Tubal Transfer Techniques for Assisted Reproduction
Authors
INTRODUCTION
GIFT Gamete intrafallopian transfer was performed for the first time in 1984,1 when in vitro fertilization (IVF) was in its inception. At that time pregnancy rates were very low, oocyte aspiration required laparoscopy, and laboratory embryo culture was in its early learning stages. It is therefore understandable that when the transfer of sperm and oocytes into the fallopian tubes resulted in higher pregnancy rates than IVF and GIFT, this was received enthusiastically.2, 3, 4
Earlier attempts to transfer oocytes and sperm into the fallopian tubes via minilaparotomy were replaced by laparoscopy.5, 6 The laparoscopic technique of elevating the fimbriated end of the fallopian tube and transabdominal cannulation of the fallopian tube were perfected (Fig. 1). Although GIFT was not applicable to patients with damaged fallopian tubes, this procedure could be successful in patients with mild oligozoospermia, idiopathic infertility and more.
The early GIFT pregnancy rates, two to three times higher than IVF pregnancy rates, prompted many beginning IVF centers to start with GIFT programs.6, 7 Performing GIFT required only basic laboratory skills, including oocyte identification, sperm washing and concentration, adequate insemination, and very short incubation of the oocytes in culture media.
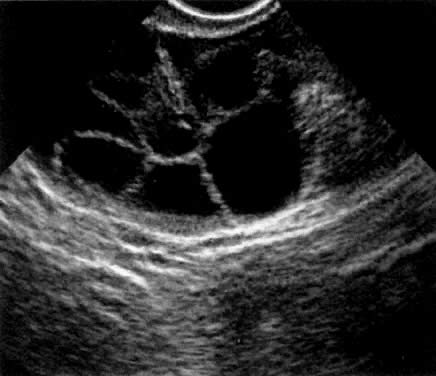
In the late 1980s, vaginal probe real-time sonography became available, with improving resolution. Transvaginal oocyte aspiration was less invasive and more efficient than laparoscopic oocyte aspiration.8 The GIFT technique was modified again to include vaginal aspiration of oocytes (Fig.2), followed by laparoscopy, tubal cannulation, sperm and oocyte deposition into the ampulla. Minilaparotomy for GIFT was almost completely abandoned in the late 1980s, and the superiority of laparoscopy was recognized by most GIFT programs. This technique became the standard approach to GIFT. Vaginal oocyte aspiration allowed for the first time a delay in embryo transfer and the birth of the ZIFT and TET procedures (zygote intrafallopian transfer and tubal embryo transfer).
The development of small diameter minilaparoscopes refined in the 1990s with improved fiber optics allowed the elimination of general anesthesia required for laparoscopic GIFT. Thus, tubal transfer of gametes could be performed in a clinical outpatient set up.9 Minilaparoscopy and conscious sedation remain difficult to perform because of a small visual field and in a less cooperative patient.
The higher than IVF-ET pregnancy rates of GIFT prompted early investigators to replace laparoscopically zygotes in the pronuclear stage in patients with normal fallopian tubes.10, 11 The placement of zygotes inside the fallopian tube was reassuring and verfiied fertilization but it was unclear whether ZIFT was better than IVF. The last development in tubal transfer occurred when embryo culture techniques improved and embryos were replaced into the fallopian tubes at the 4–8 cell stage.12
Once GIFT, ZIFT, and TET became well accepted in the medical community, additional applications were proposed for tubal gamete transfer. GIFT oocyte donation was successfully performed in patients with premature ovarian failure.13 GIFT was also extended to patients who repeatedly failed donor insemination.13, 14, 15, 16
PATIENT PREPARATION FOR GIFT
Patient preparation for GIFT, ZIFT, or TET is similar to any IVF procedure. Those patients assigned for tubal procedures must have normal fallopian tubes. Some suggest that saline ultrasound guided hysterography may not be sufficient and an additional evaluation of the fallopian tube may be necessary, using radiologic HDG and/or laparoscopy. Unreported violations of this "common sense" requirement might have contributed to isolated reports of heterotopic pregnancies following GIFT.14, 15 The placement of several oocytes/embryos in the fallopian tube may explain the higher tubal pregancy rates associated with tubal gamete transfer procedures.16 The post GIFT treatment includes progesterone support of the luteal phase and early pregnancy ultrasound thereafter. Only a few centers prefer repeat injections of low dose hCG for luteal support. Most IVF programs use progesterone supplementation to avoid OHSS. There have been some concerns that progesterone support in the luteal phase may compromise tubal motility and result in higher ectopic pregnancy rates. This theoretical concern was never corroborated by convincing supportive data.
