of 53 patients receiving between 10 and 20 mCi of 32P in addition to 40 Gy of external pelvic irradiation, only 35 patients (66%) received 32P, and 10 of those (29%) suffered significant bowel toxicity, including bowel obstruction, enterovesical fistula, and chronic diarrhea. Nonetheless, used independently, this agent is well tolerated and requires only a single administration.
The GOG evaluated this agent in a phase III protocol of patients with stage I (grade III) or stage II (grade I and II with capsular rupture or ascites) ovarian cancer.75 Patients were randomized either to 32P or oral melphalan after surgical staging. 32P was administered intraperitoneally as a single dose of 15 mCi. In this study, 6% of the patients receiving 32P required bowel surgery for related complications. Survival analysis failed to demonstrate any survival difference between these two arms (5-year survival rate, 81% vs. 78%); however, given the relative low risk of administration and favorable side-effect profile, 32P was adopted as “standard therapy” for this group of patients. A follow-up study from the GOG compared 32P with combination cisplatin and cyclophosphamide.76 In this trial, 204 stage IC, II, and “high-risk” stage IA and IB patients were randomized either to 15 mCi of intraperitoneal 32P (n = 98) or to 3 cycles of cisplatin (100 mg/m2) and cyclophosphamide (1000 mg/m2, n = 106). The 60 month progression-free survival rate was 66% in the 32P arm and 78% in the chemotherapy arm (p = 0.08). The magnitude for reducing the risk of disease recurrence by chemotherapy administration was 31%, which was not significant (90% CI: 0.45–1.07). In a similar study from the Italian Cooperative Group on Ovarian Cancer (GICOG), 104 patients with stage IC or stage IA or IB with capsular rupture or surface extension were randomized to either 32P or cisplatin (50 mg/m2 for 6 cycles).77 Recurrence was reduced by 61% if cisplatin was administered (HR = 0.39; 95% CI = 0.19–0.77, p = 0.007; Cox Model). However, the impact of this treatment on overall survival was questioned. The 5-year survival rates were 81% for those given cisplatin and 79% for those given 32P (HR = 0.72; 95% CI = 0.37–1.43; p = 0.354; Cox Model). In this trial, patients recurring after cisplatin were significantly more likely to die of their disease than if given 32P. None of these trials included a cohort in which observation alone was used, so the benefit of adjuvant treatment in this group of “high-risk” early stage patients was not addressed directly. However, the risk of recurrence is suspected to be significant enough to justify treatment. Ahmed and colleagues presented data from 194 patients with stage I (all grades) ovarian cancer who were observed after surgical therapy until recurrence.78 From these data, patients in the aforementioned studies would be expected to exhibit a 38% to 60% chance of recurrence at 5 years of follow-up without adjuvant treatment.
Recently, data from a randomized trial of chemotherapy (single-agent carboplatin, area under the curve = 7 mg/mL/min) versus observation were presented.79 Although this trial included patients not entirely comparable to those aforementioned (no stage II patients, included stage IA and IB grade 2 and aneuploid grade 1 patients), all were labeled “high risk” on the basis of increased recurrence risk. Of the 142 randomized patients, no reduction in disease recurrence is being observed for treated patients (n = 67). These preliminary data underscore the importance of defining appropriate treatment groups and addressing treatment-related bias for clinical trials. Currently, chemotherapy has replaced 32P as primary therapy for early-stage, high-risk ovarian cancer, although its benefit to long-term survival is unknown.
