Radiology in the Diagnosis, Staging, and Management of Gynecologic Malignancies
Authors
INTRODUCTION
Routine clinical, laboratory, and radiologic examinations for gynecologic malignancies are performed for the purpose of tumor detection, diagnosis, and accurate staging, so as to enable optimal treatment planning. Specific radiologic examinations for each malignancy depend on the site, histology, grade, and pattern and extent of spread based on clinical evaluation. In this chapter, we will discuss each of the three major gynecologic malignancies: cervical, uterine, and ovarian cancers. We will give a brief background, review imaging (ultrasound and magnetic resonance) anatomy, present the most common sites of spread, and evaluate the effectiveness of clinical staging. We will also discuss the roles of the various imaging techniques in terms of the following: indications, advantages, limitations in diagnosis and staging, use in treatment planning, evaluation of tumor regression and recurrence, and detection of treatment complications. Finally, we will discuss the role of radiologically guided percutaneous procedures such as fine-needle aspiration in the diagnosis of tumor, its spread, and recurrence; percutaneous nephrostomy and ureteral stenting for draining obstructed renal units; and aspiration and drainage of abscesses and other postoperative fluid collections.
CERVICAL CANCER
Background
Carcinoma of the cervix is the third most common malignancy of the female reproductive tract and the second most common malignancy in women 15 to 34 years of age, with a peak incidence at 45 to 55 years. Risk factors include low socioeconomic class, black race, early sexual activity, multiple sexual partners, multiparity, and infection with herpes simplex virus type 2. Because of extensive Papanicolaou smear screening, the incidence of invasive cervical cancer continues to decline. In the United States, there are an estimated 15,700 new cases diagnosed every year.1 The vast majority are squamous cell carcinomas. Other histologies include adenocarcinoma, small cell carcinoma, adenoid cystic carcinoma, sarcoma, and lymphoma.
Magnetic Resonance Imaging and Ultrasound Anatomy
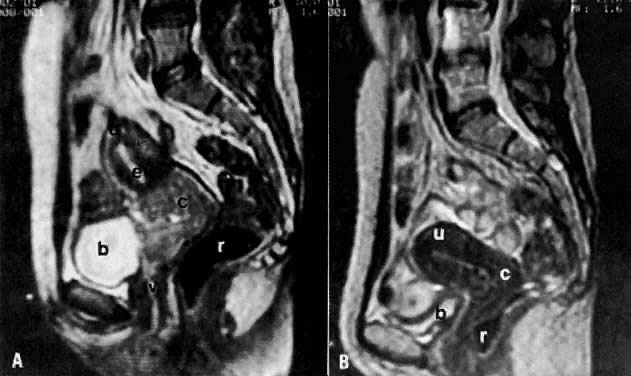
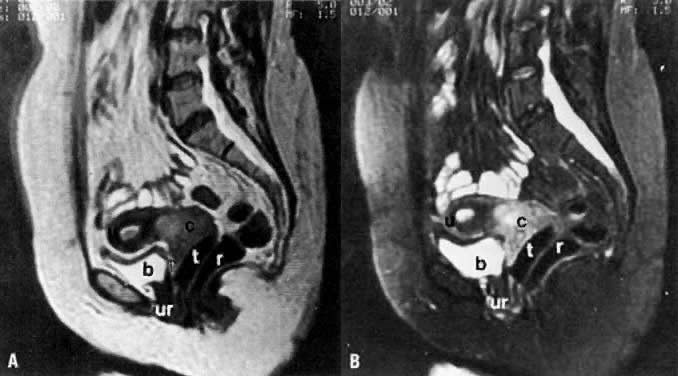
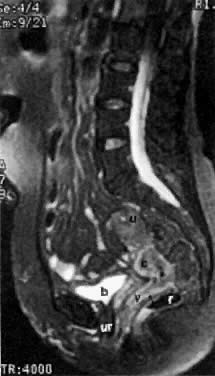
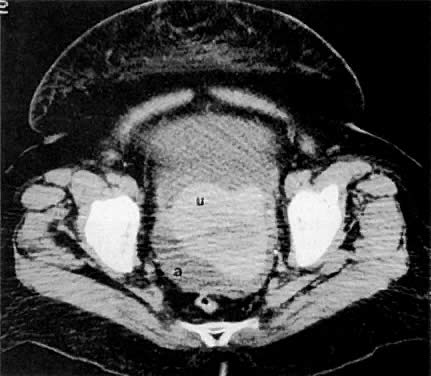
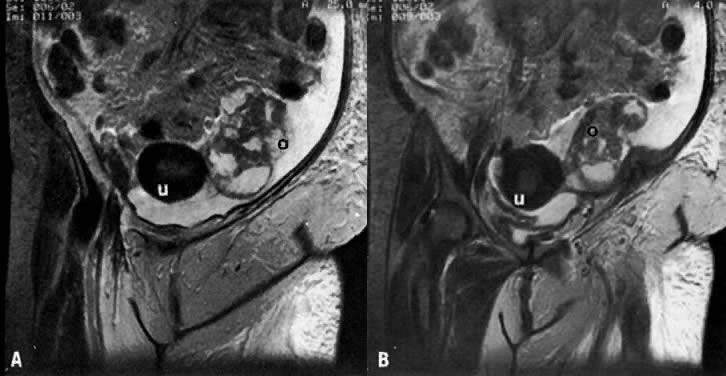
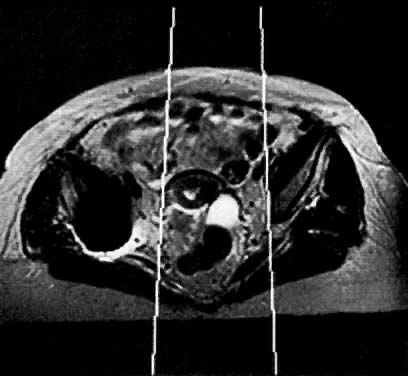
On T2-weighted magnetic resonance imaging (MRI), the normal cervix has two distinct zones (Fig. 1A, Fig. 1B, and Fig. 1C). The central 2- to 3-mm stripe of high signal intensity represents the epithelium and mucous glands in the cervical canal. This stripe is surrounded by a 3- to 8-mm zone of low signal intensity representing the fibromuscular stroma, which is continuous with the junctional zone of the uterus.2,3,4 A third peripheral, 2- to 8-mm zone of intermediate signal intensity is only seen in 15% of patients.2,3 The signal intensity of the cervix does not change with the menstrual cycle, but does change with pregnancy. As reported by Holland and associates,5 the predominant signal of the cervix is dark in the nonpregnant woman and in the first trimester, isointense in the second and third trimesters, bright at 72 hours before labor, and isointense postpartum. The parametria are depicted as two thin stripes of moderate signal intensity lateral to the cervix (Fig. 2A).6
|
Two distinct layers are seen in the vagina on T2-weighted images: an inner zone of high signal intensity, which represents the vaginal epithelium and mucous glands; and an outer zone of low signal intensity, which represents the vaginal wall. The vaginal wall can be separated easily from the urethra and rectum on T2-weighted images (Fig. 1D).6
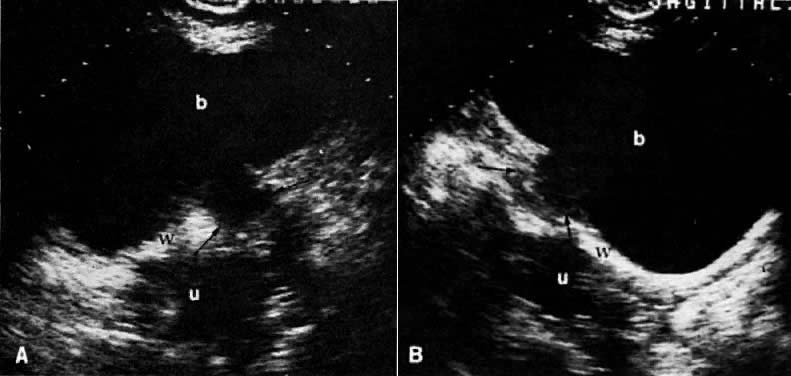
On transvaginal ultrasound (TVUS), the cervix is seen as a medium-echogenicity, 3- to 4-cm long, 3-cm thick cylindrical structure. Two layers can be identified in the cervix: the hyperechoic cervical canal and the medium-echogenicity stroma (Fig. 3A and Fig. 1D). Neither of these layers varies markedly in appearance or thickness during the menstrual cycle. Two distinct layers are seen in the vagina on TVUS: an inner hyperechoic zone, which represents the epithelium and mucous glands; and an outer hypoechoic zone, which represents the muscular wall (Fig. 3B and Fig. 1E).
Diagnosis
Diagnosis of cervical carcinoma is usually made by history, physical examination, Pap smear, and in certain cases, dilatation and curettage. Radiology plays a complementary role in the more difficult cases. On MRI7 and other cross-sectional imaging techniques, the cervix is usually normal in appearance in early cervical cancer. Locally confined cancer appears as a focal mass of medium signal intensity, which either distends the endocervical canal or focally disrupts the normal low-signal-intensity cervical stroma on T2-weighted MRI (Fig. 4A).
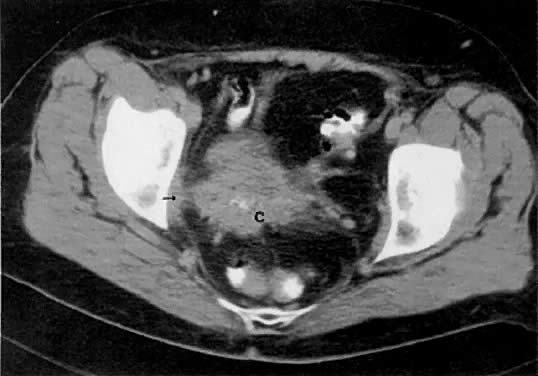
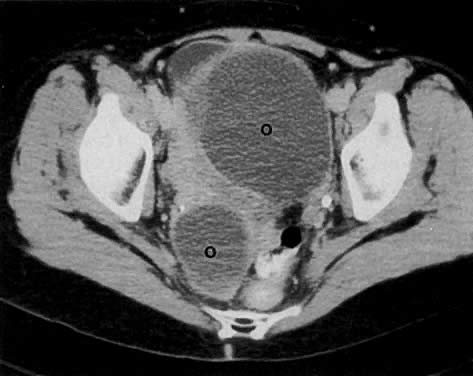
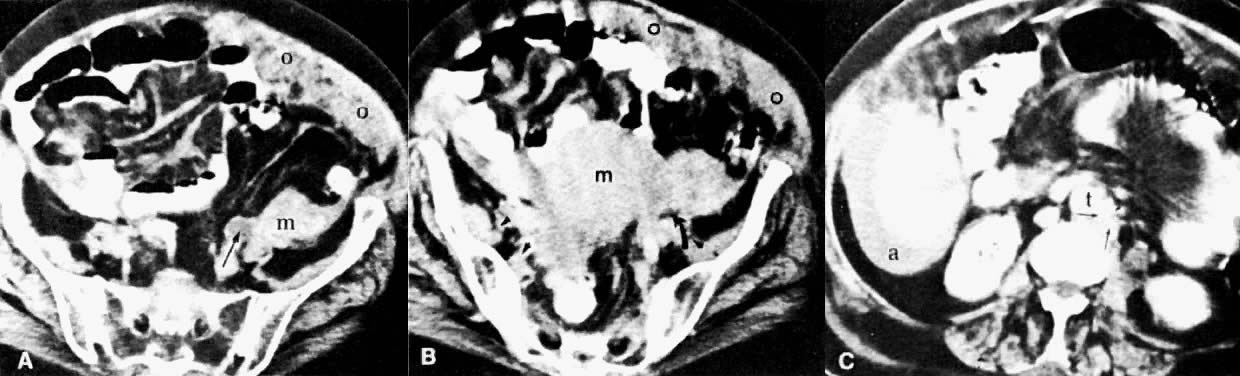
Cervical enlargement (see Fig. 2B; Fig. 5A) is a typical finding of cervical carcinoma on computed tomography (CT). After intravenous contrast material administration, necrotic soft-tissue masses of the cervix can be identified as low attenuation areas.8 Despite technical advances in CT, its accuracy for detecting cervical tumors, which has been reported to be 63% to 80%, remains less than that of the clinical, ultrasound, or MRI examination.9,10
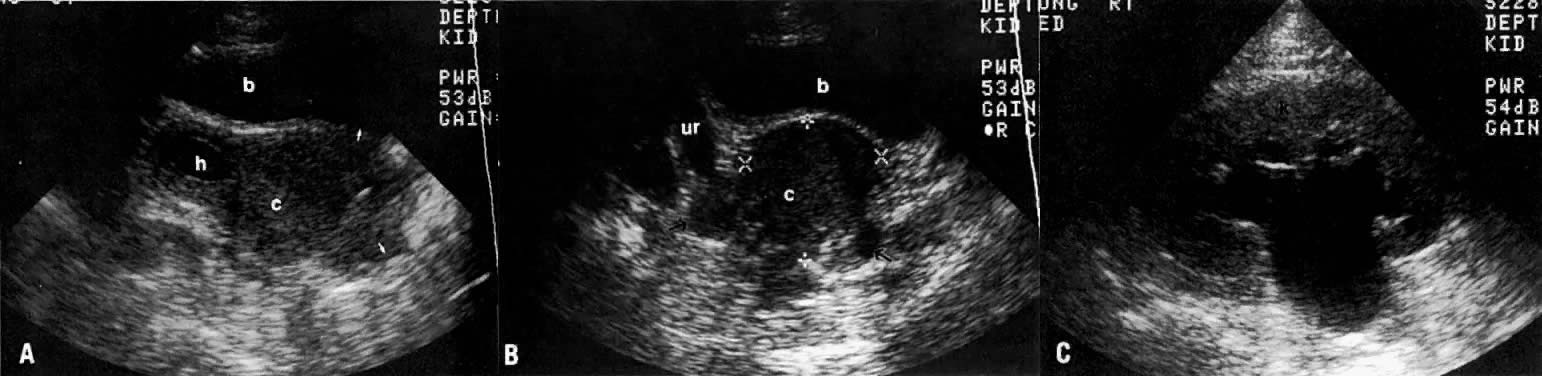
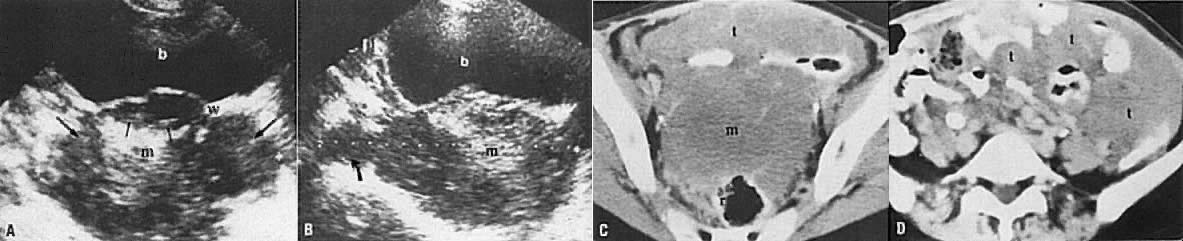
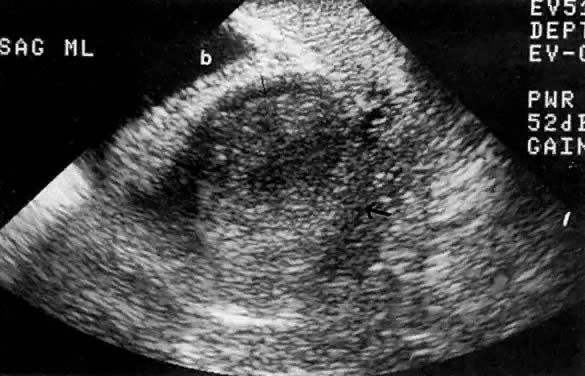
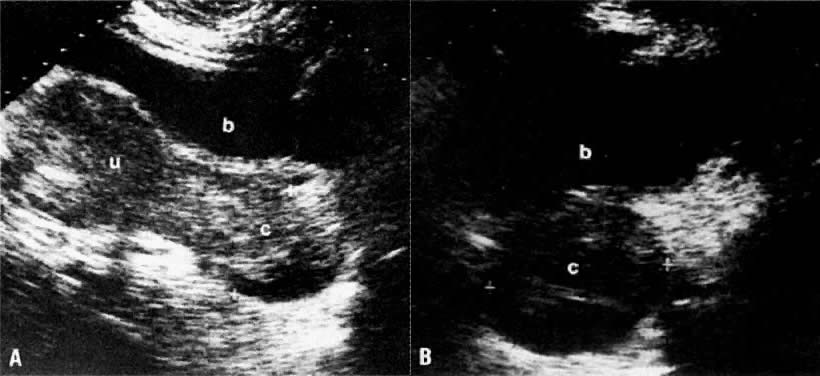
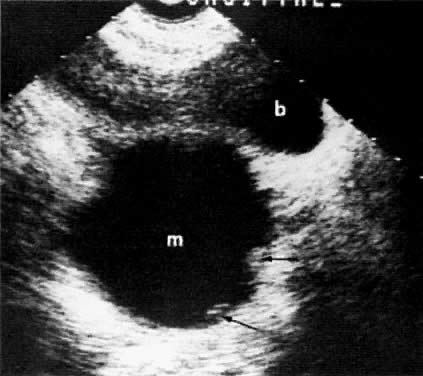
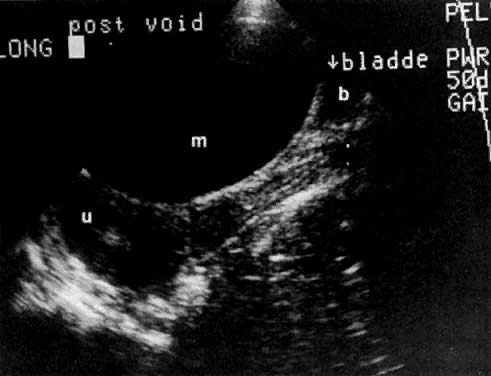
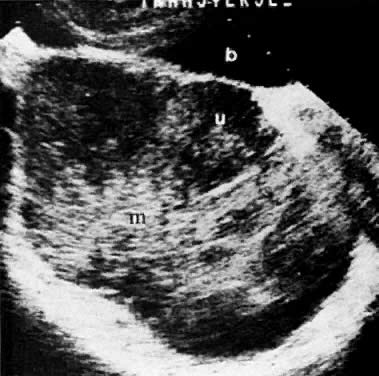
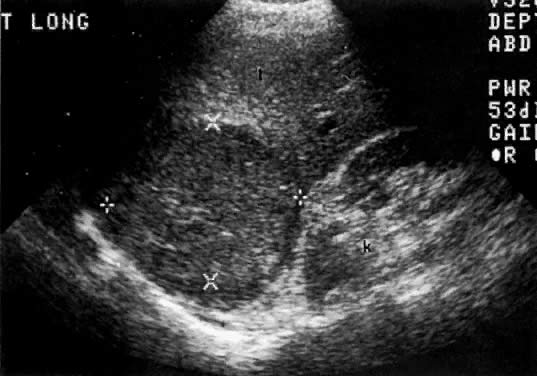
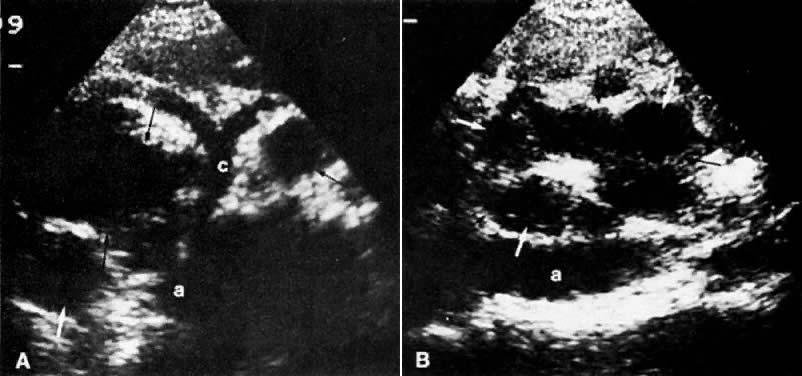
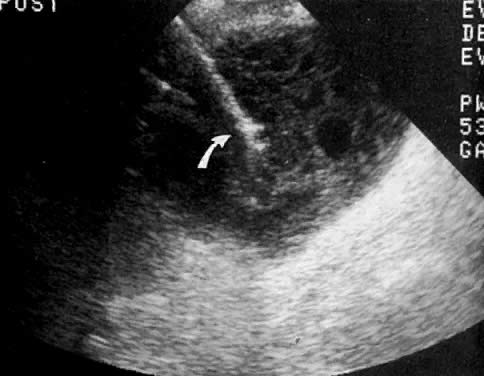
In locally confined disease, a cervical mass may be seen by ultrasound (Fig. 5B and C). In locally advanced disease, the cervix may become diffusely enlarged, inhomogeneous, and irregularly marginated (Fig. 6A and B).
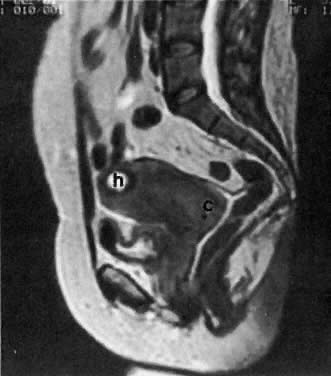
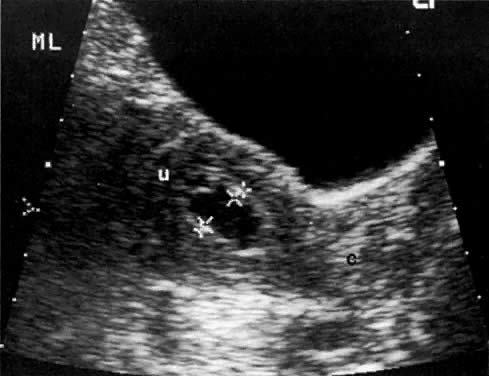
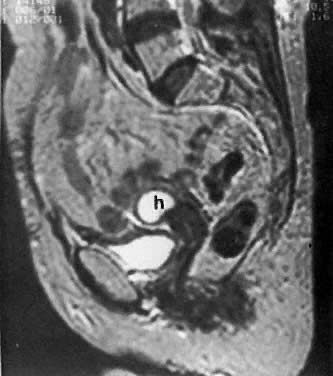
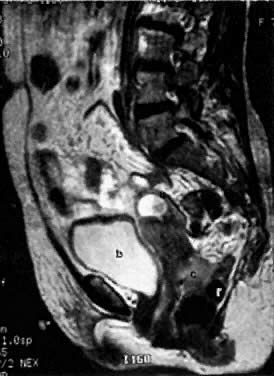
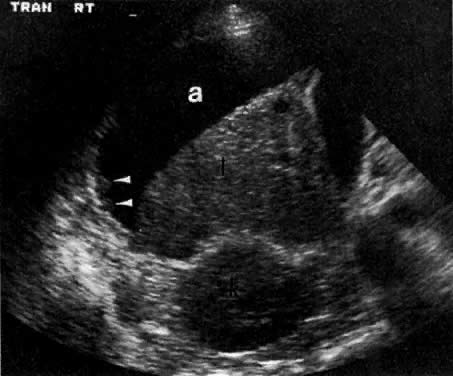
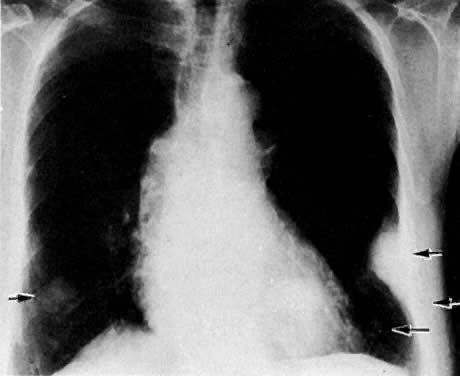