Since laparoscopy is needed during the conventional performance of GIFT, adding pre-GIFT laparoscopy to assess peritubal damage in the presence of a normal hysterosalpingogram has never received wide acceptance. The notion that GIFT can be combined with diagnostic laparoscopy to exclude peritubal damage was infrequently applied because suboptimal visualization of the hyperstimulated ovaries and peritoneal surfaces limit the ability to chromotubate and assess the peritoneal factor. Corrective intervention and tubal repair at the time of GIFT were clearly suboptimal. It is a routine practice to evaluate semen parameters prior to tubal transfer procedures and reculture the patient's cervix for sexually transmitted agents.
Gamete Tubal Transfer Technique
The access into the abdominal cavity during GIFT, ZIFT, and TET requires two-puncture laparoscopy. One trocar is placed in the umbilical region following C02 insufflation of the peritoneal cavity. The second trocar entry port is used to introduce a nontraumatic fimbria grasping instrument. The GIFT catheter is introduced through a third and smaller needle size port hole typically located in the selected site for embryo transfer. The final decision on selecting either the right or left fallopian tubes many be made intraoperatively at the time of laparoscopy.17 On occasion, one fallopian tube is easier to access while the other one is more difficult to cannulate because of the anatomical configuration of the patient, and her hyperstimulated ovaries. The tube of choice is usually the one easier to cannulate and with a healthier anatomy.
Multiple-catheter systems have been evaluated for tubal transfer procedures. Most of these catheter systems involve a rigid introducing trocar with a secondary curved catheter and a secondary embryo or gamete transfer catheter to access the ampullary portion of the fallopian tube. Most laparoscopic tubal transfer procedures can be performed with various simple coaxial catheter systems and do not require the more elaborate steerable catheter systems.18 The embryo transfer catheter is pretested with mouse embryos to confirm that embryo toxicity does not occur. Prior to the procedure the catheter is rinsed with culture media and the oocytes and sperm mixture, or embryos are loaded under a stereo microscope using a fluid bubble chamber. During GIFT procedures, 3–4 oocytes are inseminated with 100,000 motile spermatozoa.
Once the laparoscopic trocars are in place, the fallopian tube is visualized and the ampullary portion is elevated above the pelvic floor, a curved plastic or metal catheter is introduced gently into the ampullary portion of the fallopian tube. A gentle grasping of the fimbriated end of the fallopian tube is mandatory to avoid fimbrial damage and bleeding. The preferable location of the tubal grasper is at the antimesenteric portion of the fallopian tube, thus avoiding damage to the fimbria ovarica and minimizing false catheterization. Once the curved catheter is placed inside the isthmoampullary junction of the fallopian tube, the embryologist is asked to load the gametes into the transfer catheter. Within seconds, the catheter is introduced gently through the guiding catheter, and the gametes are flushed out using 5–10 microliters of culture medium. During the transfer procedure, the ampullary portion is preferably suspended in a vertical position to avoid leakage. Once complete expulsion of the gametes is verified under stereo microscope, the fallopian tube is gently placed in a horizontal position and the fimbria are checked carefully to detect any leakage of fluid. If small amounts of transfer medium are utilized, reflux from the fallopian tube is unlikely to occur.