Radioactive Chromic Phosphate in Consolidation Therapy
Radioactive chromic phosphate also has been evaluated, to a lesser degree, in the consolidation setting. The rationale for use of consolidation therapy has been presented earlier. Several retrospective studies have championed its use in this setting. Spencer and associates reported a nonsignificant reduction in recurrence after negative second-look laparotomy among 14 patients treated with 32P compared with 17 patients observed expectantly.80 In this retrospective trial, no recurrences were seen in the 32P group compared with 4 (24%) in the observation group (p = 0.07). The sequential nature of patient acquisition may have biased the two groups. Rogers and colleagues presented data on 69 high-risk patients with early- and advanced-stage ovarian carcinoma who were clinically without persistent disease after chemotherapy (50% platinum-based) and who had a pathologic negative second-look evaluation.81 In this study, 15 mCi was administered within 3 hours postoperatively. Disease-free survival rate at 5 years (excluding 5 patients with stage I disease) was 81% for patients receiving 32P versus 65% for those under observation alone (p = 0.05). Although not randomized, multivariate analysis for predictors of survival identified only grade and 32P administration as significant to successful disease-free status. In addition, no significant difference in bowel complications were observed between the treatment groups (6% vs. 5.5%). The authors81 attribute immediate administration via twin catheters placed at surgery as a reason for no observed increased toxicity. In a small prospective randomized trial, Vergote and colleagues administered 32P to 25 patients with stages IB to III ovarian cancer who underwent negative second-look laparotomy.83 All patients were treated upfront with cisplatin-based combinations. In comparison to 25 patients randomized to expectant management, no significant improvement was observed for patients treated with 32P (5-year disease-free survival rate: 95% vs. 82.5%, p = 0.61). Based on these conflicting results, the Gynecologic Oncology Group launched and completed a randomized trial of 32P versus observation in stage III ovarian cancer patients following negative second-look evaluation. This trial accrued 202 eligible patients and closed in late 1996. Although it is too early to determine the effects of treatment from this trial, the regimen was well tolerated.
From available studies of patients with microscopic and small-volume macroscopic residual disease treated with 32P after second-look laparotomy, it is clear that volume of residual disease is an important predictor of response. For reasons discussed subsequently, patients with macroscopic residual disease are expected to be poor candidates for this type of therapy. However, for those with less than 3-mm disease or those with macroscopic residual disease debulked to no visible residual, 32P is a reasonable option. Data from single-arm studies suggest that disease-free survival for these two groups is similar. Soper and colleagues reported that 6 of 10 patients with microscopic residual disease were disease free 5 years after 32P administration compared with 4 of 8 patients with larger residuals debulked to no residual.83 Similarly, Potter and associates84 reported that 6 of 13 patients with microscopic residual disease were disease free after 32P administration compared with 7 of 15 with completely resected tumors. Although these authors83,84 have argued for its activity, 32P remains the subject of randomized prospective trials to determine whether it offers improved survival over conventional strategies or APRT.
Explanations for the reduced benefits of this modality may not be only the technique of administration but also the characteristics of the compound itself. Radiobiologically, this compound emits beta particles with a maximum energy of 1.7 MeV. From a dosimetry study of 32P in the peritoneal cavity, a dose of 19 mCi would be expected to deliver a dose of 40 Gy to the peritoneal surfaces.85 This study demonstrated two features of 32P that may explain theoretical limitations of its use in ovarian cancer. First, depth of penetration is limited to a few millimeters. Although, in general, microscopic surface disease should be amenable to this dose and depth of penetration, a significant number of patients (10%–27%) have retroperitoneal disease that would not receive a significant dose from intraperitoneal 32P.86,87 Second, radiation dose distribution is heterogeneous throughout the peritoneal cavity, even with body position changes. This study demonstrated up to 10-fold differences in dose distribution throughout the peritoneal cavity. In addition, significant host factors, such as adhesion formation after surgery, would be expected to reduce uniform distribution further. An intraperitoneal technetium study to predict adequate distribution of 32P before instillation is recommended to reduce risk from these factors. Careful consideration of these characteristics is required to make an informed recommendation for or against this modality of therapy.
PALLIATIVE RADIOTHERAPY
As is observed in other metastatic solid tumors, radiation therapy can play an effective palliative role in advanced ovarian cancer. These situations arise from recurrent or progressive chemoresistant lesions causing dominant symptoms in the abdomen or pelvis or from extra-abdominal progression sites such as the brain, bone, the spinal cord, and lymphatics. In addition, skin metastases have been observed and provide relatively easy targets for treatment. The success of palliation is best measured by the successful relief of symptoms from dominant sites. Although little data are available specific to ovarian cancer, published reports of varying techniques and goals provide some insight into the ability to effect meaningful palliation with limited field radiotherapy.
In a report from the M. D. Anderson Cancer Center, Adelson and associates presented data from 42 patients treated with one to three (4-week) courses of large-fraction (10 Gy) “one-shots” to the pelvis.88 The most common indications for palliative consideration were bleeding (24%) and pain (20%). Most patients (62%) received just one course, although eight (19%) received three fractions. Success of palliation, measured loosely as a “reduction or resolution in symptoms” occurred in 71% of the patients reporting bleeding and in 55% of the patients reporting pain. Transient nausea and vomiting occurred in 26% of patients. Intestinal complications were frequent, however. Overall, 22 of 42 (52%) patients had severe intestinal complications, 13 of which (31%) required laparotomy. In six patients, radiation injury was noted. Median survival after treatment was 5.1 months. More commonly, however, a course of palliative radiotherapy is fractionated. Nausea, vomiting, and intestinal complications are expected to be less severe and less frequent with a fractionated course of radiotherapy.