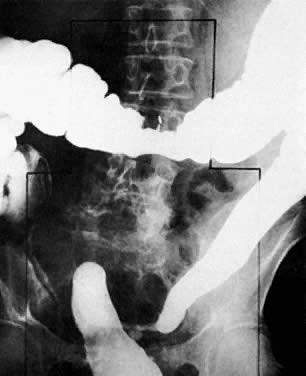
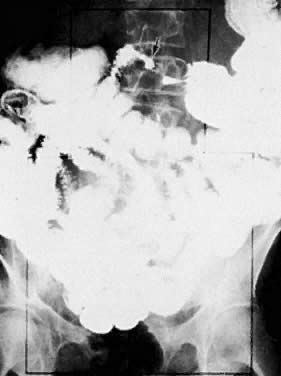
The presence of hydrometras should raise the suspicion of cervical or endometrial carcinoma. It is best demonstrated by ultrasound (see Fig. 6A), but can be seen by both MRI (Fig. 7) and CT (Fig. 8).11,12 This finding, however, is nonspecific because it can also be seen in benign cervical strictures caused by either previous cervical inflammation (Fig. 9) or pelvic irradiation (Fig. 10).
|
|
|
|
Methods of Spread
Cervical cancer spreads in a stepwise fashion by either local infiltration into parametria, pelvic sidewall, and adjacent organs (vagina, ureters, bladder, and rectum); or by lymphatic spread to the pelvic (obturator and external, internal, and common iliac) and para-aortic lymph nodes.13 In advanced cervical cancer and small cell cervical cancer, hematogenous metastases to the lungs, liver, brain, and bones can occur.14
Clinical Staging
Standard clinical workup for cervical cancer staging includes a history and physical examination with pelvic and rectal examinations. Ideally, the pelvic examination is performed under anesthesia along with proctoscopy and cystoscopy in advanced cases, with or without dilatation and curettage. Laboratory studies include a complete blood count and blood chemistries. The staging system (Table 1) was last revised in 1984 by FIGO.15
TABLE 1. Staging System for Carcinoma of the Uterine Cervix (FIGO 1995)
Stage | Characteristics |
IA | Invasive cancer identified only microscopically. (Depth of invasion less than 5 mm, width less than 7 mm) |
IB | Clinical lesion confined to the cervix or preclinical lesion greater than stage IA. |
IIA | Extension to the vagina (not the lower third), with no parametrial extension |
IIB | Parametrial invasion (but not to pelvic sidewall) |
IIIA | Tumor involves lower third of the vagina, with no extension to pelvic sidewall |
IIIB | Tumor extends to the pelvic sidewall or causes hydronephrosis or nonfunctioning kidney |
IVA | Tumor invades mucosa of bladder or rectum and/or extends beyond the true pelvis |
IVB | Distant metastases |
(Adapted from International Federation of Gynecology and Obstetrics staging announcement. FIGO staging of gynecologic cancers, cervical, and vulva. Int J Gynecol Cancer 5:319, 1995)
The tumor prognosis, as well as the choice and success of therapy, depends on accurate clinical staging and volume assessment of the tumor.16,17 For example, the differentiation between stage I and stage IIB disease is of special significance because patients with parametrial spread are treated with primary radiation therapy, whereas those with lesser stages may be treated with either a radical hysterectomy or radiation therapy. Also, the presence of nodal involvement would change patient management by adding radiation therapy to the para-aortic area and/or chemotherapy. Clinical examination may not always provide optimal evaluation of the tumor extent and volume.18,19,20,21 It differs from surgical staging in approximately 30% to 40% of cases,18,19 and its accuracy decreases as the disease advances in stage.
Local Radiologic Staging
Radiologic studies that have been used to evaluate the locoregional extent of cervical cancer include excretory urography (EU), lymphangiography, barium enema, CT, MRI, and ultrasound. Abdominopelvic CT has replaced EU and lymphangiography, especially in locally advanced disease, because it provides accurate information about ureteral encasement, parametrial and pelvic sidewall extension, and pelvic and para-aortic nodal involvement. Barium enema should be considered for the evaluation of patients with stage III and stage IV disease or when symptoms suggest bowel involvement. MRI and ultrasound are complementary studies that may provide useful information about local extension to the parametria, bladder, ureters, and rectosigmoid.
LOCAL CT STAGING.
Poor soft-tissue contrast and restriction to the axial plane limit the ability of CT to determine the depth of stromal invasion or to assess tumor volume. Tumor confined to the cervix may be seen as an inhomogeneous, hypodense area in an enlarged cervix; however, the size of the cervix cannot be used in staging cervical carcinoma. The criteria used to stage confined disease include smooth, well-defined cervical margins; no prominent parametrial soft-tissue strands; no parametrial soft-tissue mass; and preservation of the periureteral fat planes (see Fig. 5A).22 CT has a limited role, however, in the detection of tumor extension into the vagina and body of the uterus.
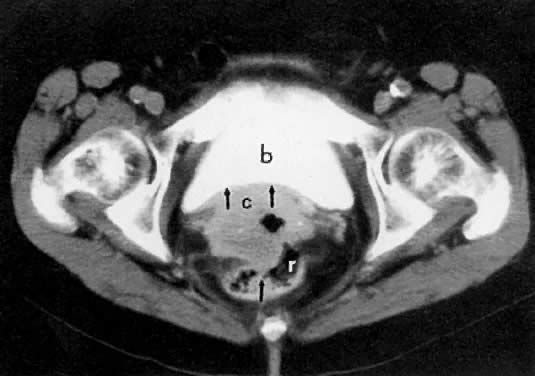
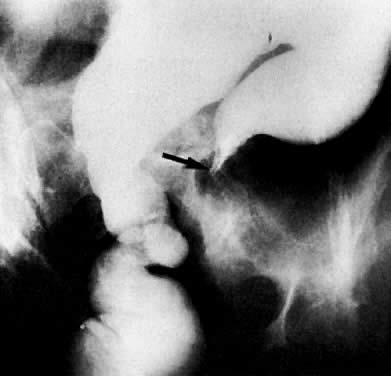
CT can be helpful in the diagnosis of parametrial invasion and can be used in conjunction with the physical examination in differentiating stage I cervical carcinoma from stage IIB or more advanced disease. The criteria used to diagnose parametrial invasion include irregularity or poor definition of the lateral cervical margins, prominent parametrial soft-tissue strands, eccentric soft-tissue mass (see Fig. 2B), and obliteration of the periureteral soft-tissue planes without hydroureter (Fig. 11). Prominent strands can be seen with inflammation, but are more commonly seen with tumor invasion. One should be careful in diagnosing parametrial invasion in patients with asymmetry of the cervix because tampon insertion occasionally causes angulation of the uterus. The reported accuracy of parametrial involvement by CT is 70%, positive predictive value 33%, and negative predictive value 67%.9
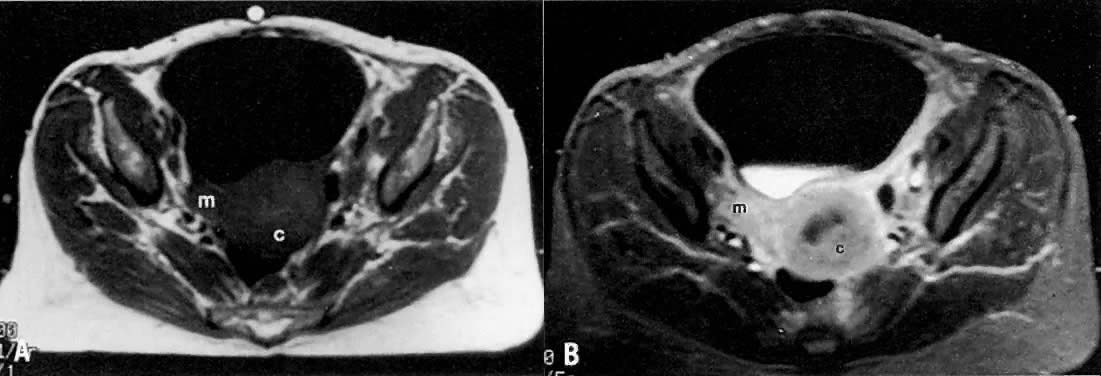
Signs of pelvic sidewall invasion (stage IIIB) on CT include irregular soft-tissue strands extending to pelvic muscles (Fig. 12), a fat plane of less than 3 mm between these muscles and the tumor, muscle encasement by the tumor, and ureteral obstruction.23 CT is much more accurate than clinical examination in detecting pelvic sidewall invasion. Its reported accuracy ranges from 66% to 80%. Although CT can detect invasion of the bladder and colon by tumor, it may be difficult to differentiate invasion from mere contiguity to these structures based on the loss of fat planes.24,25 The presence of bladder or rectal wall thickening and intraluminal mass in association with focal fatty plane obliteration may help in differentiation (Fig. 13).23
|
|
LOCAL MRI STAGING.