The patient's recovery post tubal transfer procedure is similar to diagnostic laparoscopy performed for different indications. The follow-up of early pregnancy is performed similar to IVF gestation. Early β-hCG level detection and early sonographic monitoring of the pregnancy are directed towards identification of abnormal pregnancies and ectopic pregnancies.
TRANSVAGINAL GAMETE AND EMBRYO TRANSFER TO THE FALLOPIAN TUBES
Once oocyte retrieval was converted into an outpatient transvaginal sonographically guided needle aspiration, the added laparoscopy to perform tubal transfer became an obvious disadvantage compared to transcervical uterine embryo transfer.19 Noninvasive transvaginal catheterization of the fallopian tubes was reported with and without sonographic guidance.20, 21 The transcervical GIFT procedure requires a reliable access into the fallopian tube either under tactile impression, ultrasound or hysteroscopically. The ultrasound-guided approach is attractive because of the less invasive nature of this procedure; however, live visualization is not easy to accomplish.20 Tubal cannulation under tactile impression requires significant operator experience and failures to catheterize the fallopian tubes occurred in about 12% of the patients.19 A GIFT procedure performed under ultrasound guidance yielded very low pregnancy rates following different tubal gamete transfer procedures. This report was one of the few reports that documented pregnancy rates above 20% using the transcervical approach. Tubal gamete-embryo deposition performed under ultrasound guidance initially had only a 2% pregnancy rate, probably reflecting an early learning curve.
Table 1 demonstrates the variability in pregnancy rates following different transcervical procedures. This inconsistency in pregnancy rates is probably related to various catheterization techniques, operator skills in transcervical cannulation, patient selection, small sample sizes, and differences in laboratory quality.22, 23
Table 1. Transcervical fallopian, gamete, or embryo transfer summarized from references 19 through 23
Cycles (#) | Pregnancies (#) | Pregnancy rates (%) | |
Fallopian oocyte + sperm transfer (US-GIFT) | 28 | 7 | 25.0 |
Fallopian zygote or embryo transfer | 148 | 31 | 20.9 |
Fallopian oocyte + sperm transfer (US-GIFT) | 14 | 1 | 7.1 |
Fallopian oocyte + sperm transfer (US-GIFT) | 7 | 3 | 42.9 |
Fallopian zygote transfer | 5 | 1 | 20.0 |
Fallopian zygote transfer | 25 | 8 | 32.0 |
Fallopian zygote transfer | 57 | 7 | 12.3 |
Fallopian embryo transfer | 18 | 3 | 16.7 |
Fallopian embryo transfer | 28 | 5 | 17.9 |
US, ultrasound; GIFT, gamete intrafallopian transfer
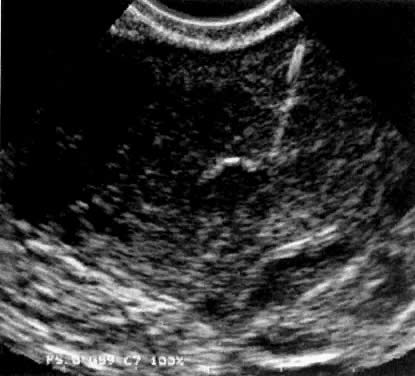
Transcervical and transmyometrial (Fig. 3) deposition of gametes and embryos remain experimental techniques. Unless technological novelty simplifies transcervical tubal cannulation, and superiority of tubal deposition changes, this technology will remain of historic interest only.
ARE INTRATUBAL GAMETE DEPOSITION PROCEDURES INDICATED?
The efficacy of transcervical procedures such as GIFT, ZIFT, and TET largely depends on the comparison of their pregnancy rates to IVF-ET. Practice variations, lack of large randomized clinical trials and different patient populations make the comparison of GIFT, ZIFT and TET unreliable.