In another report, May and colleagues administered 55 courses of palliative radiation therapy to 38 patients.89 The most frequent indications for treatment were pain (18 courses) and intestinal obstruction (11 courses). Techniques of administration were fairly standard. For whole pelvis radiotherapy, doses of 180 to 200 cGy/day were delivered; for smaller sites, fraction sizes increased to 250 to 300 cGy/day. For patients with intestinal obstruction, 7 of 11 received “clear-relief” of symptoms for a mean of 1.9 months. Of patients receiving palliative radiotherapy for pain, the majority (67%) obtained a measurable relief of symptoms, lasting a mean 2.1 months. These authors89 recommend a careful evaluation of life expectancy and a review of the likelihood to induce a short-term response before beginning treatment.
Similarly, Corn and colleagues treated 33 patients with symptomatic progressive ovarian cancer who had prior exposure to platinum-based chemotherapy.90 Complete palliation of all symptoms was observed in 17 (51%) patients. Bleeding and pain were controlled in 90% and 83%, respectively. The median duration of palliation was 4 months but entailed the entire rest of life for 90% of the cohort. Although multivariate analysis of parameters affecting successful palliation could not be performed, a relationship to dose and initial Karnofsky score was observed. Despite these limitations, apparently in certain situations, radiotherapy serves a functional role in effective palliation.
TOXICITY OF ABDOMINOPELVIC RADIATION
Traditionally, the toxic side effects of radiotherapy have been categorized as early and late—a reflection on the time to appearance and histopathologic characteristics found in affected tissues. In general, early effects are common but not severe whereas late effects are uncommon but generally severe and sometimes life-threatening. Many factors, including tissue site, fraction size, total dose, time to completion, prior surgery, prior or concomitant use of chemotherapy, and host susceptibility, are known to alter the frequency of observed toxicity. Early treatment effects of primary whole abdominal irradiation usually appear within 2 to 3 weeks of treatment initiation and persist for about that same duration after the completion of treatment. The most common site for toxicity is the gastrointestinal tract, manifesting primarily as nausea, vomiting, and diarrhea. Table 5, compiled from 12 studies reporting on nearly 1200 patients treated with primary abdominopelvic radiation, lists the common early and late toxicities and their approximate frequency.17,22,24,28,29,32,91,92,93,94,95,96 Although many patients suffer short-term complications during therapy, relatively few require treatment breaks.
TABLE 5. Acute and Chronic Toxic Side Effects of Primary Abdominal Pelvic Irradiation in Patients with Ovarian Cancer
Type | % |
Early |
|
Nausea and vomiting | 69–95 |
Diarrhea | 32–69 |
Neutropenia (grade I–II) | 11–33 |
Neutropenia (grade III–IV) | 0–5 |
Thrombocytopenia (<70,000) | 11–22 |
Interruption in treatment | 5–18 |
Required hospitalization | 0–11 |
Late |
|
Asymptomatic basal pneumonitis | 15–20 |
Diarrhea | 10–15 |
Small bowel obstruction | 1.4–14 |
Abnormal hepatic enzymes | 3 |
Ascites | <1 |
Malabsorption | <5 |
Death from toxicity | <0.5 |
Late treatment effects usually present after a “grace period,” which varies from 6 months to 3 years depending on the tissue and amount of injury. Many of these effects are asymptomatic, albeit recognizable. Others, such as injury to the small intestine, can be symptomatic from treatment initiation and demonstrate an evolving process from malabsorption to stricture or perforation. Currently, little correlation has been demonstrated between the development of early treatment side effects and the development of severe late complications. However, a number of primary treatment factors have correlated with the development of late gastrointestinal complications. The frequency and severity appear to be dependent on the total dose of radiation, the dose per fraction, the extent and number of previous operations, and the performance of retroperitoneal lymphatic sampling.22,95,96 Fortunately, treatment-related mortality is distinctly uncommon (<0.5%) for patients treated with modern abdominopelvic irradiation protocols.