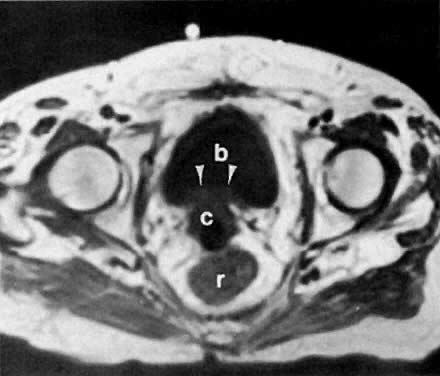
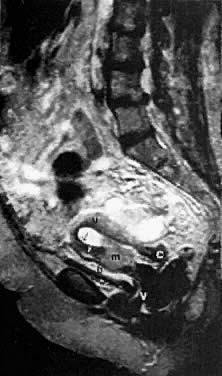
With its improved soft-tissue contrast resolution and multiplanar capability, MRI is very useful in local staging of cervical carcinoma. Low signal intensity surrounding the tumor in the cervix and preservation of fat planes are consistent with tumor confined to the cervix (Fig. 14A). Stage IIA is indicated by the loss of the normal low signal intensity of the vaginal wall (Fig. 15). Stage IIB is indicated by the presence of abnormal thickening of the parametrium on T1-weighted MRI (see Fig. 2A; Fig. 16A). On T2-weighted images, stage IIB is depicted as increased signal intensity in the parametrium with the loss of the normal low signal intensity of the cervical stroma.9 Stage IIIA is indicated by the loss of low signal intensity of the wall of the lower third of the vagina. In stage IIIB, the lateral pelvic wall muscles lose their normal low signal intensity, with loss of the fat planes separating these muscles from the tumor on T1-weighted images (Fig. 17A and B). Stage IVA is indicated by loss of the normal low signal intensity of the bladder wall (see Fig. 17D; Fig. 18) or rectal wall (Fig. 19), associated with wall thickening and/or intraluminal mass.26,27
|
Several studies have shown that MRI improves the accuracy of parametrial assessment over that of the clinical examination.28,29,30,31 In one study with surgical correlation, the accuracy of detecting parametrial involvement by MRI was 92% compared with 78% by clinical examination, and the positive predictive value was 89%, and negative predictive value was 92%.9 Most errors occur because of overstaging. In a study of 57 patients by Hricak and colleagues,26 MRI overstaged the tumor in 11 patients and understaged it in 3 patients, yielding an overall accuracy of 81%. In this study, the accuracy of MRI was 88% for detecting parametrial extension, 95% for vaginal extension, 93% for pelvic sidewall extension, and 96% for bladder wall invasion. In another study of 67 patients, Togashi and co-workers27 overstaged the tumor in 6 patients and understaged it in 10 patients. Accuracy was 89% for parametrial tumor extension and 83% for vaginal involvement. In patients with large tumors or tumors located in an area difficult to evaluate, MRI can accurately assess tumor volume and extent.2,6,28,29,30,31,32 As tumor diameter approaches 3 cm, approximately 50% of these patients will have lymph node metastasis.
Peritumoral inflammatory reaction can mimic parametrial involvement, causing tumor overstaging on T2-weighted imaging.33 In addition to edema, factors including volume averaging, bowel peristalsis artifact, close proximity of structures, obliquity of planes, and anatomic variations may obliterate fat planes between the tumor and the surrounding organs, thus increasing the false-positive rate, especially in advanced disease.34 Gadolinium-enhanced T1-weighted MRI techniques do not seem to improve visualization of stromal invasion of cervical carcinoma, but do improve staging in advanced cases (see Fig. 16A and Fig. 16B).35 Recent studies have shown that gadolinium-enhanced dynamic MRI may be superior to T2-weighted MRI in the evaluation of stromal invasion.12 Fat suppression techniques may also better delineate the tumor margins (Fig. 14B) and detect bladder or rectal wall invasion.36 MRI using endorectal surface coils may also prove to be a promising technique because of improved resolution over conventional MRI techniques.37
LOCAL ULTRASOUND STAGING.
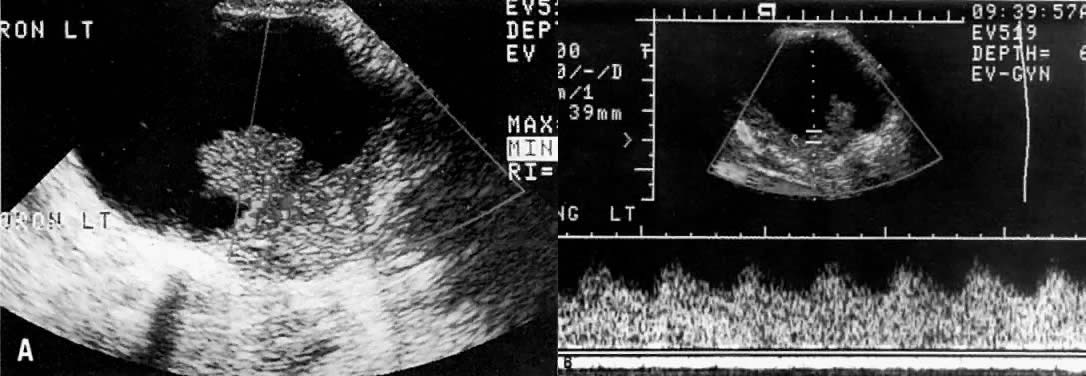
Ultrasound may play a role in the evaluation of tumor extension to the parametrium and the pelvic sidewall in patients with an equivocal pelvic examination.38,39 In stage IB, the tumor is confined to the cervix on ultrasound, without parametrial extension (see Fig. 5B and C). In stage IIB, a tongue of the hypoechoic soft-tissue mass extends laterally from the cervix (see Fig. 6B). More recently, high-resolution transrectal ultrasound was found to be more sensitive in assessing parametrial tumor spread compared to clinical evaluation (78% vs 52%, respectively).40 Ultrasound is also capable of detecting tumor extension into the bladder (Fig. 20),39,41 hydronephrosis (Fig. 6C),38 and/or ureteral obstruction (see Fig. 6B). Unlike CT, however, it cannot always reliably evaluate the level and cause of the obstruction.
ENDOMETRIAL CARCINOMA
Background
Endometrial carcinoma is the most common gynecologic cancer: 34,000 new cases were estimated for 1996, with 6000 patients dying during the same period. Peak age was 55 to 65 years. Risk factors include obesity, nulliparity, estrogen replacement, estrogen-secreting ovarian tumors, late-onset menopause, diabetes mellitus, anovulatory cycling, polycystic ovaries, and adenomatous hyperplasia. Patients with endometrial cancer generally present with uterine bleeding. Most endometrial cancers are adenocarcinomas. Uterine sarcomas constitute less than 10% of uterine body tumors and are considered separately because of their different clinical behavior.42,43
MRI and Ultrasound Anatomy
On MRI, uterine zonal anatomy is best depicted with T2-weighted imaging. Three distinct zones are seen (see Fig. 1A and Fig. 1B):
- The central, high-signal-intensity zone represents the endometrium and its cavity. During the menstrual cycle, the width of this stripe ranges from 5 mm early in the cycle to 10 mm late in the cycle.44,45
- The layer of low signal intensity that underlies the endometrium, called the junctional zone, corresponds to the inner layer of the myometrium. Its width does not vary during the menstrual cycle.
- The peripheral intermediate-signal-intensity zone represents the outer portion of the myometrium. The latter varies in width and signal intensity during the menstrual cycle, reaching a maximum width of 25 mm and its highest signal intensity during the midsecretory stage.45,46 In postmenopausal women, both endometrium and myometrium decrease in thickness, with a consequent decrease in uterine size. At this stage, the endometrium should not exceed 5 mm in width.44,46
On ultrasound, the uterus is seen as a medium-echogenicity, pear-shaped structure with a central hyperechoic zone, called an endometrial stripe (see Fig. 3A and F). The thickness of this stripe and its echo pattern vary during the menstrual cycle. In the early proliferative phase, it appears as a thin, 2- to 5-mm echogenic stripe. In the late proliferative phase, it has a multilayered appearance: a central hyperechoic line representing the endometrial cavity, and subjacent isoechoic and hyperechoic layers representing the endometrium and its glands. In the late secretory phase, the endometrial stripe appears uniformly hyperechoic and fairly thick (up to 15 mm). Frequently seen subjacent to the endometrial stripe is a hypoechoic junctional zone that is somewhat thinner than the junctional zone seen by MRI. The outer thick medium-echogenicity zone represents the outer myometrium. In postmenopausal women, the endometrial stripe decreases in thickness and should not exceed 5 mm. Similarly, the myometrium decreases in thickness with considerable decrease in the size of the uterus.
Diagnosis
The diagnosis of endometrial carcinoma is usually made by endometrial biopsy or by dilatation and curettage. TVUS is the preferred method of screening for endometrial abnormalities in high-risk patients such as those on estrogen replacement therapy and tamoxifen treatment for breast carcinoma. The appearance of endometrial carcinoma on ultrasound is variable, ranging from a completely normal-appearing uterus; to echogenic thickening of the endometrium (greater than 15 mm in premenopausal and greater than 5 mm in postmenopausal women); to an irregular, heterogeneous, hyperechoic endometrium with ill-defined hypoechoic or isoechoic areas (Fig. 21A).47 The latter pattern is highly suggestive of endometrial carcinoma. Although the former pattern is seen in endometrial carcinoma, it is seen more often in association with adenomatous hyperplasia and endometrial polyps.48,49 It can also be seen with the proliferative endometrium associated with estrogen replacement therapy and tamoxifen treatment for breast carcinoma.50,51
Submucosal fibroids and intrauterine hematomas may also have a hyperechoic appearance that may simulate endometrial carcinoma. The less frequently encountered hypoechoic pattern of endometrial carcinoma can be differentiated from that of intramural fibroids by the association of variable degrees of shadowing with the latter. Other suggestive but nondiagnostic signs of uterine malignancy are a rapid increase or any postmenopausal increase in the size of a uterine mass, extrauterine extension, and coexistence of a uterine mass with abdominal metastases.52 Spectral and color Doppler can enhance the ability of ultrasound to make a more specific diagnosis. Although no flow will be demonstrated in blood clots, high-resistivity flows are typically seen in benign disease compared with low-resistivity flows seen in endometrial carcinoma (Fig. 21B). However, some overlap exists between the Doppler findings of the two disease groups. Endometrial carcinoma may occasionally obstruct the uterus, causing variable degrees of pyometra, hydrometra, or hematometra.
The T2-weighted MRI appearance of noninvasive endometrial cancer is nonspecific. The uterus may appear normal on MRI. Thickening of the endometrial stripe-focal or diffuse, hyperintense or isointense, homogeneous or heterogeneous-may be seen (Fig. 22). As with ultrasound, it is not always possible to differentiate noninvasive endometrial cancer from adenomatous hyperplasia, endometrial polyps, or blood clots by MRI.53,54 In more advanced cases, it may be difficult to differentiate endometrial cancer from the more common uterine fibroids by MRI. The use of contrast media helps to differentiate viable tumor from retained debris within the endometrial cavity.35,55
Endometrial carcinoma is demonstrated as a central area of hypodensity relative to the enhanced normal myometrium on postcontrast CT (Fig. 23).56 More often, CT shows diffuse uterine enlargement. It is often impossible to differentiate endometrial carcinoma from benign causes of uterine enlargement (e.g., fibroids) by CT.
|
Leiomyosarcomas constitute 3% of uterine body tumors. They are very invasive neoplasms that frequently present at an advanced stage. The typical ultrasound appearance is that of a very irregular mass with bizarre areas of degeneration, high-level echoes, and invasion of the surrounding pelvic structures (Fig. 24A and B).52 CT shows a large, irregular uterine tumor with invasion of the surrounding pelvic organs, peritoneum, and omentum (Fig. 24C and D).
Methods of Spread
Endometrial carcinoma spreads by local infiltration from the endometrium to the myometrium and along the lower uterine segment to the cervix.57 Other routes of spread follow lymphatic channels.13 The incidence of pelvic lymph node metastases in patients with tumors confined to the uterus ranges from 1% to 36% and is related to the histologic grade of the tumor and depth of myometrial invasion.58,59 Peritoneal dissemination and hematogenous metastases occur more commonly in poorly differentiated endometrial carcinomas60 and uterine sarcomas.61
Clinical Staging
Preoperative clinical and laboratory evaluation includes a history and physical examination with pelvic and rectal examinations, which may be done under anesthesia, and dilatation with fractionated curettage. Approximately 80% of endometrial carcinomas have clinical stage I disease.62 This accounts for the overall high cure rate for endometrial cancer. In contrast to cervical cancer staging, endometrial cancer staging is achieved surgically, according to histologic criteria (Table 2). The staging system is based on extent of involvement of the uterus, adjacent structures, and lymph nodes. In stage I, the tumor is limited to the uterine corpus, including endometrial involvement in stage IA; less than 50% myometrial invasion in stage IB; and greater than 50% myometrial invasion in stage IC. In stage II, there is cervical involvement. The differentiation between stages I and II is important because stage I tumors are commonly treated surgically, whereas stage II tumors may be treated with preoperative radiation therapy. Stages IIIA, IIIB, and IIIC signify extension to the adnexa, vagina, and lymph nodes, respectively. The prognosis depends on the depth of myometrial extension, tumor histology and grade, and nodal involvement.2,13,63 Clinical evaluation understages tumors in 20% of patients. In patients with endometrial adenocarcinomas, it is frequently very difficult to determine whether the tumor arises from the endometrium or the cervical area. Treatment for these two conditions is different (total abdominal hysterectomy with bilateral salpingo-oophorectomy versus radical hysterectomy with preoperative radiation therapy).
TABLE 2. Staging System for Endometrial Cancer (FIGO 1988)
Stage | Characteristics |
IA | Tumor limited to endometrium |
IB | Invasion to < 1/2 myometrium |
IC | Invasion to > 1/2 myometrium |
IIA | Endocervical glandular involvement only |
IIB | Cervical stromal invasion |
IIIA | Tumor invades serosa or adnexa, or positive peritoneal cytology |
IIIB | Vaginal spread |
IIIC | Metastases to pelvic or para-aortic lymph nodes |
IVA | Tumor invasion to bladder and/or bowel mucosa |
IVB | Distant metastases including intra-abdominal and/or inguinal lymph nodes |
(Adapted from International Federation of Gynecology and Obstetrics classification and staging of malignant tumors of the female pelvis. Annual report on the results of treatment in gynecologic cancers. Int J Gynecol Obstet 28:189, 1989)
Local Radiologic Staging
Standard radiologic studies for local staging include EU, MRI, and ultrasound. Abdominopelvic CT is indicated for patients with clinically advanced tumors to evaluate nodal, peritoneal, bowel, or liver spread. MRI and ultrasound can provide additional information for accurate delineation of local spread of uterine body tumors.
LOCAL CT STAGING.
CT plays a less important role in local staging of endometrial cancer because of its poor contrast resolution and poor multiplanar capability. Myometrial invasion can best be demonstrated on postcontrast scans.25 Uterine size alone cannot be used as an indicator for myometrial invasion. In one study, extension into the cervix (stage II disease) was present when the cervix was larger than 6 cm in diameter; all patients with a cervical diameter of 4.5 cm or less were diagnosed with stage I disease.62 The reported accuracy of CT in staging endometrial carcinoma is 83% for confined disease and 86% for extrauterine tumor spread.64
LOCAL MRI STAGING.
In noninvasive endometrial cancer, the junctional zone on T2-weighted MRI should be intact. The disruption of the junctional zone by a mass of higher signal intensity on T2-weighted imaging suggests myometrial invasion (see Fig. 22). In stage II, tumor extension to the cervix is usually indicated by the increased size and signal intensity of the cervix on all MRI planes. In stage III, extension to the vagina is indicated by an increase in the signal intensity of its wall. In stage IV, there is a focal loss of normal low signal intensity between the rectum or bladder and the tumor on T2-weighted images.