Tubal transfer procedure involving laparoscopy requires that this procedure demonstrate superior pregnancy rates to justify its more invasive nature. Observation of the reproductive technology practice preferences in the United States and Canada in the last two decades demonstrates that most practitioners prefer IVF-ET treatment, and very few patients are subjected to either laparoscopic or transcervical GIFT procedures.24, 25, 26Table 2 depicts assisted reproductive technology choices in the United States. In the years 1988–1993 both IVF and GIFT pregnancy rates have improved. While IVF reported cycles to SART (Society of Assisted Reproductive Technology) doubled, the GIFT cycles have dwindled to less than 1% in 2004.26 Pregnancy rates associated with GIFT were higher than the pregnancy rates reported with IVF only in the early 1980s. A comparison of GIFT with no treatment among women with unexplained infertility generates initial calculation of seven fold improvement when GIFT is applied compared to no treatment or inseminations.27The cycle fecundity of GIFT ranges between 12% and 28%, and that of patients who underwent intrauterine inseminations and superovulation between 1% and 36%. Different followup schedules made such comparison inconclusive. The very large overlapping rates demonstrate that it is difficult to claim superiority of GIFT compared to less invasive and inexpensive interventions such as superovulation and IUI.28 In a prospective randomized study comparing IVF, GIFT and TET in 150 couples with unexplained infertility, male factor infertility and minimal endometriosis, the pregnancy rates per transfer were 46% for IVF, 38% for TET and 26% for GIFT. If the number of eggs and embryos transferred were controlled for, GIFT had a significantly lower implantation rate of 7% compared to IVF (13%). This finding suggests that implantation rates per embryo transfer are not superior in tubal procedures.28, 29 Evaluating multiple studies suggests that it would be preferable to perform IVF rather than GIFT in couples with unexplained infertility.29, 30, 31, 32, 33
Table 2. The number of IVF and GIFT cycles, pregnancy, and delivery rates in the United States
Year | IVF/GIFT cycles (#) | IVF/GIFT pregnancies per retrieval (%) | IVF/GIFT deliveries per retrieval (%) | IVF/GIFT pregnancies per cycle (%) | IVF/GIFT deliveries per cycle (%) |
1988 | 17,496/3,949 | 16/27 | 12/21 | 13/21 | 9/17 |
1990 | 19,070/4,439 | 19/29 | 14/22 | 16/25 | 12/19 |
1993 | 31,900/4,992 | 23/35 | 19/28 | 20/29 | 16/24 |
IVF, in vitro fertilization; GIFT, gamete intrafallopian transfer
The costs of the procedure also determines the final selection of ART in patients with normal fallopian tubes. If 1–4 cycles of superovulation and intrauterine insemination is as effective as one cycle of IVF in producing a pregnancy, three cycles were superior to IVF or GIFT and comparable to GIFT.34 Using the same algorithm, four cycles of superovulation and intrauterine inseminations are superior to IVF or GIFT. Cost assessment would favor 4 cycles of hMG combined with inseminations over IVF, GIFT or ZIFT. It is possible that the significant decline in GIFT procedures reflects also the understanding of ART practitioners that GIFT may not be as cost effective as IVF.
Until superiority of tubal transfer procedures is unequivocally demonstrated, this procedure cannot be advocated without considering IVF/ET. GIFT may be indicated only in patients who demonstrate cervical stenosis and inaccessible uterus to catheters, and in patients with religious reservations precluding IVF/ET. The added costs of laparoscopy mandates a careful use of patient healthcare resources. Laboratory improvements and delivery of high quality embryos into the uterine cavity renders tubal transfer procedures obsolete. The final place of those procedures in clinical practice remains unclear.