One radiation strategy devised to improve tumor control without increasing late tissue toxicity through an increased biologic-therapeutic ratio is dose hyperfractionation. In this strategy, lower doses per fraction (60–100 cGy) are delivered two or three times daily, separated by 6 to 8 hours, yielding higher daily total doses. Withers and associates have postulated that tumors with a large population of actively dividing clonogenic cells should respond like acutely reacting normal tissues whereas late effects on normally dividing tissues should remain unaltered.97 Hyperfractionated radiation therapy has been evaluated in several other disease sites, particularly head and neck tumors and in nonsmall cell lung cancer. A recent meta-analysis of 12 randomized clinical trials published since 1980 evaluating hyperfractionated radiation in a number of primary sites suggested that improved local control and prolonged survivorship could be induced over conventional fractionation.98 Tissue late effects were not significantly increased in those studies reporting long-term treatment effects. Similarly Morgan and colleagues completed a trial of hyperfractionation in the salvage treatment of ovarian cancer patients.99 In this trial, 3060 cGy was delivered to the whole abdomen and pelvis at a dose of 80 cGy twice daily. The pelvis was boosted to standard doses. This treatment was well tolerated, and although premature in follow-up, it did not appear to appreciably increase late effects of treatment.
CONCLUSION
Radiotherapy in the management of ovarian cancer continues to intrigue clinicians because of the durable survivorship observed in selected, properly treated patients. These data are unparalleled by current standards but have called into question patient selection. A wide variety of techniques and methods have been used, inducing a confusing tangle of reports that has limited the applicability to current situations. This chapter has attempted to clarify these issues and provide “evidence-based” recommendations from the literature. Work continues on technique refinement, reduction, and management of long-term effects and patient selection. The information gained from prior studies has demonstrated the necessity of appropriate cohort comparison and critical methodology evaluation. It is through these avenues that the future role of radiotherapy in ovarian cancer will be defined.
REFERENCES
Cannistra SA: Cancer of the ovary. N Engl J Med 329: 1550, 1993 |
|
Partridge EE, Phillips JL, Menk HR: The National Cancer Data Base report on ovarian cancer treatment in United States hopsitals. Cancer 78: 2236, 1996 |
|
Eymer H: Beeinflussug ovn proliferierenden ovarialtumoren durch roentgenstrahlen. Strahlen 1: 358, 1912 |
|
Fyles A, Bolis G, Ferraris C, Parazzini F, Bolla M: Is abdomino-pelvic radiation therapy the optimal treatment for completely resected stage I and II high risk ovarian cancer? Eur J Cancer 33: 12, 1997 |
|
Mychalaczak B, Fuks Z: The current role of radiotherapy in the management of ovarian cancer. Hematol Oncol Clin North Am 6: 895, 1992 |
|
Thomas G: Radiotherapy in early ovarian cancer. Gynecol Oncol 55: S73, 1994 |
|
Bush R: Ovarian cancer: Contribution of radiation therapy to patient management. Radiology 153: 17, 1984 |
|
Dembo A: Epithelial ovarian cancer: The role of radiotherapy. Int J Radiat Oncol Biol Phys 22: 835, 1992 |
|
Thomas G, Dembo A: Integrating radiation therapy into the management of ovarian cancer. Cancer 71: 1710, 1993 |
|
Bertelsen K, Jakobsen A: Radiotherapy for gynecologic cancer. Curr Opin Oncol 5: 885, 1993 |
|
Dembo AJ: Radiation therapy in the management of ovarian cancer. Clin Obstet Gynecol 10: 261, 1983 |
|
Dembo A, Bush R, Beal F et al: The Princess Margaret Hospital study of ovarian cancer: Stage I, II and asymptomatic III presentations. Cancer Treat Rep 63: 249, 1979 |
|