Several studies addressing the accuracy of MRI staging of endometrial carcinoma have been published. Hricak and associates65 concluded that MRI accuracy is 74% for stage I disease, 89% for superficial invasion, and 54% for deep invasion. Their data also showed a sensitivity of 17% for stage III and stage IV disease and a specificity of 97%. Positive predictive value for stages III and IV was 50%.65 Most errors in MRI were caused by overestimation rather than underestimation. This has been attributed to atrophic myometrium in postmenopausal women or to incorrect interpretation of blood clots or polypoid lesions distending the uterus.66
Gadolinium-enhanced T1-weighted imaging improves the accuracy of MRI in assessing the depth of myometrial invasion from approximately 68% to approximately 87%.55,67,68 It can also differentiate tumor extension from residual blood clots and debris within the uterus. Minimal or microscopic invasion was the most common reason for underestimating myometrial invasion, regardless of imaging technique.68 Dynamic-enhanced MRI,12 variable-flip-angle spin-echo MRI,36 body coils, and endorectal coils37,69 have also been shown to offer substantial improvements in the evaluation of the depth of myometrial invasion and the staging of endometrial cancer.
LOCAL ULTRASOUND STAGING.
Ultrasound may play a significant role in local staging. The size of the uterus, which can be accurately evaluated by ultrasound, is not helpful in staging endometrial carcinoma. There is, however, a statistically significant difference in the echo pattern and shape of the uterus between stages I and II and stages III and IV. Of patients with tumor stages I and II, 94% had a normal or bulbous uterus and a normal or decreased echo pattern; however, of patients with tumor stages III and IV, 80% had a lobular and/or mixed-echo pattern.47 On TVUS, stage IA disease is diagnosed when there is no disruption of the normal hypoechoic halo that surrounds the hyperechoic endometrial stripe (see Fig. 21A). Muscular invasion (stage IB and IC) is indicated by the lack of uniformity of this halo (Fig. 25). The degree of invasion is evaluated by comparing the depth of the endometrium to the depth of the myometrium. Using these criteria, Cagnazzo and associates70 found TVUS to be 78% accurate in assessing myometrial invasion, with a sensitivity of 80%, specificity of 77%, positive predictive value of 87%, and negative predictive value of 66%. Similar values for MRI in the same study were 83%, 87%, 78%, 82%, and 84%, respectively.70 Others have also found no difference in the staging accuracy of TVUS and T2-weighted MRI (68% vs 68% to 74%).68,71 In another study, TVUS was found to be accurate for detecting both myometrial invasion and cervical extension.72 Errors of depth overestimation of myometrial invasion by ultrasound can occur when the tumor has a significant intraluminal component, atrophic myometrium, associated fibroids, pyometra, and poor tumor/myometrial contrast.68,73 Errors of underestimation resulted predominantly from microscopic invasion. Gross cervical involvement by endometrial carcinoma causes enlargement of the cervix, which can also be accurately assessed by ultrasound (Fig. 26).
OVARIAN CANCER
Background
Ovarian carcinoma is the second most common gynecologic malignancy and the leading cause of death, with 26,700 new cases and 14,800 deaths per year in the United States.1 It is the fourth most common cause of cancer death in women in the United States, with a peak incidence at 40 to 65 years. Risk factors include multiparity, late menopause, early menarche, and family history of ovarian or endometrial cancer.
Eighty percent of ovarian neoplasms are benign. Only 10% to 15% are primary malignant neoplasms, and 5% are metastatic lesions from bowel tumors, malignant melanoma, or lung or breast carcinoma.74 The most common primary malignant tumors are the epithelial ones, which arise from the serosal mesothelial layer of the gonads. They constitute 80% to 90% of all malignant ovarian tumors. The remainder are of germ cell or stromal origin. Epithelial tumors include serous cystadenocarcinoma (42%), mucinous cystadenocarcinoma (12%), endometrioid carcinoma (15%), undifferentiated carcinoma (17%), and clear cell carcinoma (6%).75
MRI and Ultrasound Anatomy
Ovaries are seen in 96% of premenopausal females on MRI. They have a homogeneous texture of moderately low signal intensity on T1-weighted MRI.76 They are distinguishable from surrounding fat, but not as well from bowel. On T2-weighted MRI, ovarian stroma becomes isointense to fat, and the fluid-filled follicles become hyperintense (see Fig. 1B). Simple cysts are isointense to urine on T2-weighted images and hyperintense to urine on T1-weighted images if they contain protein or mucus.
On TVUS, the ovaries are seen as medium-echogenicity, almond-shaped structures on both sides of the uterus. In menstruating females, each ovary contains several small cystic structures called follicles (Fig. 3C), one of which may become dominant, reaching a size of up to 25 mm before ovulation. After ovulation, this becomes the corpus luteum, which decreases in size and acquires a complex echotexture. Each ovary has an approximate volume of 9 mL (range, 6 to 12 mL). After menopause, the ovaries lose their follicles and become smaller (1.5 to 3 mL) and harder to see.
Diagnosis
Because ovarian cancer remains asymptomatic for long periods of time, it is frequently discovered at an advanced stage (III or IV). Patients generally present with vague abdominal discomfort, distention, and/or weight loss. A pelvic mass may be felt on physical examination.
Ultrasound plays a significant role in the diagnosis of ovarian tumors. This modality can determine the presence or absence of a mass with an accuracy of 97%.77 The following features of an ovarian mass can be assessed: size, site of origin, relationship to adjacent organs, shape, wall thickness, margin sharpness, internal echotexture, homogeneity, presence and thickness of septations, presence and size of solid components, presence and coarseness of debris, and presence of acoustic enhancement or shadowing. Ultrasound is 84% accurate in the tissue characterization of ovarian masses.77 The ultrasound appearance correlates very well with the morphology of ovarian neoplasms, but not with the histology. This modality is highly accurate in differentiating benign from malignant neoplasms (87% to 91%) if the criteria of malignancy and benignancy are strictly followed.77,78 The criteria that favor malignancy include a multiloculated cystic mass greater than 5 cm in diameter; thick, irregular septations; coexistent solid projections (Fig. 27 and Fig. 28A); a complex mass inseparable from the uterus; a thick, irregular capsule; invasion of contiguous organs; ascites; and presence of omental, mesenteric, or liver metastases.79 The presence of ascites, however, is nonspecific, since it can be associated with benign ovarian tumors such as fibromas.80 The criteria of a benign mass are a well-defined cystic lesion with thin walls, few or no septations, and minimal fine papillary projections (Fig. 29).80 In a study of 106 patients with ovarian neoplasms, Moyle and associates81 found a high correlation between the percentage of solid components and their malignant nature. However, with few exceptions (Fig. 30), solid or mostly solid masses are more likely to be benign. Teratomas, 65% of which are highly echogenic with “dirty” shadowing, showing variable degrees of calcifications and fat-fluid levels (Fig. 31), are also predominantly benign.81
|
|
TVUS was found to improve the image quality of the pelvic organs and provide additional information to characterize ovarian masses.82,83 It affords the sonologist added confidence in diagnosing hemorrhagic cysts such as endometriomas and corpus luteum cysts, differentiating teratomas from stool-filled bowel loops, and distinguishing between benign and malignant cystic masses. TVUS establishes the exact relationship of masses to various pelvic organs by its high resolution and use of transducer-manual compression. The overall accuracy of TVUS in making a specific diagnosis of pelvic masses is 83% compared with the 70% accuracy of MRI.84
Despite its initial promise, TVUS was found not to be cost-effective for ovarian carcinoma screening because of its low yield85 and because both ovaries are imaged by TVUS in less than 60% of postmenopausal women.86 In two studies, TVUS missed 30% to 46% of ovarian masses in postmenopausal women.86,87 In another study in which 5479 patients were screened by TVUS, Crane and associates88 found the cost of detecting each ovarian cancer to be $355,044, compared to $25,000 for each breast cancer. However, TVUS screening for high-risk patients is more cost-effective ($10,144 for each ovarian cancer detected). Manual pelvic examination is currently the most effective screening method for the general population.
Incidental, small (less than 5 cm), purely cystic masses have recently been discovered in postmenopausal women with increasing frequency on TVUS examinations (10% to 20%).89 Ultrasound-pathologic correlation in several studies has shown the rate of malignancy to be very low in such cysts.90,91,92 Follow-up ultrasound studies have shown that most of these cysts either resolve, decrease in size, or remain the same size.93 Ultrasound is also helpful in characterizing ovarian masses detected incidentally on CT or other imaging techniques.
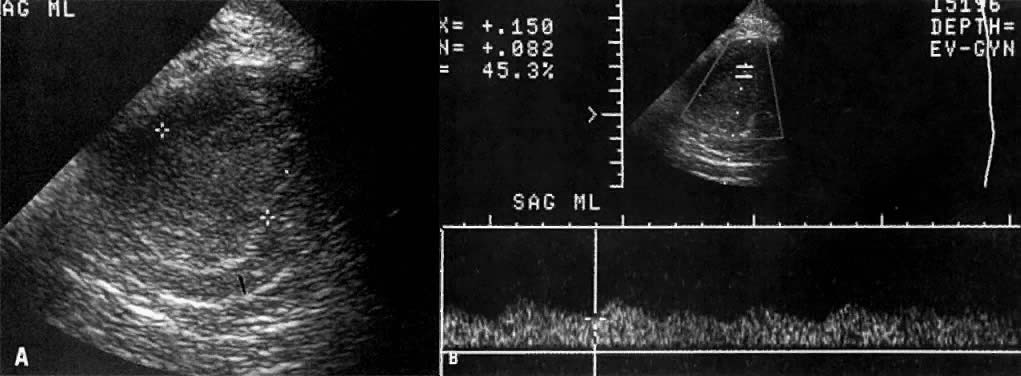
Spectral duplex and color Doppler, another new development in ultrasound, has been found to be helpful in differentiating benign from malignant tumors by demonstrating a neovascular flow pattern. Typically, because of arteriovenous shunting and the loss of muscular wall in the arterioles that supply these tumors, the resistance to blood flow in the peripheral circulation is decreased. This is depicted on spectral Doppler as a relative increase in diastolic flow causing a decrease in the resistive index to 0.4 or less (Fig. 28B).94,95 In benign tumors, this value would typically be greater than 0.5. Because there is considerable overlap in the values between benign and malignant tumors, this value cannot be used alone without morphologic criteria.96,97,98,99
MRI has been reported to be superior to CT in determining whether a pelvic mass is ovarian in origin; however, MRI lacks histologic specificity.2 MRI can aid in tumor characterization and in differentiating various benign lesions (e.g., teratomas, endometriomas) from malignant lesions.100,101 Gadolinium has improved the specificity of MRI for the detection of ovarian carcinoma.101 Thurnher and associates100 reported an accuracy of 92% in the ability of contrast-enhanced MRI to differentiate benign from malignant neoplasms. MRI characteristics of ovarian carcinoma include thickened wall, irregular margins, thickened irregular septa, endocystic vegetations, irregular solid portions, and increased tumor vessels (Fig. 32). There is no difference, however, between benign and malignant tumors in terms of signal intensity of vegetations. Enlarged, tortuous tumor vessels that are hypointense on both T1- and T2-weighted images can be seen in 33% of malignant tumors.102 These vessels are not seen in benign tumors or in those with low malignant potential.
CT plays a less important role in establishing the diagnosis of ovarian cancer. Cystic malignant tumors characteristically have irregular walls, thickened septa (greater than 3 mm), papillary projections, and solid portions that may have regions of necrosis on CT (Fig. 33).103 As ovarian masses increase in size, they may distort pelvic structures, making it difficult to determine the organ of origin (Fig. 34).104
Methods of Spread
Ovarian cancer most commonly spreads by the shedding of tumor cells from gross or microscopic excrescences on the surface of the primary tumor. This seeding leads to intraperitoneal dissemination13 involving the serosal surfaces of the uterus, bladder, bowel, cul-de-sac, diaphragm, and omentum. Less commonly, ovarian cancer spreads through lymphatic channels involving primarily the external, internal, and para-aortic lymph nodes.105,106 In advanced disease, distant metastases to the lung, pleura, bone, adrenal glands, and liver can occur.
Clinical Staging
The prognosis depends on the histologic subtype, tumor grade, and extent of disease. Preoperative staging of ovarian masses includes a history and physical examination to exclude other primary cancers, pelvic examination, Pap smear, complete blood count, blood chemistries, and chest radiography. Ovarian cancer is usually staged surgically via an exploratory laparotomy. The FIGO staging system is shown in Table 3.
TABLE 3. Staging System for Ovarian Cancer (FIGO 1986)
Stage | Characteristics |
IA | Limited to one ovary; no ascites; no tumor on the external surface, capsule intact |
IB | Limited to both ovaries; no ascites, no tumor on the external surface, capsule intact |
IC | Stage IA or stage IB, but with tumor on the surface of one or both ovaries, or with capsule ruptured, or |
| with ascites containing malignant cells |
IIA | Extension or metastases to the uterus or tubes |
IIB | Extension to other pelvic organs |
IIC | Stage IIA or stage IIB with tumor on the surface of one or both ovaries, or with capsule(s) ruptured, or |
| with ascites containing malignant cells |
IIIA | Tumor grossly limited to the true pelvis with negative nodes, but with histologically confirmed |
| microscopic seeding of abdominal peritoneal surfaces |
IIIB | Tumor of one or both ovaries; histologically confirmed implants of abdominal peritoneal surfaces, none |
| exceeding 2 cm in diameter; nodes negative |
IIIC | Abdominal implants >2 cm in diameter or positive retroperitoneal or inguinal nodes |
IV | Growth involving one or both ovaries with distant metastases; if pleural effusion is present, there must |
| be positive cytologic test results to assign a case to stage IV; parenchymal liver metastases equals |
| stageIV |
(Adapted from the International Federation of Gynecology and Obstetrics. Changes in definitions of clinical staging for carcinoma of the cervix and ovary. Am J Obstet Gynecol 156:263, 1987)
Local Radiologic Staging
Radiologic procedures, in general, play a limited role in the local staging of ovarian carcinoma.
LOCAL CT STAGING.
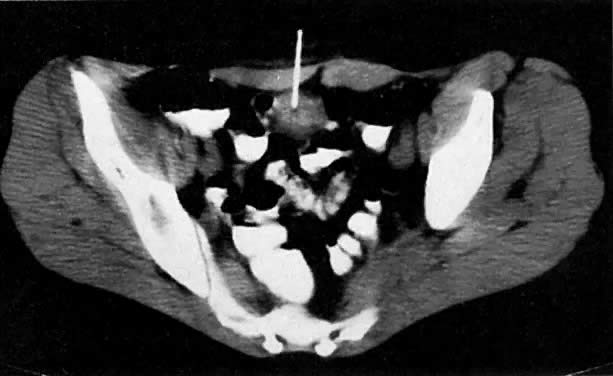
Normal adult ovaries are not necessarily seen on CT as separate structures from the adnexae, which are generally shown as symmetric soft-tissue densities on both sides of the uterus. It may also be difficult to distinguish the ovaries from adjacent bowel loops unless these are well opacified by contrast material. The detailed internal ovarian architecture seen on ultrasound or MRI is not usually seen on CT. Mere contiguity of ovarian masses to the uterus may be difficult to differentiate from uterine invasion (see Fig. 33 and Fig. 35A). CT is more useful in detecting ascites, pelvic or mesenteric masses, large peritoneal implants, nodal involvement, and liver metastases.
LOCAL MRI STAGING.
The role of MRI in the staging of ovarian carcinoma is unclear. MRI may play a more important role in detecting recurrence. An elevated serum CA125 may initiate an MRI examination. According to one report, MRI has been able to diagnose tumor in 60% of patients with recurrence, thus obviating the need for second-look laparotomy.107
LOCAL ULTRASOUND STAGING.
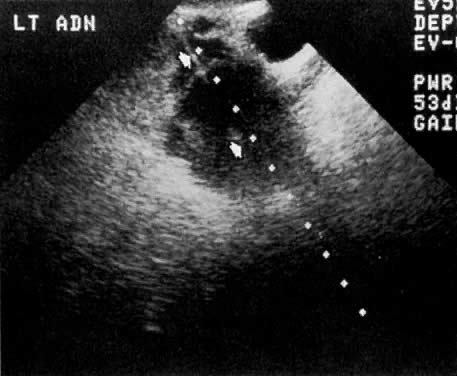
Ultrasound also plays a very limited role in ovarian tumor staging. The staging accuracy of ovarian neoplasms by ultrasound is poor (48%). Pelvic ultrasound examinations can show the size and local extent of the tumor, involvement of the contralateral ovary, uterine or bladder invasion, and local peritoneal spread. The presence of a uterus inseparable from an ovarian mass (Fig. 35B and Fig. 35C) correlated with uterine involvement in 74% of the cases, whereas the presence of a uterus separable from an ovarian mass correlated with a lack of uterine involvement in 93% of the cases.77
SPREAD TO BLADDER AND URETERS
Methods of Spread
The predominant method of local spread from cervical carcinoma is by direct extension to the distal ends of the ureters and the bladder base. Encasement of the ureters by metastases to the retroperitoneal and common iliac lymph nodes is also a common path of spread to the urinary system. Hydronephrosis along with deterioration of renal function consequently develops.
Griffin and co-workers108 found that 7.3% of their patients with carcinoma of the cervix demonstrated incomplete ureteral obstruction causing hydronephrosis or complete ureteral obstruction with nonvisualization of the kidney on EU. Van Nagell and co-workers109 reported that 2.8% of patients with stage I disease had obstructive uropathy demonstrated on EU. The incidence of ureteral obstruction was 32% in patients with stage III disease. Patients with ureteral obstruction also have an unfavorable prognosis, with a 5-year survival of 18%.
With anterior extension of carcinoma of the cervix or corpus, a bladder fistula can occur, caused by neoplastic invasion of the supporting tissues and shrinking of the tumor after treatment.
Radiologic Examinations and Findings
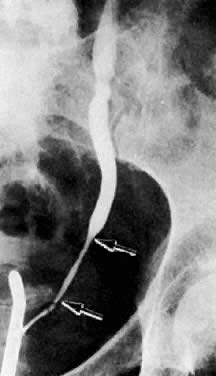
Previously, EU and retrograde pyelography have been the principal radiologic methods for examination of the urinary tract. As the tumor encases the ureter, a narrowed segment occurs (Fig. 36). The renal pelvis and calices and the ureter proximal to the involved area dilate (Fig. 37). With further involvement, the kidney ceases to function. Patients who have had a radical hysterectomy frequently have proximal ureterectasis to just below the pelvic brim. This radiologic finding lasts 6 to 12 months, and findings then gradually return to normal. The normal or narrowed distal ureters also are pulled medially secondary to the surgical procedure.
|
|
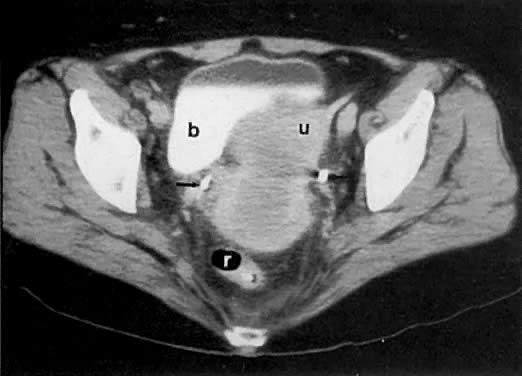
Currently ultrasound, CT, and MRI have replaced EU for the detection of urinary tract obstruction. Both CT and MRI can accurately demonstrate local tumor spread. CT has been shown to be superior to EU in the evaluation of the urinary system.24,110,111 Although the specificity of EU ranges from 79% to 97%, its sensitivity ranges from 7% to 33% in depicting the spread of malignant diseases.110 EU demonstrates the course and caliber of the ureters, but it is inaccurate in diagnosing nonobstructive extension of malignancy to the ureter. The superiority of CT is more noticeable in stage IIB cervical cancer and at more advanced stages.24 CT not only can diagnose ureteral obstruction, but also can detect its level and cause. In one study, ureteral involvement without hydronephrosis was detected in 21% of cases (see Fig. 11).9 CT can also demonstrate involvement of unopacified obstructed ureters (Fig. 38A and Fig. 38B).
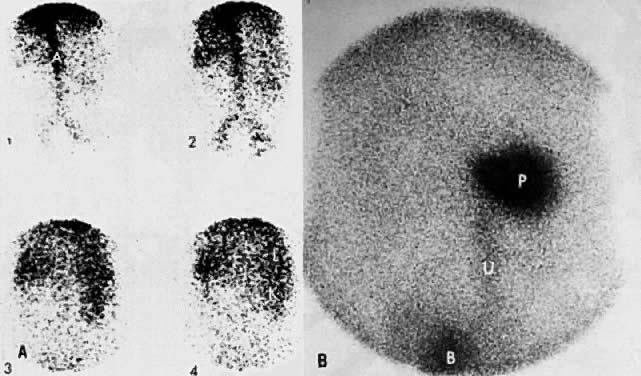
Ultrasound is the preferred method of screening for and assessing the degree of hydronephrosis (see Fig. 6C). It has an accuracy of 97%, a sensitivity of 90%, and a specificity of 98%.112 Although it is not as accurate as CT, it can sometimes show the level and cause of obstruction (see Fig. 6B). Renal and perirenal abnormalities can be accurately evaluated by ultrasound, CT, and MRI. 99mTc - diethylenetriamine-pentaacetic acid (DTPA) radionuclide imaging has also been used to complement other studies in the evaluation of renal perfusion, residual function, and presence and degree of urinary obstruction (Fig. 39).113
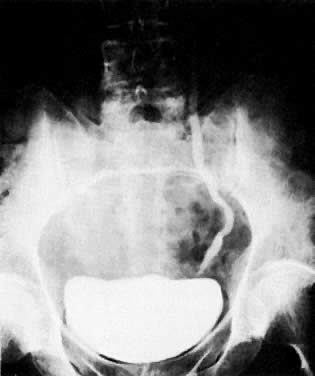
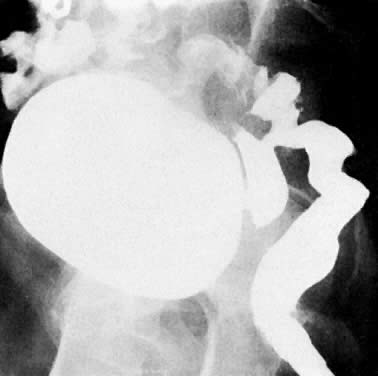
Air in the bladder seen on plain film examination (Fig. 40A) may indicate a vesicovaginal fistula. Placing a tampon in the vagina before a cystogram is an effective method of demonstrating this fistula (Fig. 40B). The contrast medium instilled into the bladder fills the tampon as it flows through the fistula, and an oblique projection shows the opacified vaginal tampon. Contrast medium absorbed by the tampon can also be seen after drainage of the bladder. A colon examination can demonstrate a vesicovaginorectal fistula. Barium introduced into the rectum fills the vaginal vault and urinary bladder (Fig. 41). MRI and ultrasound are more accurate in assessing bladder wall infiltration by tumor (see Fig. 17C, Fig. 18, Fig. 20, and Fig. 24A).
SPREAD TO BOWELS
Methods of Spread
Gynecologic tumors infrequently spread to the gastrointestinal tract. Patterns of bowel spread vary with the type of gynecologic cancer. Spread of cervical and uterine tumors occurs by direct extension to the rectosigmoid and to the small bowel, which becomes adherent to the uterus. Spread of ovarian tumors occurs predominantly by peritoneal seeding.
Radiologic Examinations and Findings
In patients presenting with symptoms of bowel obstruction, supine and upright or decubitus plain films are usually obtained. Dilated bowel loops, with multiple air-fluid levels, and lack of gas in the distal small bowel and/or colon are radiographic signs of bowel obstruction. Free intraperitoneal air in the absence of recent surgery suggests bowel perforation.
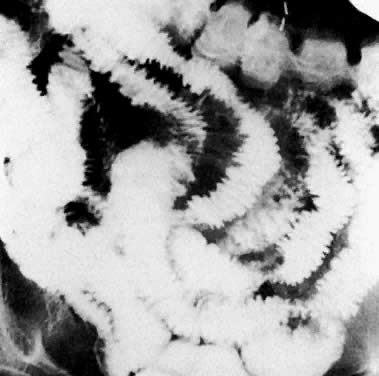
Patients with suspected mechanical bowel obstruction should be examined by a barium enema followed, if necessary, by small-bowel enteroclysis. Besides confirming the presence and site of obstruction (Fig. 42), these studies may show bowel loop displacement (Fig. 43) or may sometimes identify the cause of the obstruction, such as metastatic deposits to the bowel wall (Fig. 44).
|
|
|
More recently, CT with opacification of the bowel by diatrizoate sodium diluted to 2% or barium sulfate diluted to 2% was shown to be capable of establishing the presence or absence of small-bowel obstruction. In addition, in most patients, CT has the benefit of determining the cause of obstruction and the presence or absence of metastatic tumor to the small-bowel serosa and mesentery (see Fig. 24D).114,115 Both MRI (see Fig. 19) and CT (see Fig. 24C) may be helpful in detecting local spread to the rectum.
SPREAD TO LIVER
Methods of Spread
Tumor spread to the liver from gynecologic malignancies is not common. It occurs predominantly by peritoneal seeding, and rarely by hematogenous metastases.
Radiologic Examinations and Findings
CT is a reasonably sensitive and specific modality for the detection of liver metastases.116 Most metastases are less dense than the normal liver parenchyma and are relatively well demarcated (Fig. 45A). Occasionally, however, the margins will merge imperceptibly with the liver parenchyma. Cystic metastases are seen with rapidly growing tumors, such as leiomyosarcoma. Peritoneal seeding on the liver capsule from ovarian neoplasms gives a characteristic scalloped appearance to the liver margin. Punctate or homogeneous calcifications can be seen in metastatic mucinous cystadenocarcinoma. New CT techniques, including spiral CT and CT angiography, are able to detect most hepatic metastases. The latter involves the introduction of contrast material directly into the superior mesenteric artery and allows improved contrast opacification of the liver and detection of very small metastatic deposits.
Ultrasound is also fairly accurate in detecting liver metastases. Both ultrasound and MRI are predominantly used for further characterization of lesions seen on CT. Ultrasound can readily differentiate true cysts and hemangiomas from solid and cystic metastases (Fig. 46). The introduction of color and spectral Doppler has increased the specificity of ultrasound in differentiating benign from malignant solid liver tumors in some cases. In malignant tumors in general, spectral Doppler shows an increase in diastolic flow and, to a lesser extent, in systolic flow, resulting in a decrease in the resistive index to 0.4 or less. Benign tumors, however, show a higher resistive index because of the absence of the characteristic neovascular flow pattern. However, because there is considerable overlap in the Doppler findings, this modality cannot be relied on by itself for the differentiation of benign from malignant tumors.
|
The use of different pulse sequences and gadolinium enhancement has further improved characterization of liver lesions by MRI. However, because of its high cost, it should be reserved for cases with uncharacteristic ultrasound findings.
SPREAD TO SKELETON
Methods of Spread
In autopsy studies by Henriksen117 and Bergman,118 metastatic involvement of bone occurred in 16% of patients with cervical carcinoma, in 7.6% of those with endometrial carcinoma, and in 13.9% of those with ovarian carcinoma (Table 4). However, the incidence of metastases at the time of presentation was low (4%).119 The spine was the most common area of involvement, followed by the pelvic bones. Distant bony metastases were uncommon, and most of the bones had been involved by direct extension. The lesions were usually lytic.
TABLE 4. Distant Metastases from Female Genital Cancer (%)
| Cervix |
| ||
Site | Untreated | Treated | Uterus† | Ovary‡ |
Lung | 13.9 | 14.0 | 30.2 | 37.2 |
Pleura | 2.4 | 4.9 | 7.9 | 37.2 (Right) |
|
|
|
| 29.0 (Left) |
Liver | 24.5 | 16.4 | 28.5 | 33.7 |
Vertebrae | 8.1 | 8.2 |
|
|
Sigmoid | 6.5 |
|
|
|
Large bowel |
| 7.2 | 9.5 |
|
Brain | 0.8 |
|
|
|
Supraclavicular nodes | 1.6 | 0.6 |
|
|
Bones* | 8.0 | 6.0 |
| 13.9 |
Adrenals | 1.6 | 1.8 | 7.9 | 8.1 |
Spleen | 1.6 | 1.2 | 3.1 |
|
Kidney | 1.6 | 3.0 | 3.1 | 7.0 |
Peritoneum |
|
| 27.0 | 87.2 |
Ovary |
|
| 14.2 | 70.9 |
Skin | 0.8 | 1.2 | 3.1 |
|
Omentum |
|
|
| 70.9 |
Uterus |
|
|
| 18.6 |
*Total excluding vertebrae.
(Data from †Henriksen E: The lymphatic spread of carcinoma of the cervix and of the body of the uterus. Am J Obstet Gynecol 58:924, 1949; and ‡Bergman F: Carcinoma of the ovary. Acta Obstet Gynecol Scand 45:211, 1966)
Radiologic Examinations and Findings
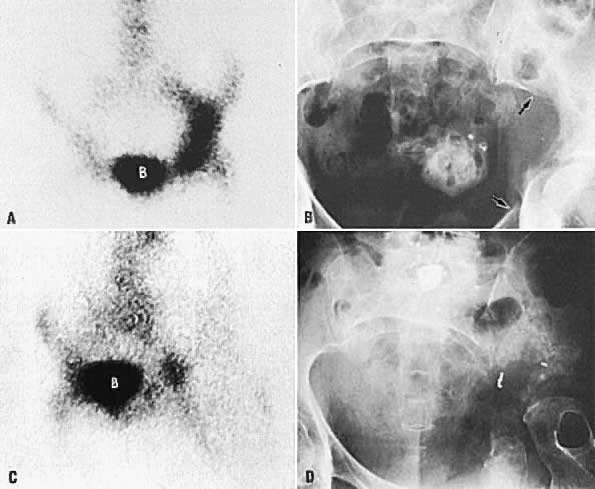
Bone scintigraphy and radiographic skeletal bone survey are the standard imaging procedures for skeletal metastases. Neither imaging modality is indicated in asymptomatic patients. In patients with bone pain, bone scintigraphy should be obtained first in order to identify areas of metastases. Bone radiography is obtained for correlation with positive bone scans. Currently, bone scintigraphy is more sensitive than skeletal survey for detecting early skeletal metastasis.120 The abnormal bone is usually visualized as areas of increased radiotracer activity (Fig. 47A and Fig. 47B); however, in a few cases, the abnormality is imaged as an area of decreased activity (Fig. 47C and Fig. 47D).121 The radiotracer uptake depends on the amount of new osteoid tissue formation. Although CT and MRI are not primary screening techniques for bone metastases, they can detect tumor spread to bone in the pelvis or spine when used for staging of gynecologic malignancies (Fig. 48).
SPREAD TO LYMPH NODES
Methods of Spread
Cancer of the female genital organs frequently spreads via lymphatics to the pelvic (obturator and internal, external, and common iliac) and retroperitoneal lymph nodes.