Hreshchyshyn M, Park R, Blessing J et al: The role of adjuvant therapy in stage I ovarian cancer. Am J Obstet Gynecol 138: 139, 1980 |
|
Delclos L, Smith J: Tumors of the ovary. In Fletcher G (ed): Textbook of Radiotherapy, 2nd ed, pp 690–702. Philadelphia, Lea & Febiger, 1973 |
|
Martinez A, Schray M, Howes A, Bagshaw M: Post-operative radiation therapy for epithelial ovarian cancer: The curative role based on a 24-year experience. J Clin Oncol 3: 901, 1985 |
|
Peters L, Ang K: Unconventional fractionation schemes in radiotherapy. Important Adv Oncol 269, 1986 |
|
Calkins A, Rosenshein N, Fox M, Order S: Delayed split whole abdominal irradiation in the combined modality treatment of ovarian cancer. Int J Radiat Oncol Biol Phys 20: 661, 1991 |
|
Kong J, Peters L, Wharton J: Hyperfractionation split-course whole abdominal radiotherapy for ovarian carcinoma. J Radiat Oncol Biol Phys 14: 1, 1988 |
|
Fazekas J, Maier J: Irradiation of ovarian carcinomas: A prospective comparison of the open-field and moving-strip techniques. Am J Roentgenol 120: 118, 1974 |
|
Dembo A, Bush R, Beale F: A randomized clinical trial of moving strip versus open field whole abdominal irradiation in patients with invasive epithelial cancer of ovary (abstr 52). Int J Radiat Oncol Biol Phys 9: 97, 1983 |
|
Fowler J, Margan R, Wood C: Biological and physical advantages and problems of neutron therapy. Br J Radiol 36: 79, 1963 |
|
Schray M, Martinez A, Howes A: Toxicity of open field whole abdominal irradiation as primary post-operative treatment in gynecologic malignancy. Int J Radiat Oncol Biol Phys 16: 397, 1988 |
|
Cmelak A, Kapp D: Long-term survival with whole abdominopelvic irradiation in platinum-refractory persistent or recurrent ovarian cancer. Gynecol Oncol 65: 453, 1997 |
|
Dembo A: Abdominopelvic radiotherapy in ovarian cancer: A 10-year experience. Cancer 55: 2285, 1985 |
|
Bush R, DeBoer G, Hill R: Long term survival with gynecological cancer. In Stoll BA (ed): Prolonged Arrest of Cancer, pp 27–58. Wiley, New York, 1982 |
|
Carey M, Dembo AJ, Gyles AW, Simm J: Testing the validity of a prognostic classification in patients with surgically optimal ovarian carcinoma: A 15-year review. Int J Gyncol Cancer 3: 24, 1993 |
|
Lindner H, Willich H, Atzinger A: Primary adjuvant whole abdominal irradiation in ovarian carcinoma. Int J Radiat Oncol Biol Phys 19: 1203, 1990 |
|
Sell A, Bertelsen K, Andersen J et al: Randomized study of whole-abdomen irradiation versus pelvic irradiation plus cyclophosphamide in treatment of early ovarian cancer. Gynecol Oncol 37: 367, 1990 |
|
Goldberg N, Peschel R: Postoperative abdominopelvic radiation therapy for ovarian cancer. Int J Radiat Biol Oncol Phys 14: 425, 1988 |
|
Chiara S, Conte P, Franzone P et al: High-risk early ovarian cancer-randomized trial comparing cisplatin plus cyclophosphamide versus whole abdominal radiotherapy. Am J Clin Oncol (CCT) 17: 72, 1994 |
|
Redman C, Mould J, Warwick J et al: The West Midlands epithelial ovarian cancer adjuvant therapy trail. Clin Oncol 5: 1, 1993 |
|
Klaassen D, Shelley W, Starreveld A et al: Early stage ovarian cancer: A randomized clinical trial comparing whole abdominal radiotherapy, melphalan and intraperitoneal chromic phosphate: A National Cancer Institute of Canada clinical trials group report. J Clin Oncol 6: 1254, 1988 |
|
Dembo A, Davy M Stenwig A et al: Prognostic factors in stage I epithelial ovarian cancer. Obstet Gynecol 75: 263, 1990 |
|
Licter A, Ozols R, Myers C et al: The treatment of advanced stage ovarian carcinoma with a combination of chemotherapy, radiotherapy, and radiosensitizer: Report of a pilot study from the National Cancer Institute. Int J Radiat Oncol Biol Phys 13: 1225, 1987 |
|
Ho A, Beller U, Speyer J et al: A reassessment of the role of second-look laparotomy in advanced ovarian cancer. J Clin Oncol 5: 1316, 1987 |
|