Radiologic Examinations and Findings
Both CT and MRI have replaced lymphography in detecting tumor spread to the lymphatic system. CT has a reported accuracy of 83% and a specificity of 86% in detecting metastases to the para-aortic lymph nodes.122 Although CT is useful in detecting small lymph nodes within the pelvis, it is slightly less accurate (70% to 80%) in detecting metastases to pelvic lymph nodes compared to para-aortic lymph nodes, because of the sparsity of fat around the former.25
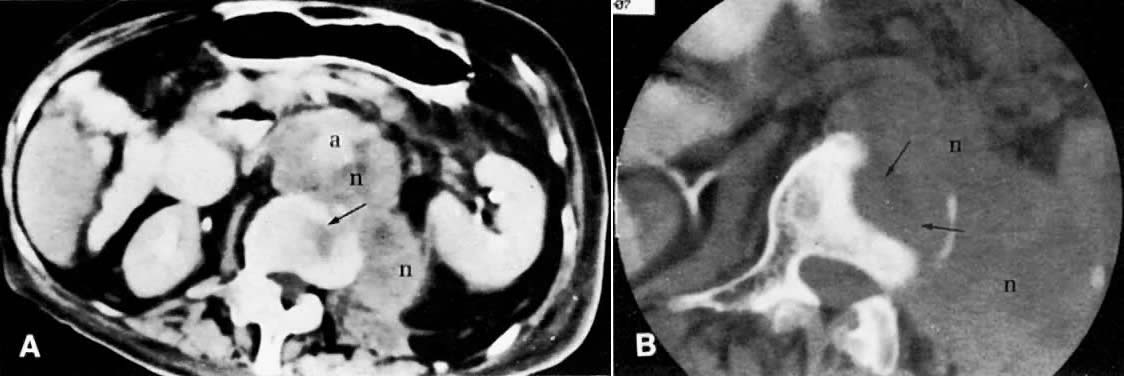
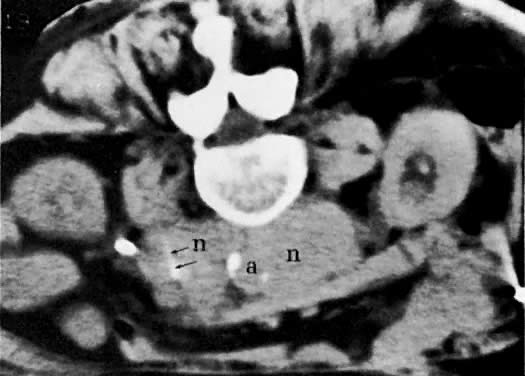
The diagnosis of lymph node metastases by CT depends predominantly on the increase in their size, but occasionally there is also a change in density (see Fig. 45B and Fig. 45C). Normal lymph nodes measure less than 1 cm in diameter. An isolated para-aortic or iliac lymph node measuring 1 to 1.5 cm should be considered suspicious (see Fig. 45C), and a clustering of small lymph nodes should be considered abnormal (see Fig. 38C). Lymph nodes larger than 1.5 cm in diameter also should be considered abnormal (see Fig. 45B).123 Metastatic lymphadenopathy is occasionally visualized as a conglomerate group of contiguous enlarged lymph nodes similar in size to the aorta or inferior vena cava, or as a large, homogeneous mass (see Fig. 48A).124 A false-negative diagnosis by CT is caused by the technique's inability to diagnose metastases in normal-sized lymph nodes. Because metastases from cervical carcinoma often replace part of a lymph node without enlarging it, the reported false-negative rate has been as high as 40%.25 A false-positive diagnosis results from the inability of CT to differentiate enlarged nodes caused by hyperplasia from those caused by metastases. CT-guided percutaneous fine-needle aspiration biopsy of the enlarged lymph nodes may be required (Fig. 49). Extension of tumor into lymph node groups not opacified by lymphography (e.g., mesenteric lymph nodes) can also be detected by CT, and biopsy specimens of these nodes can be taken during CT scanning.
|
MRI is also helpful in identifying metastatic spread to lymph nodes and has an accuracy rate similar to that of CT (78% for MRI vs 77% for CT).125,126 As with CT, MRI is able to rely only on the size of the lymph nodes to determine the presence or absence of a tumor. A variety of particulate contrast materials are now being tested with MRI to detect lymph nodes that are only partially involved by tumor. Unfortunately, these agents currently are only experimental and are not available for routine use. More recently, Kim and colleagues127 found the accuracy of MRI to be fairly high in detecting pelvic lymph node metastases from cervical carcinoma. By using a minimum axial diameter of 1 cm, they were able to achieve an accuracy of 93%, a sensitivity of 62.2%, and a specificity of 97.9%. Biopsy is difficult using MRI as a guidance technique. In most patients, CT is a satisfactory means of detecting the presence or absence of involved lymph nodes and is used for guiding biopsy of those nodes suspicious for tumor involvement.
Metastases can be detected by ultrasound when the nodes are larger than 2 cm in diameter (Fig. 50). Ultrasound is less accurate in detecting metastases to the pelvic and para-aortic lymph nodes. In a study of 32 patients with malignant ovarian tumors, Requard and colleagues77 found ultrasound to be 25% accurate in detecting lymph node metastases.
SPREAD TO PERITONEUM
Methods of Spread
Seeding of the peritoneum and bowel serosa from gynecologic malignancies occurs by direct spread. It is frequent in ovarian carcinomas, uterine sarcomas, and more advanced cervical and endometrial carcinomas.
Radiologic Examinations and Findings
Bowel obstruction (see Fig. 42 and Fig. 44), ascites, and deviation of the course of the bowel (see Fig. 43) are common radiologic findings with metastatic involvement of the bowel and peritoneum. CT is the method of choice for demonstrating tumor extension to the mesentery, omentum, peritoneum, and bowel when the lesions are greater than 2 cm in diameter. High-resolution CT can detect 50% of peritoneal implants 5 mm in size.128 CT can detect psammomatous calcification and plaque-like peritoneal metastases from papillary cystadenocarcinoma of the ovary.129,130 Despite the ability of CT to detect extrapelvic extent of tumor, 40% to 45% of patients with a negative CT are later found to have disease at exploratory laparotomy.131,132 In patients with leiomyosarcomas and high-grade endometrial carcinomas, CT is capable of detecting unsuspected metastases to the peritoneum and omentum that are greater than 2 cm (see Fig. 38A and Fig. 38B). Ascites can also be easily detected by this technique.
Abdominal ascites, which can be associated with peritoneal spread, is also detected with high accuracy by MRI. MRI is less sensitive than CT in detecting small serosal and mesenteric implants because of motion artifact.2
Metastases to the mesentery and omentum can also be identified by ultrasound if larger than 2 cm. In the presence of ascites, ultrasound may be able to detect smaller peritoneal implants (Fig. 51). In their study of 32 patients with malignant ovarian tumors, Requard and colleagues77 found ultrasound to be 20% accurate in detecting omental and peritoneal metastases. As with CT, the “omental cake” typical of omental metastases can also be demonstrated by ultrasound (Fig. 52).
SPREAD TO CHEST
Methods of Spread
Metastatic spread to the chest from gynecologic malignancies is uncommon and occurs predominantly by the hematogenous route. Lymphatic spread is rare.133,134,135 At autopsy, metastatic pulmonary lesions were found in 27.9% of patients with cervical carcinoma,117 30.2% with endometrial carcinoma,117 and 37.2% with ovarian carcinoma (see Table 4).118 In pretreatment chest radiographs, however, the incidence of chest metastases was found to be less than 1% in two separate studies.108,136 Ovarian carcinoma can spread via the peritoneal cavity through the diaphragm into the pleural cavity, causing malignant pleural deposits and effusions.
Radiologic Examinations and Findings
The posteroanterior and lateral chest radiographic examination is a standard procedure in staging gynecologic malignancies. Decubitus views are performed to confirm the presence of free pleural effusion. The side with the suspected effusion is placed in the dependent position; if free fluid is present, it will layer along the lateral chest wall.
Chest CT is a more sensitive and accurate test that can be used to confirm the presence of lesions and to differentiate benign from malignant ones. CT better visualizes lesions in the apices of the lungs, costophrenic sinuses, cardiophrenic angles, mediastinum, and supradiaphragmatic areas. In some cases, pleural-based lesions (Fig. 53) and loss of rib detail are also better demonstrated by CT.137 CT can identify diffuse calcification by assigning a higher number to the nodule. Unfortunately, CT cannot differentiate benign from malignant nodules when they are not calcified. Recent advances in CT technology, including spiral CT, have been used to reconstruct three-dimensional images of the chest and detect tumor spread to the pleura. In addition, spiral CT probably has a higher rate of detection of metastatic nodules than conventional CT, because of the lack of respiratory motion artifacts. CT-guided fine-needle percutaneous aspiration biopsy is used to differentiate benign from malignant chest nodules with a high degree of accuracy.
|
Hematogenous metastases have a nodular appearance and usually occur in the lower lung fields because of their preferential blood flow in the erect posture (Fig. 54 and Fig. 55). Occasionally, metastatic lesions from squamous cell carcinoma cavitate (Fig. 56). Solitary lesions in the upper lobes are unlikely to be metastases.138 The presence of central nodular, concentric, or diffuse popcorn calcifications in a nodule denotes benignity.
|
|
Lymphangitic spread of gynecologic tumors to the chest appears radiographically as Kerley's A-lines, which radiate from the pulmonary hilum; and/or as Kerley's B-lines, which are seen as short (20 mm long), thin (1 mm thick), peripheral basilar lines perpendicular to the pleural surface.
TREATMENT PLANNING
Radiologic procedures play a significant role not only in diagnosing gynecologic malignancies, but also in planning their treatment. In cervical and endometrial cancer, plain radiographs, ultrasound, and CT are commonly used for planning radiation therapy. The delineation of radiation treatment fields is generally performed under fluoroscopic guidance. Radiopaque stainless steel markers are placed into the cervix, and rectal contrast material, with or without a small-bowel follow-through study, is used to identify the cervix and radiosensitive normal structures, such as the small- and large-bowel loops that may be partially excluded from the radiation field.139 With the use of bipedal lymphangiograms, radiation fields to cover the pelvic and para-aortic lymph nodes can be individually designed based on the involved nodal areas.139 More recently, CT examinations have been used to delineate soft tissues of the pelvis and abdomen for radiation treatment planning.
In cervical and endometrial cancer, intraoperative ultrasound is a valuable tool in guiding the placement of an intrauterine tandem for intracavitary irradiation. Dilation of the tumor-involved cervix or uterus is frequently difficult because of anatomic distortion by the tumor, and the cervical os is often difficult to identify. Intraoperative ultrasound can provide information on the position of the uterus and the endocervical canal, thus helping to avoid uterine perforation. Plain orthogonal isocentric radiographs with rectal and bladder contrast material are used in radioactive implants for the purpose of radiation-dose computations.
More recently, MRI was found to provide better depiction of tumor volume and better delineation of extent of tumor involvement compared to physical or CT examinations.31 The information provided by MRI was found to be helpful in the placement of radiation ports, especially when small radiation boosts were used (Fig. 57).
|
For whole-abdomen irradiation in ovarian cancer, plain anteroposterior radiographs are obtained to plan the radiation fields. Intravenously administered contrast material is used to delineate the kidneys for the purpose of shielding in order to reduce the radiation dose to these organs.140 CT is also frequently used to localize the kidneys, spinal cord, and cauda equina in order to limit the radiation dose to these radiosensitive structures.
Before intraperitoneal instillation of radioactive phosphorus (32P) for the treatment of ovarian cancer, plain anteroposterior and lateral abdominal radiographs are obtained after intraperitoneal instillation of water-soluble contrast media to demonstrate adequate intra-abdominal distribution. Alternatively, scintigraphy after instillation of 99mTc sulfur colloid is also used.141
MONITORING TUMOR TREATMENT
Physical examination after radiation treatment may be limited. Radiologic procedures are helpful in monitoring the size of the tumor in patients undergoing radiation or chemotherapy. MRI (see Fig. 4A and Fig. 4B) and CT can be used to monitor tumor response to treatment and to assess tumor recurrence. It may be difficult, however, to differentiate inflammatory changes and fibrosis caused by surgery or radiation from tumor recurrence with CT. MRI may help differentiate radiation fibrosis from recurrent tumor with fair accuracy 6 to 12 months after treatment.142,143 Early postirradiation and postsurgical fibrosis and inflammatory changes may be difficult to differentiate from tumor because of the similarity of their signal intensity on T2-weighted MRIs. Overall accuracy of MRI for detecting recurrence from cervical cancer was 78%, but it improved to 88% more than 6 months after irradiation compared with 69% 6 months or less after irradiation. Ultrasound cannot replace a second-look laparotomy in patients with ovarian carcinoma because it cannot differentiate patients without residual disease from those with microscopic disease or disease less than 2 cm.144 Ultrasound is helpful in the evaluation of recurrent cancer by screening for hydronephrosis. More recently, TVUS was found to be superior to CT in detecting recurrent pelvic tumors, with a sensitivity of 100% and a specificity of 91%.145 Masses detected at follow-up pelvic examination can also be evaluated by TVUS for tissue characterization. Although ultrasound is helpful in differentiating postsurgical scarring from recurrent tumor,38,39 CT- or ultrasound-guided fine-needle aspiration biopsy is often necessary for confirming the diagnosis.146
TREATMENT COMPLICATIONS
Pulmonary Complications
Chemotherapeutic agents used in the treatment of gynecologic malignancies may cause diffuse interstitial or reticulonodular pulmonary infiltrates that could mimic lymphangitic metastases. Bleomycin, methotrexate, and cyclophosphamide are three chemotherapeutic agents used in gynecologic cancer that cause this complication. The chest radiographic examination is helpful for the detection of lymphangitic spread. Lung biopsy may occasionally be necessary to differentiate metastatic tumor from iatrogenic disease.147
Urinary Complications
UTERER.
Slater and Fletcher148 reviewed 1749 patients treated with radiation therapy for carcinoma of the cervix. A total of 194 patients had strictures before treatment and were therefore excluded, yielding 1555 patients. Of 1416 patients with radiographically proven normal ureters and 139 without initial urograms but presumed normal, 134 had ureteral obstructions after therapy, of whom 108 had recurrent disease. Of the 26 without evidence of recurrent disease, 16 had surgical procedures after the irradiation that may have contributed to fibrosis around the ureter, 5 had fibrosis at the site of the tumor, and 5 had narrowing attributable solely to radiation. This group of five patients in whom ureteral narrowing developed constituted 0.3% of the total 1555 patients. However, transient ureterectasis caused by ureteral edema sometimes can be seen 3 weeks after radiation therapy. Radiographically, these acute changes appear as areas of ureteral narrowing and medial deviation of the ureters.149
BLADDER.
Acute radiation cystitis complicating pelvic irradiation or chemotherapy can occur after 4 months. Loss of epithelium and ulceration are chronic changes that may occur 1 to 20 years after irradiation. On cystography, the chronically damaged bladder appears small, with a thick wall. Ureteral reflux and elevation of the bladder floor may also be present. With severe ulceration, the bladder wall may rupture and develop a fistula to adjacent organs (see Fig. 41). Rarely, the damaged bladder wall contains dystrophic calcification. The use of cyclophosphamide can also result in hemorrhagic cystitis. The bleeding can be life threatening, and blood clots frequently fill the bladder (Fig. 58).150
|
Bowel Complications
Radiation damage of the bowel occurs most commonly in the terminal ileum, sigmoid colon, and rectum. The rectosigmoid tolerates higher doses of radiation than the small bowel. With external-beam radiation doses of 6500 cGy to the rectosigmoid, the incidence of severe proctitis is 2% and the incidence of severe rectal strictures is 0.5%.151 The incidence of injury (perforation, obstruction, and fibrosis) to the small bowel or gastric mucosa is 1% to 5% with radiation doses of 4500 to 5000 cGy. The risk of injury also depends on the volume of small bowel irradiated and the energy of the megavoltage beam. Previous abdominal surgery, pelvic inflammatory disease, adhesions, and tumors that cause fixation of the bowel are predisposing factors to radiation injury. Certain diseases, such as hypertension, diabetes mellitus, and collagen vascular disease, cause arterial circulatory problems and obliterative endarteritis, which may also increase the likelihood of radiation injury.
Schmitz and associates152 reviewed 1200 patients treated with external irradiation for either cervical carcinoma or endometrial carcinoma and found 37 patients with radiation injury to the bowel: 14 to the terminal ileum, 3 to the sigmoid colon, and 20 to the rectum. Of the 37 patients, 29 had cervical carcinoma and 8 had endometrial carcinoma.
Edema, ulceration of the mucosa, and damage to the vasculoconnective tissue of the submucosa are the principal forms of bowel injury. When healing occurs, atrophy, cicatrization, and stenosis of the bowel result from obliterative endarteritis.
The radiographic appearance of radiation injury to the bowel is similar to that of ischemia. Involved areas are rigid, and the valvulae conniventes are straightened and thickened (Fig. 59); the usual pattern of the ileum, therefore, becomes irregular and pointed. “Thumbprinting” and nodular filling defects may also be seen, owing to focal areas of edema.