Lund B, Williamson P: Prognostic factors for outcome of and survival after second-look laparotomy in patients with advanced ovarian carcinoma. Obstet Gynecol 76: 619, 1990 |
|
Rubin S, Hoskins W, Saio P et al: Prognostic factors for recurrence following negative second-look laparotomy in ovarian cancer patients treated with platinum-based chemotherapy. Gynecol Oncol 42: 137, 1991 |
|
Bolis G, Villa A, Guarnereo P et al: Survival of women with advanced ovarian cancers and complete pathologic response and second-look laparotomy. Cancer 76: 128, 1996 |
|
Seltzer V, Vogl S, Kaplan B: Recurrent ovarian carcinoma: retreatment utilizing combination chemotherapy including cis-diamminedichloroplatinum in patients previously responding to this agent. Gynecol Oncol 21: 167, 1985 |
|
Hagopian GS, Lurain JR: Options for managing recurrent of persistent ovarian cancer. Contemp Oncol (Special Report):5, 1995 |
|
Muggia FM, Liu PY, Alberts DS et al: Intraperitoneal mitoxantrone or fluxuridine: Effect on time-to-failure and survival in patients with minimal residual ovarian cancer after second-look laparatomy: A randomized phase II study by the Southwest Oncology Group. Gynecol Oncol 61: 395, 1996 |
|
Bookman MA: Biologic therapies for gynecologic cancer. Curr Opin Oncol 7: 478, 1995 |
|
Goff BA, Blake J, Bamberg MP, Hasan T: Treatment of ovarian cancer with photodynamic therapy and immunoconjugates in a murine ovarian cancer model. Br J Cancer 74: 1194, 1996 |
|
Stiff PJ, Bayer R, Kerger C et al: High-dose chemotherapy with autologous transplantation for persistent/relapsed ovarian cancer: A multivariate analysis of survival for 100 consecutively treated patients. J Clin Oncol 15: 1309, 1997 |
|
Lambert HE, Rustin GJ, Gregory WM, Nelstrop A: A randomized trial comparing single-agent carboplatin with carboplatin followed by radiotherapy for advanced ovarian cancer: A North Thames Ovary Group study. J Clin Oncol 11: 440, 1993 |
|
Fuks Z, Rizel S, Anteby S, Biran S: The multimodality approach to the treatment of stage III ovarian carcinoma. Int J Radiat Oncol Biol Phys 8: 903, 1982 |
|
Thomas G: Is there a role for consolidation or salvage radiotherapy after chemotherapy in advanced epithelial ovarian cancer? Gynecol Oncol 51: 97, 1993 |
|
Ledermann J, Dembo A, Sturgeon J et al: Outcome of patients with unfavorable optimally cytoreduced ovarian cancer? Gynecol Oncol 41: 30, 1991 |
|
Bruzzone M, Repetto L, Chiara S et al: Chemotherapy versus radiotherapy in the management of ovarian cancer patients with pathological complete response or minimal residual disease at second look. Gynecol Oncol 38: 392, 1990 |
|
Lawton F, Luesley D, Blackledge G et al: A randomized trial comparing whole abdominal radiotherapy following cisplatinum cytoreduction in epithelial ovarian cancer: West Midlands Ovarian Cancer Group Trial II. Clin Oncol (R Coll Radiol) 2: 4, 1990 |
|
Menczer J, Ben-Baruch G, Modan M, Brenner H: Intraperitoneal cisplatin chemotherapy vs. abdominopelvic irradiation in ovarian carcinoma patients after second-look laparotomy. Cancer 63: 1509, 1989 |
|
Ozols R, Rubin S, Thomas G, Robboy S: Epithelial ovarian cancer. In Hoskins W, Perez C, Young R (eds): Principles and Practice of Gynecologic Oncology, pp 969–70. Lippincott-Raven, Philadelphia, 1997 |
|
Pickel H, Lahousen M, Petru E et al: Consolidation radiotherapy following carboplatin-based chemotherapy in radically operated advanced ovarian cancer. Gynecol Oncol 68:119 (abstract 192), 1998 |
|
Ben-Baruch G, Menczer J, Feldman B et al: Intraperitoneal cisplatin chemotherapy versus abdominopelvic irradiation in ovarian carcinoma patients after second look laparotomy: A reassessment based on mature data. Eur J Gynecol Oncol 15: 272, 1994 |
|
Haisworth J, Malcolm A, Johnson D et al: Advanced minimal residual ovarian carcinoma: Abdominopelvic irradiation following combination chemotherapy. Obstet Gynecol 61: 619, 1983 |
|
Pickel H, Lahousen M, Petru E et al: Consolidation radiotherapy following carboplatin-based chemotherapy in radically operated advanced ovarian cancer. Gynecol Oncol 68:119 (abstract 192), 1998 |
|
Hacker N, Berek J, Burnison C et al: Whole abdominal radiation as salvage therapy for epithelial ovarian cancer. Obstet Gynecol 65: 60, 1985 |
|
Peters W, Blaski J, Bagle C et al: Salvage therapy with whole-abdominal irradiation in patients with advanced carcinoma of the ovary previously treated by combination chemotherapy Cancer 58:880, 1986 |
|
Solomon H, Atkinson K, Coppleson J et al: Ovarian carcinoma: Abdominopelvic irradiation following reexploration. Gynecol Oncol 31: 396, 1988 |
|
Green J, Warenius H, Errington R et al: Sequential cisplatin/cyclophosphamide chemotherapy and abdominopelvic radiotherapy in the management of advanced ovarian cancer. Br J Cancer 58: 635, 1988 |
|
Kersh C, Randall M, Constable W et al: Whole abdominal radiotherapy following cytoreductive surgery and chemotherapy in ovarian carcinoma. Gynecol Oncol 31: 113, 1988 |
|
Kong J, Peters L, Wharton J et al: Hyperfractionated split-course whole abdominal radiotherapy for ovarian carcinoma: Tolerance and toxicity. Int J Radiat Oncol Biol Phys 14: 1, 1988 |
|
Fuks Z, Rizel S, Biran S: Chemotherapeutic and surgical induction of pathological complete remission and whole abdominal irradiation for consolidation does not enhance the cure of stage III ovarian carcinoma. J Clin Oncol 6: 509, 1988 |
|
Reddy S, Lee MS, Yordan E et al: Salvage whole abdomen radiation therapy: Its role in ovarian cancer. Int J Radiat Oncol Biol Phys 27: 879, 1993 |
|
Fink D, Zheng H, Nebel S et al: In vitro and in vivo resistance to cisplatin in cells that have lost DNA mismatch repair. Cancer Res 57: 1841, 1997 |
|
Ozols R, Hamilton T, Masuda H et al: Manipulation of cellular thiols to influence drug resistence. In: Wolley P, Tew K (eds): Mechanisms of Drug Resistance in Neoplastic Cells, p 289. San Diego, Academic Press, 1988 |
|
De Pooter CM, Scalliet PG, Elst HJ et al: Resistance patterns between cis-diamminedichloroplatinum(II) and ionizing radiation. Cancer Res 51: 4523, 1991 |
|
Oshita F, Fujiwara Y, Saijo N: Radiation sensitivities in various anticancer-drug-resistant human lung cancer cell lines and mechanism of radiation cross-resistance in a cisplatin-resistant cell line. J Cancer Res Clin Oncol 119: 28, 1992 |
|
Behrens B, Hamilton T, Masuda H et al: Characterization of a cis-diamminedichloroplatinum (II)-resistant human ovarian cancer cell line and its use in evaluation of platinum analogues. Cancer Res 47: 414, 1987 |
|
Hamilton T, Masuda H, Young R: Modulation of cisplatin cytotoxicity by inhibition of DNA repair in a cisplatin resistant human ovarian carcinoma. Proc Am Assoc Cancer Res [abstract] 28: 291, 1987 |
|
Card R, Cole D, Henschke U: Summary of ten years of the use of radioactive colloids in intracavitary therapy. J Nucl Med 1: 195, 1960 |
|
Soper J, Wilkinson R, Bandy L et al: Intraperitoneal chromic phosphate P32 as salvage therapy for persistent carcinoma of the ovary after surgical restaging. Am J Obstet Gynecol 156: 1153, 1987 |
|
Tharp M, Hornback N: Complications associated with intraperitoneal 32 P. Gynecol Oncol 53: 170, 1994 |
|
Klaassen D, Starreveld A, Shelly W et al: External beam pelvic radiotherapy plus intraperitoneal radioactive chromic phosphate in early stage ovarian cancer: A toxic combination. Int J Radiat Oncol Biol Phys 11: 1801, 1985 |
|
Young R, Walton L, Ellenberg S et al: Adjuvant therapy in stage I and stage II epithelial ovarian cancer. N Engl J Med 322: 1021, 1990 |
|
Young R, Brady M, Hnieberg R et al: Randomized clinical trial of adjuvant treatment of women with early (FIGO I-IIA high-risk) ovarian cancer-GOG #95. Int J Gynecol Cancer 7 (Supp 2): 17, 1997 |
|