The sequelae of radiation injury to the small bowel are obstruction resulting from the narrowing of the bowel segments and perforation with peritonitis and fistula formation. The radiographic appearance of radiation injury must be differentiated from that of regional enteritis, lymphoma, and recurrent tumor. CT can be helpful in the differentiation. Rectal fistula formation and loss of colon haustra with smooth narrowing are some of the sequelae of radiation injury (Fig. 60). Gastric or duodenal ulcers caused by radiation therapy of the upper abdomen can occur 2 months to 6 years after treatment (Fig. 61).153 Small- and large-bowel changes can occur as much as 20 years afterward.
Skeletal Complications
Atrophic bony changes can be seen radiographically after doses of 4500 to 5200 cGy.154 Osteoradionecrosis, however, is uncommon and usually occurs only after much higher doses or in patients with preexisting osteoporosis. This is mainly caused by the use of megavoltage radiation and multiple field techniques. The first bony changes, patchy osteoporosis followed by thickened bony trabeculae, are seen 6 to 12 months after treatment. Lack of bone expansion and lack of cortical thickening differentiate radiation injury from Paget's disease (Fig. 62A and Fig. 62B). Lytic areas can be confused with pathologic fractures; however, postirradiation fractures occur 2 to 3 years after treatment and only within the radiation field. Areas of stress (e.g., ribs, clavicle, hips) are especially prone to postirradiation fractures. Fracture of the femoral neck occurs in 2% of patients irradiated for carcinoma of the cervix. Hall and co-workers155 reported a case of bilateral protrusio acetabuli after the administration of 3800 cGY for treatment of a “poorly differentiated adenocarcinoma of probable ovarian origin.” Radiation changes are seen as areas of increased signal intensity in the spine or muscles on MRI because of the edema and bone marrow replacement by fat (see Fig. 17A and D).
Postoperative Fluid Collections
Lymphoceles, abscesses, urinomas, and hematomas are not uncommon complications of surgical therapy for invasive gynecologic cancer. Disruption of the lymphatic vessels during radical hysterectomy and pelvic lymphadenectomy leads to formation of lymphoceles (Fig. 63). Ultrasound or CT can evaluate these and other postoperative complications, such as abscesses, urinomas, or hematomas. It is difficult to differentiate among the various types of fluid collections without ultrasound- or CT-guided aspiration.
RADIOLOGY-GUIDED INTERVENTIONAL PROCEDURES
Percutaneous Fine-Needle Aspiration of Masses and Collections
Percutaneous fine-needle aspiration biopsy procedures for suspicious mass lesions can be performed under ultrasound and CT guidance. These procedures can differentiate benign from malignant lesions, seen by either modality, with a high degree of accuracy. They are performed under local anesthesia, with or without sedation, using a 22- to 25-gauge needle. More than one pass may be necessary to obtain an adequate sample for cytologic analysis. Complications associated with these procedures are usually minor and uncommon. They include hemorrhage, infection, needle-track tumor seeding, and pneumothorax. Cooperation between the radiology and cytology teams is necessary to ensure a successful outcome. These procedures include CT-guided aspiration of chest masses; CT- or ultrasound-guided fine-needle aspiration of hepatic, retroperitoneal (see Fig. 49), abdominal (Fig. 64), and pelvic masses; TVUS-guided biopsies of pelvic masses (Fig. 65); and ultrasound-guided thoracentesis and paracentesis.
|
|
Percutaneous Nephrostomy/Ureteral Stent Placement
Percutaneous nephrostomy and/or ureteral stent placement procedures are performed in patients with ureteral obstruction to provide drainage for obstructed kidneys.156 These procedures are performed under a combination of ultrasound and fluoroscopic guidance using local anesthesia and sedation. For the initial needle placement, ultrasound guidance may be used. Then, under fluoroscopic guidance, a guidewire is introduced through the needle, which is then exchanged with dilators of increasing diameters, followed by placement of a 9-French catheter within the renal pelvis or a variable-caliber stent to bypass the obstruction. Contrast medium injected through the nephrostomy tube can help demonstrate the level and cause of obstruction.
Percutaneous Drainage of Abscesses and Other Fluid Collections
CT-, ultrasound-, and/or fluoroscope-guided percutaneous or transvaginal procedures can be performed to drain postoperative fluid collections, such as urinomas, abscesses, or lymphoceles. Abscess drainage is usually accomplished using a large catheter, which is left in place for at least 10 to 12 days, depending on the patient's clinical condition. It is much more difficult to treat lymphoceles with percutaneous drainage techniques. The lymphocele must be completely aspirated and maintained on negative suction until total obliteration of the cavity is achieved. Long-term drainage is usually necessary for complete resolution of lymphoceles. Recently, drainage of fluid collections using the transvaginal route and ultrasound guidance was found to be very helpful (Fig. 66).
|
We have included portions of the previous (1987) chapter and would like to acknowledge Dr. R.C. Brown, the co-author of that chapter, for his contribution.
REFERENCES
Parker SL, Tong T, Bolden S, Wungo PA: Cancer statistics, 1996. CA 65: 5, 1996 |
|
Scoutt LM, McCarthy SM: Application of magnetic resonance imaging to gynecology. Top Magn Reson Imaging 2: 37, 1990 |
|
Scoutt LM, McCauley TR, Flynn SD et al: Zonal anatomy of the cervix: Correlation of MR imaging and histologic examination of hysterectomy specimens. Radiology 186: 159, 1993 |
|
Hricak H: MRI of the female pelvis: A review. AJR 146: 1115, 1986 |
|
Holland GA, Ludmin J, Holland LD et al: Evaluation of the uterine cervix by MR imaging in the non pregnant, pregnant, and post partum state (abstr). Proc Soc Magn Reson Med 1: 341, 1993 |
|
Olson MC, Posniak HV, Tempany CM, Dudiak CM: MR imaging of the female pelvic region. Radiographics 12: 445, 1992 |
|
Togashi K, Nishimura K, Itoh K et al: Uterine cervical cancer: Assessment with high-field MR imaging. Radiology 160: 431, 1986 |
|
Walsh JW, Vick CW: Staging of female genital tract cancer. In Walsh JW (ed): Contemporary Issues in Computed Tomography. Edinburgh, Churchill Livingstone, 1985 |
|
Kim SH, Choi BI, Lee HP et al: Uterine cervical carcinoma: Comparison of CT and MR findings. Radiology 175: 45, 1990 |
|
Cobby M, Browning J, Jones A et al: Magnetic resonance imaging, computed tomography, and endosonography in the local staging of carcinoma of the cervix. Br J Radiol 63: 673, 1990 |
|
Scott WW Jr, Rosenshein NB, Siegelman SS, Sanders RC: The obstructed uterus. Radiology 141: 767, 1981 |
|
Yamashita Y, Takahashi M, Sawada T et al: Carcinoma of the cervix: Dynamic MR imaging. Radiology 182: 643, 1992 |
|
Plenti AA, Friedman EA: The Morphologic Basis of Oncologic Diagnosis and Therapy, p 75. Philadelphia, WB Saunders, 1971 |
|
Carlson V, Delclos L, Fletcher GH: Distant metastases in squamous-cell carcinoma of the uterine cervix. Radiology 88: 961, 1967 |
|
Changes in definitions of clinical staging for carcinoma of the cervix and ovary: International Federation of Gynecology and Obstetrics. Am J Gynecol 156:263, 1987 |
|
Kovalic JJ, Perez CA, Grigsby PW, Lockett MA: The effect of volume of disease in patients with carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys 21: 905, 1991 |
|
Lanciano RM, Won M, Hanks GE: A reappraisal of the International Federation of Gynecology and Obstetrics staging system for cervical cancer: A study of patterns of care. Cancer 69: 482, 1992 |
|
van Nagell JR, Roddick JW, Lowin DM: The staging of cervical cancer: Inevitable discrepancies between clinical staging and pathologic findings. Am J Obstet Gynecol 110: 973, 1971 |
|
Averette HE, Ford JH, Dudan RC et al: Staging of cervical cancer. Clin Obstet Gynecol 18: 215, 1975 |
|
Brunschwig A: The surgical treatment of cancer of the cervix: Stage I and II. AJR 102: 147, 1968 |
|
Heyman J, Kottmeier H-L, Segerdahl C-O: An investigation of the reliability of stage grouping in cancer of the uterine cervix. Acta Obstet Gynecol Scand 32: 65, 1953 |
|
Vick CW, Walsh JW, Wheelock JB, Brewer WH: CT of the normal and abnormal parametria in cervical cancer. AJR 143: 597, 1984 |
|
Ascher SM, Silverman PM: Applications of computed tomography in gynecologic diseases. Urol Radiol 13: 16, 1991 |
|
Goldman SM, Fishman EK, Rosenshein NB et al: Excretory urography and computed tomography in the initial evaluation of patients with cervical cancer: Are both examinations necessary? AJR 143: 991, 1984 |
|
Lee JKT, Balfe DM: Pelvis. In Lee JKT, Sagel SS, Stanley RJ (eds): Computed Body Tomography, p 257. New York, Raven Press, 1983 |
|
Hricak H, Lacey CG, Sandles LG et al: Invasive cervical carcinoma: Comparison of MR imaging and surgical findings. Radiology 166: 623, 1988 |
|
Togashi K, Nishimura K, Sagoh T et al: Carcinoma of the cervix: Staging with MR imaging. Radiology 171: 245, 1989 |
|
Greco A, Mason P, Leung AWL et al: Staging of carcinoma of the uterine cervix: MRI-surgical correlation. Clin Radiol 40: 401, 1989 |
|
Hricak H, Quivey JM, Campos Z et al: Carcinoma of the cervix; predictive value of clinical and magnetic resonance (MR) imaging assessment of prognostic factors. Int J Radiat Oncol Biol Phys 27: 791, 1993 |
|
Sironi S, Belloni C, Taccagni GL, Del Maschio A: Carcinoma of the cervix: Value of MR imaging in detecting parametrial involvement. AJR 156: 753, 1991 |
|
Mayr NA, Tali ET, Yuh WTC et al: Cervical cancer: Application of MR imaging in radiation therapy. Radiology 189: 601, 1993 |
|
Burghardt E, Hofmann HMH, Ebner F et al: Magnetic resonance imaging in cervical cancer: A basis for objective classification. Gynecol Oncol 33: 61, 1989 |
|
Sironi S, De Cobelli F, Scarfone G et al: Carcinoma of the cervix: Value of plain and gadolinium-enhanced MR imaging in assessing degree of invasiveness. Radiology 188: 797, 1993 |
|
Waggenspack GA, Amparo EG, Hannigan EV, O'Neal MF: MRI of cervical carcinoma. Semin Ultrasound CT MR 9: 158, 1988 |
|
Hricak H, Hamm B, Semelka RC et al: Carcinoma of the uterus: Use of gadopentetate dimeglumine in MR imaging. Radiology 181: 95, 1991 |
|
Mitchell DG, Vinitski S, Burk DL Jr et al: Variable-flip-angle spin-echo MR imaging of the pelvis: More versatile T2-weighted images. Radiology 171: 525, 1989 |
|
Milestone BN, Schnall MD, Lenkinski RE, Kressel HY: Cervical carcinoma: MR imaging with an endorectal surface coil. Radiology 180: 91, 1991 |
|
Gottesfeld KR: The role of ultrasound in gynecologic diagnosis. In Rosenfield AT (ed): Genitourinary Ultrasonography, p 207. New York, Churchill Livingstone, 1979 |
|
Brascho DJ: Detecting and assessing cancer with ultrasound. Contemp Obstet Gynecol 14: 21, 1979 |
|
Innocenti P, Pulli F, Savino L et al: Staging of cervical cancer: Reliability of transrectal US. Radiology 185: 201, 1992 |
|
Walsh JW, Rosenfield AT, Jaffe CC et al: Prospective comparison of ultrasound and computed tomography in the evaluation of gynecologic pelvic masses. AJR 131: 955, 1978 |
|
Rose PG, Piver MS, Tsukada Y, Lau T: Patterns of metastasis in uterine sarcoma: An autopsy study. Cancer 63: 935, 1989 |
|
Doss LL, Llorens AS, Henriquez EM: Carcinosarcoma of the uterus: A 40-year experience from the state of Missouri. Gynecol Oncol 18: 43, 1984 |
|
McCarthy SM, Tauber C, Gore J: Female pelvic anatomy: MR assessment of variations during the menstrual cycle and with the use of oral contraceptives. Radiology 160: 119, 1986 |
|
Haynor DR, Mack LA, Soules MR et al: Changing appearance of the normal uterus during the menstrual cycle: MR studies. Radiology 161: 459, 1986 |
|
Demas BE, Hricak H, Jaffe RB: Uterine MR imaging: Effects on hormonal stimulation. Radiology 159: 123, 1986 |
|
Requard CK, Wicks JD, Mettler FA Jr: Ultrasonography in the staging of endometrial adenocarcinoma. Radiology 140: 781, 1981 |
|
Sheth S, Hamper UM, Kurman RJ: Thickened endometrium in the postmenopausal woman: Sonographicpathologic correlation. Radiology 187: 135, 1993 |
|
Hulka CA, Hall DA, McCarthy K, Simeone JF: Endometrial polyps, hyperplasia, and carcinoma in postmenopausal women: Differentiation with endovaginal sonography. Radiology 191: 755, 1994 |
|
Lin MC, Gosink BB, Wolf SI et al: Endometrial thickness after menopause: Effect of hormone replacement. Radiology 180: 427, 1991 |
|
Hulka CA, Hall DA: Endometrial abnormalities associated with tamoxifen therapy for breast cancer: Sonographic and pathologic correlation. AJR 160: 809, 1993 |
|
Gross BH, Callen PW: Ultrasound of the uterus. In Callen PW (ed): Ultrasonography in Obstetrics and Gynecology, p 227. Philadelphia, WB Saunders, 1982 |
|
Hricak H, Stern JL, Fisher MR et al: Endometrial carcinoma staging by MR imaging. Radiology 162: 297, 1987 |
|
Long F, Scoutt L, McCarthy S et al: MRI & synthetic imaging of endometrial carcinoma (abstr). Magn Reson Imaging 6: 62, 1988 |
|
Sironi S, Colombo E, Villa G et al: Myometrial invasion by endometrial carcinoma: Assessment with plain and gadolinium-enhanced MR imaging. Radiology 185: 207, 1992 |
|
Hamlin DJ, Burgener FA, Beecham JB: CT of intramural endometrial carcinoma: Contrast enhancement is essential. AJR 137: 551, 1981 |
|
Tak WK: Carcinoma of the endometrium with cervical involvement (stage II). Cancer 43: 2504, 1979 |
|
Boronow RC, Morrow CP, Creasman WT et al: Surgical staging in endometrial cancer: Clinical-pathologic findings of a prospective study. Obstet Gynecol 63: 825, 1984 |
|
Creasman WT, Boronow RC, Morrow CP et al: Adenocarcinoma of the endometrium: Its metastatic lymph node potential: A preliminary report. Gynecol Oncol 4: 239, 1976 |
|