Bolis G, Colombo N, Pecorelli S et al: Adjuvant treatment for early epithelial ovarian cancer: Results of two randomized clinical trials comparing cisplatin to no further treatment or chromic phosphate (32 P). G.I.C.O.G.: Gruppo Interregionale Collaborativo in Ginecologia Oncologica. Ann Oncol 6: 887, 1995 |
|
Ahmed F, Wiltshaw E, A'Hern R et al: Natural history and prognosis of untreated stage I epithelial ovarian carcinoma. J Clin Oncol 14: 2968, 1996 |
|
Trope C, Kaern J, Vergote I et al: Randomized trial on adjuvant carboplatin versus no treatment in stage I high risk ovarian cancer by the Nordic Ovarian Cancer Study Group (NOCOVA). Proc ASCO 16:352a [abstract 1260], 1997 |
|
Spenser T, Marks R, Fenn J et al: Intraperitoneal 32 P after negative second-look laparotomy in ovarian carcinoma. Cancer 63: 2434, 1989 |
|
Rogers L, Varia M, Halle J et al: 32 P following negative second-look laparotomy for epithelial ovarian cancer. Gynecol Oncol 50: 141, 1993 |
|
Vergote I, Winderen M, De Vos L, Tropé C: Intraperitoneal radioactive phosphorus therapy in ovarian carcinoma. Cancer 71: 2250, 1993 |
|
Soper T, Wilkinson R, Bandy L: Intraperitoneal chromic phosphate-32 as salvage therapy for persistent carcinoma of the ovary after surgical restaging. Am J Obstet Gynecol 156: 1153, 1987 |
|
Potter ME, Partridge EE, Shingleton HM et al: Intraperitoneal chromic phosphate in ovarian cancer: Risks and benefits. Gynecol Oncol 32: 314, 1989 |
|
Ott R, Flower M, Jones A, McCready V: The measurement of radiation doses from 32P chromic phosphate therapy of the peritoneum using SPECT. Eur J Nucl Med 11: 305, 1985 |
|
Young R, Decker D, Wharton J et al: Staging laparotomy in early ovarian cancer. JAMA 250: 3072, 1983 |
|
Di-Re F, Fonanelli R, Raspagliesi F et al: Pelvic and paraortic lymphadenectomy in cancer of the ovary. Baillieres Clin Obstet Gynaecol 3: 131, 1989 |
|
Adelson M, Wharton J, Delclos L et al: Palliative radiotherapy for ovarian cancer. Int J Radiat Oncol Biol Phys 13: 17, 1987 |
|
May L, Belinson J, Roland T: Palliative benefit of radiation therapy in advanced ovarian cancer. Gynecol Oncol 37: 408, 1990 |
|
Corn B, Lanciano R, Boente M et al: Recurrent ovarian cancer: effective palliation of chemotherapy failures by irradiation. Cancer 74: 2979, 1994 |
|
Powlis W, Mauch P, Ehrmann R et al: The role of postoperative local or regional irradiation in the treatment of stage I ovarian cancer. Radiology 142: 747, 1982 |
|
Fuller B, Sause W, Plenk H, Menlove R: Analysis of post-operative radiation therapy in stage I through III epithelial ovarian carcinoma. J Clin Oncol 5: 897, 1987 |
|
Weiser E, Burke T, Heller P et al: Determinants of survival of patients with epithelial ovarian carcinoma following whole abdominal irradiation (WAR). Gynecol Oncol 30: 201, 1988 |
|
Smith J, Rutledge F, Delclos L: Post-operative treatment of early cancer of the ovary: A random trial between post-operative irradiation and chemotherapy. Natl Cancer Inst Monogr 42: 149, 1975 |
|
Perez C, Korba A, Zivnuska F et al: 60 Co moving strip technique in the management of carcinoma of the ovary: Analysis of tumor control and morbidity. Int J Radiat Oncol Biol Phys 4: 379, 1978 |
|
van Bunnigen B, Bouma J, Kooijman C et al: Total abdominal irradiation in stage I and II carcinoma of the ovary. Radiother Oncol 11: 305, 1988 |
|
Withers H, Thames H, Peters L: Differences in fractionation response of acutely and late responding tissues. In Karcher KH et al (eds): Progress in Radio-oncology II, p 287. New York: Raven Press, 1982. |
|
Stuschke M, Thames HD: Hyperfractionated radiotherapy of human tumors: Overview of the randomized clinical trials. Int J Radiat Oncol Biol Phys 37: 259, 1997 |
|
Morgan L, Chafe W, Mendenhall W, Marcus R: Hyperfractionation of whole-abdomen radiation therapy: Salvage treatment of persistent ovarian carcinoma following chemotherapy. Gynecol Oncol 31: 122, 1988 |