Creasman WT, DiSaia PJ, Blessing J et al: Prognostic significance of peritoneal cytology in patients with endometrial cancer and preliminary data concerning therapy with intraperitoneal radiopharmaceuticals. Am J Obstet Gynecol 141: 921, 1981 |
|
Salazar OM, Bonfiglio TA, Patten SF et al: Uterine sarcomas: Analysis of failures with special emphasis on the use of adjuvant radiation therapy. Cancer 42: 1161, 1978 |
|
Balfe DM, Van Dyke J, Lee JKT et al: Computed tomography in malignant endometrial neoplasms. J Comput Assist Tomogr 7: 677, 1983 |
|
DiSaia PJ, Creasman WT, Boronow RC: Risk factors and recurrent patterns in stage I endometrial cancer. Am J Obstet Gynecol 151: 1009, 1985 |
|
Gross BH, Moss AA, Mihara K et al: Computed tomography of gynecologic diseases. AJR 141: 765, 1983 |
|
Hricak H, Rubinstein LV, Gherman GM, Karstaedt N: MR imaging evaluation of endometrial carcinoma: Results of an NCI cooperative study. Radiology 179: 829, 1991 |
|
Sironi S, Taccagni G, Garancini C et al: Myometrial invasion by endometrial carcinoma: Assessment by MR imaging. AJR 158: 565, 1992 |
|
Yamashita Y, Harada M, Sawada T et al: Normal uterus and FIGO stage I endometrial carcinoma: Dynamic gadolinium-enhanced MR imaging. Radiology 186: 495, 1993 |
|
Yamashita Y, Mizutani H, Torashima M et al: Assessment of myometrial invasion by endometrial carcinoma: Transvaginal sonography vs contrast-enhanced MR imaging. AJR 161: 595, 1993 |
|
Smith RC, Reinhold C, McCauley TR et al: Multicoil high-resolution fast spin-echo MR imaging of the female pelvis. Radiology 184: 671, 1992 |
|
Cagnazzo G, D'Addario V, Martinelli G, Lastilla G: Depth of myometrial invasion in endometrial cancer: Preoperative assessment by transvaginal ultrasonography and magnetic resonance imaging. Ultrasound Obstet Gynecol 2: 40, 1992 |
|
Del Maschio A, Vanzulli A, Sironi S et al: Estimating the depth of myometrial involvement by endometrial carcinoma: Efficacy of transvaginal sonography vs MR imaging. AJR 160: 533, 1993 |
|
Karlsson B, Norstrom A, Granberg S, Wikland M: The use of endovaginal ultrasound to diagnose invasion of endometrial carcinoma. Ultrasound Obstet Gynecol 2: 35, 1992 |
|
Fleischer AC, Dudley BS, Entman SS et al: Myometrial invasion by endometrial carcinoma: Sonographic assessment. Radiology 162: 307, 1987 |
|
Sutton CL, McKinney CD, Jones JE, Gay SB: Ovarian masses revisited: Radiologic and pathologic correlation. Radiographics 12: 853, 1992 |
|
Serov SF, Scully RE, Sobin LH: Histological Typing of Ovarian Tumours. Geneva, World Health Organization, 1973 |
|
Zawin M, McCarthy S, Scoutt LM, Comite F: High-field MRI and US evaluation of the pelvis in women with leiomyomas. Magn Reson Imaging 8: 371, 1990 |
|
Requard CK, Mettler FA Jr, Wicks JD: Preoperative sonography of malignant ovarian neoplasms. AJR 137: 79, 1981 |
|
Meire HB, Farrant P, Guha T: Distinction of benign from malignant ovarian cysts by ultrasound. Br J Obstet Gynaecol 85: 893, 1978 |
|
Lewis E, Zornoza J, Jing BS et al: Radiologic contributions to the diagnosis and management of gynecologic neoplasms. Semin Roentgenol 17: 251, 1982 |
|
Williams AG, Mettler FA, Wicks JD: Cystic and solid ovarian neoplasms. Semin Ultrasound 4: 166, 1983 |
|
Moyle JW, Rochester D, Sider L et al: Sonography of ovarian tumors: Predictability of tumor type. AJR 141: 985, 1983 |
|
Leibman AJ, Kruse B, McSweeny MB: Transvaginal sonography: Comparison with transabdominal sonography in the diagnosis of pelvic masses. AJR 151: 89, 1988 |
|
Coleman BG, Arger PH, Grumbach K et al: Transvaginal and transabdominal sonography: Prospective comparison. Radiology 168: 639, 1988 |
|
Jain KA, Friedman DL, Pettinger TW et al: Adnexal masses: Comparison of specificity of endovaginal US and pelvic MR imaging. Radiology 186: 697, 1993 |
|
van Nagell JR Jr, DePriest PD, Puls LE et al: Ovarian cancer screening in asymptomatic postmenopausal women by transvaginal sonography. Cancer 68: 458, 1991 |
|
Fleischer AC, McKee MS, Gordon AN et al: Transvaginal sonography of postmenopausal ovaries with pathologic correlation. J Ultrasound Med 9: 637, 1990 |
|
DiSantis DJ, Scatarige JC, Kemp G et al: A prospective evaluation of transvaginal sonography for detection of ovarian disease. AJR 161: 91, 1993 |
|
Crane JP, Gray DL, Mutch DG: Routine ultrasound screening for ovarian cancer-Can we afford it? J Ultrasound Med 10: 543s, 1991 |
|
Wolf SI, Gosink BB, Feldesman MR et al: Prevalence of simple adnexal cysts in postmenopausal women. Radiology 180: 65, 1991 |
|
Rulin M, Preston A: Adnexal masses in postmenopausal women. Obstet Gynecol 70: 578, 1987 |
|
Andolf E, Jorgensen C: Simple adnexal cysts diagnosed by ultrasound in postmenopausal women. J Clin Ultrasound 16: 301, 1988 |
|
Hall DA, McCarthy KA: The significance of the postmenopausal simple adnexal cyst. J Ultrasound Med 5: 503, 1986 |
|
Levine D, Gosink BB, Wolf SI et al: Simple adnexal cysts: The natural history in postmenopausal women. Radiology 184: 653, 1992 |
|
Kurjak A, Zalud I, Alfirevic Z: Evaluation of adnexal masses with transvaginal color ultrasound. J Ultrasound Med 10: 295, 1991 |
|
Kurjak A, Predanic M: New scoring system for prediction of ovarian malignancy based on transvaginal color Doppler sonography. J Ultrasound Med 11: 631, 1992 |
|
Brown DL, Frates MC, Laing FC et al: Ovarian masses: Can benign and malignant lesions be differentiated with color and pulsed Doppler US? Radiology 190: 333, 1994 |
|
Levine D, Feldstein VA, Babcook CJ, Filly RA: Sonography of ovarian masses: Poor sensitivity of resistive index for identifying malignant lesions. AJR 162: 1355, 1994 |
|
Tekay A, Jouppila P: Validity of pulsatility and resistive indices in classification of adnexal tumors with transvaginal color Doppler ultrasound. Ultrasound Obstet Gynecol 2: 338, 1992 |
|
Hamper UM, Sheth S, Abbas FM et al: Transvaginal color Doppler sonography of adnexal masses: Differences in blood flow impedance in benign and malignant lesions. AJR 160: 1225, 1993 |
|
Thurnher S, Hodler J, Baer S et al: Gadolinium-DOTA enhanced MR imaging of adnexal tumors. J Comput Assist Tomogr 14: 939, 1990 |
|
Stevens SK, Hricak H, Stern JL: Ovarian lesions: Detection and characterization with gadolinium-enhanced MR imaging at 1.5 T. Radiology 181: 481, 1991 |
|
Ghossain MH, Buy JN, Lignires L et al: Epithelial tumors of the ovary: Comparison of MR and CT findings. Radiology 181: 863, 1991 |
|
Occhipinti KA, Frankel SD, Hricak H: The ovary: Computed tomography and magnetic resonance imaging. Radiol Clin North Am 31: 1115, 1993 |
|
Scoutt LM, McCarthy SM, Moss AA: CT and MRI of the pelvis. In Moss AA, Gamsu G, Genant HK (eds): CT of the Body with MRI, 2nd ed, p 1215. Philadelphia, WB Saunders, 1983 |
|
Eichner E, Bove ER: In vivo studies on the lymphatic drainage of the human ovary. Obstet Gynecol 3: 287, 1954 |
|
Burghardt E, Pickel H, Stettner H: Management of advanced ovarian cancer. Eur J Gynaecol Oncol 5: 155, 1984 |
|
Prayer L, Kainz C, Kramer J et al: CT and MR accuracy in the detection of tumor recurrence in patients treated for ovarian cancer. J Comput Assist Tomogr 17: 626, 1993 |
|
Griffin TW, Parker RG, Taylor WJ: An evaluation of procedures used in staging carcinoma of the cervix. AJR 127: 825, 1976 |
|
Van Nagell JR Jr, Donaldson EJ, Wood EG: Urinary tract involvement by invasive cervical cancer. In Buchsbaum HJ, Schmidt JD (eds): Gynecologic and Obstetric Urology, p 347. Philadelphia, WB Saunders, 1978 |
|
Hillman BJ, Clark RL, Babbitt G: Efficacy of the excretory urogram in the staging of gynecologic malignancies. AJR 143: 997, 1984 |
|
Barloon TJ, Brown RC, Berbaum KS: Current status of adult uroradiology: A survey of members of the Society of Uroradiology. AJR 154: 301, 1990 |
|
Malave SR, Neiman HL, Spies SM et al: Diagnosis of hydronephrosis: Comparison of radionuclide scanning and sonography. AJR 135: 1179, 1980 |
|
Lome LG, Pinsky S, Levy L: Dynamic renal scan in the non-visualizing kidney. J Urol 121: 148, 1979 |
|
Fukuya T, Hawes DR, Lu CC, Barloon TJ: CT of small-bowel obstruction. AJR 158: 765, 1992 |
|
Taal BG, Steinmetz R, Den Hartog J: Rectosigmoid obstruction caused by ovarian cancer. Clin Radiol 41: 170, 1990 |
|
Stanley RJ: Liver and biliary tree. In Lee JKT, Sagel SS, Stanley RJ (eds): Computed Body Tomography, p 167. New York, Raven Press, 1983 |
|
Henriksen E: The lymphatic spread of carcinoma of the cervix and the body of the uterus: A study of 420 necropsies. Am J Obstet Gynecol 58: 924, 1949 |
|
Bergman F: Carcinoma of the ovary: A clinicopathological study of 86 autopsied cases with special reference to mode of spread. Acta Obstet Gynecol Scand 45: 211, 1966 |
|
Blythe JG, Ptacek JJ, Buchsbaum HJ et al: Bony metastases from carcinoma of cervix: Occurrence, diagnosis, and treatment. Cancer 36: 475, 1975 |
|
Fordham EW: Bone scanning. In Gottschalk A, Potchen EJ (eds): Diagnostic Nuclear Medicine, Sect 13, p 505. Baltimore, Williams & Wilkins, 1978 |
|
Vieras F, Herzberg DL: Focal decreased skeletal uptake secondary to metastatic disease. Radiology 118: 121, 1976 |
|
Villasanta U, Whitley NO, Haney PJ et al: Computed tomography in invasive carcinoma of the cervix: An appraisal. Obstet Gynecol 62: 218, 1983 |
|
Ginaldi S, Wallace S, Jin BS et al: Carcinoma of the cervix: Lymphangiography and computed tomography. AJR 136: 1087, 1981 |
|
Lee JKT: Retroperitoneum. In Lee JKT, Sagel SS, Stanley RJ (eds): Computed Body Tomography, p 257. New York, Raven Press, 1983 |
|
Dooms GC, Hricak H, Crooks LE, Higgins CB: Magnetic resonance imaging of lymph nodes: Comparison with CT. Radiology 153: 719, 1984 |
|
Lee JKT, Heiken JP, Ling D et al: Magnetic resonance imaging of abdominal and pelvic lymphadenopathy. Radiology 153: 181, 1984 |
|
Kim SH, Kim SC, Choi BI, Han MC: Uterine cervical carcinoma: Evaluation of pelvic lymph node metastasis with MR imaging. Radiology 190: 807, 1994 |
|
Buy JN, Moss AA, Ghossain MA et al: Peritoneal implants from ovarian tumors: CT findings. Radiology 169: 691, 1988 |
|
Amman AM, Walsh JW: Normal anatomy and techniques of examination. In Walsh JW (ed): Computed Tomography of the Pelvis, p 1. New York, Churchill Livingstone, 1985 |
|
Bryan PJ, Cohen WN, Seidelmann FE: The pelvis. In Haaga JR, Alfidi RJ (eds): Computed Tomography of the Whole Body, 2nd ed, p 1116. St. Louis, CV Mosby, 1988 |
|
Stehman FB, Calkins AR, Wass JL et al: A comparison of findings at CT and second look laparotomy in patients with ovarian carcinoma. Gynecol Oncol 29: 37, 1988 |
|
Calkins AR, Stehman FB, Wass JL et al: Pitfalls in interpretations of computed tomography prior to second look laparotomy in patients with ovarian carcinoma. Br J Radiol 60: 975, 1987 |
|
Maulitz RM, Sahn SA: Pulmonary lymphangitic carcinomatosis from the cervix: Diagnosis by transbronchial biopsy. Arch Intern Med 139: 708, 1979 |
|
Janower ML, Blennerhassett JB: Lymphangitic spread of metastatic cancer to the lung: A radiologic-pathologic classification. Radiology 101: 267, 1971 |
|
Buchsbaum HJ: Lymphangitis carcinomatosis secondary to carcinoma of cervix. Obstet Gynecol 36: 850, 1970 |
|
Casey MJ, Watson RC: Roentgenographic techniques in the evaluation of epidermoid carcinoma of the cervix uteri. Surg Gynecol Obstet 139: 367, 1974 |
|
Fraser RG, Paré JAP, Paré PD et al: Diagnosis of Diseases of the Chest, 3rd ed, p 329. Philadelphia, WB Saunders, 1988 |
|
Fraser RC, Paré JAP: Diagnosis of Diseases of the Chest, 2nd ed, p 481. Philadelphia, WB Saunders, 1977 |
|
Fletcher GH: Textbook of Radiotherapy, 3rd ed, p 755. Philadelphia, Lea & Febiger, 1980 |
|
Martinez A, Schray MF, Howes AE: Postoperative radiation therapy for epithelial ovarian cancer. J Clin Oncol 3: 901, 1985 |
|
Rosenhein NB: Radioisotopes in the treatment of ovarian cancer. Clin Obstet Gynecol 10: 279, 1983 |
|
Ebner F, Kressel HY, Mintz MC: Tumor recurrence versus fibrosis in the female pelvis: Differentiation with MR imaging at 1.5 T. Radiology 166: 333, 1988 |
|
Hricak H, Swift PS, Campos Z et al: Irradiation of the cervix uteri: Value of unenhanced and contrast-enhanced MR imaging. Radiology 189: 381, 1993 |
|
Murolo C, Costantini S, Foglia G et al: Ultrasound examination in ovarian cancer patients: A comparison with second look laparotomy. J Ultrasound Med 8: 441, 1989 |
|
Weiner Z, Beck D, Brandes JM: Transvaginal sonography, color flow imaging, computed tomographic scanning, and CA 125 as a routine follow-up examination in women with pelvic tumor: Detection of recurrent disease. J Ultrasound Med 13: 37, 1994 |
|
Bret PM, Guibaud L, Atri M et al: Transvaginal US-guided aspiration of ovarian cysts and solid pelvic masses. Radiology 185: 377, 1992 |
|
Morrison DA, Goldman AL: Radiographic patterns of drug-induced lung diseases. Radiology 131: 299, 1979 |
|
Slater JM, Fletcher GH: Ureteral strictures after radiation therapy for carcinoma of the uterine cervix. AJR 111: 269, 1971 |
|
Green B, Libshitz HI: Bladder and ureter. In Libshitz HI (ed): Diagnostic Roentgenology of Radiotherapy Change, p 123. Baltimore, Williams & Wilkins, 1979 |
|
White DR, Cooper MR, Muss HB et al: Complications of cytotoxic antineoplastic chemotherapy. NC Med J 40: 73, 1979 |
|
Forman JD, Zinreich E, Lee JD et al: Improving the therapeutic ratio of external beam radiation for carcinoma of the prostate. Int J Radiat Oncol Biol Phys 11: 2073, 1985 |
|
Schmitz RL, Chao JH, Bartolome JS Jr: Intestinal injuries incidental to irradiation of carcinoma of the cervix of the uterus. Surg Gynecol Obstet 138: 29, 1974 |
|
Seaman WB: Nonneoplastic disease of the stomach. In Margulis AR, Burhenne HJ (eds): Alimentary Tract Roentgenology, p 636. St. Louis, CV Mosby, 1973 |
|
Howland WJ, Loeffler RK, Starchman DE, Johnson RG: Postirradiation atrophic changes of bone and related complications. Radiology 117: 677, 1975 |
|
Hall FM, Mauch PM, Levene MB, Goldstein MA: Protrusio acetabuli following pelvic irradiation. AJR 132: 291, 1979 |
|
vanSonnenberg E, D'Agostino HB, O'Laoide R et al: Malignant ureteral obstruction: Treatment with metal stents-technique, results, and observations with percutaneous intraluminal US. Radiology 191: 765, 1994 |