REFERENCES
Asch RH, Elsworth LR, Balmaceda JP, Wong PC: Pregnancy after translaparoscopic gamete intrafallopian transfer. Lancet 2: 1034, 1984 |
|
Noss U, Weideman R, Hepp H: Gamete intra-fallopian transfer (GIFT): Results of 219 treatment cycles in idiopathic sterility, andrological subinfertility and selected forms of genital pathology. Geburtshilfe Frauenheilkd 47: 797, 1987 |
|
Molloy D, Speirs AL, dePlessis Y et al: The establishment of a successful program of gamete intrafallopian transfer (GIFT): Preliminary results. Aust NZ J Obstet Gynecol 26: 206, 1986 |
|
Corson SL, Batzer F, Eisenberg E et al: Early experience with the GIFT procedure. J Reprod Med 31: 219, 1986 |
|
Asch RH, Balmaceda JP, Wong PC et. al.: Gamete Intra Fallopian Transfer (GIFT): Use of mini-laparotomy and an individualized regimen induction of follicular development. Acta Eur Fertil 1986;17:198. |
|
Edwards RG, Steptoe PC: Current status of in vitro fertilization and implantation of human embryos. Lancet 1983;2:1265. |
|
Leeton J, Trounson A, Wood C et. al.: In vitro fertilization and embryo transfer. The Monash Group Experience 1981 -1983. Acta Eur Fertil 1983;14:95. |
|
Molloy D, Speirs A, dePlessis Y et al: A laparoscopic approach to a program of gamete intrafallopian transfer. Fertil Steril 47: 289, 1987 |
|
Pellicano M, Zullo F, Fiorentino A, Tommaselli GA, Palomba S et. al.: Conscious sedation versus general anesthesia for minilaparoscopic gamete intra-fallopian transfer: a prospective randomized study. Hum Reprod 2001;2295-2297. |
|
Devroey P, Braeckmans P, Smitz J et. al.: Pregnancy after translaparoscopic zygote intra-fallopian transfer in a patient with sperm antibodies. Lancet 1987;1:1329. |
|
Yovich JL, Blackledge DG, Richardson PA, Matson PL et. al.: Pregnancies following pronuclear stage tubal transfer. Fertil Steril 1987;48:851. |
|
Yovich JL, Yovich JM, Edirisinghe WR: The relative chance of pregnancy following tubal or uterine transfer procedures. Fertil Steril 1988;49:858-864. |
|
Asch RH, Balmaceda JP, Ord T et al: Oocyte donation and gamete intrafallopian transfer as a treatment for premature ovarian failure. Lancet 1987;1:687. |
|
Li HP, Balmaceda JP, Zouves C, Cittadino E, Casas PF et. al.: Heterotopic pregnancy associated with gamete intra-fallopian transfer. Hum Reprod 1992;7:131-135. |
|
Molloy D, Deambrosis W, Keeping D, Hynes et. al.: Multiple-sited (heterotopic) pregnancy after in vitro fertilization and gamete intrafallopian transfer. Fertil Steril 1990;53:1068-1071. |
|
Craft I, Al-Shawaf T: IVF vs. GIFT. Assist Reprod Genet 1992;9:424-427. |
|
Driscoll GL, Tyler JP, Clark J, Bernstein J: Transfer of gametes into the fallopian tube - is choice of side important? Hum Reprod 1996;11:1881-1883. |
|
Confino E, Friberg J, Gleicher N: A new stirrable catheter for gamete intrafallopian tube transfer (GIFT). Fertil Steril 1986;46:1147-1149. |
|
Bauer O, Van der Ven H, Al-Hasani S, Krebs D, Gembruch U: Preliminary results on transvaginal tubal embryo stage transfer (TV-TEST) without ultrasound guidance. Hum Reprod 1990;5:552-556. |
|
Bustillo M, Schulman JD: Transcervical ultra-sound guided intrafallopian placement of gametes, zygotes, and embryos. J In Vitro Fert Embr Transf 1989;6:321-324. |
|
Scholtes MCW, Roozenburg BJ, Alberda TH, Zeilmaker GH: Transcervical intrafallopian transfer of zygotes. Fertil Steril 1990;54:283-286. |
|
Yovich JL, Draper RR, Turner SR, Cummins J: Transcervical tubal embryo-stage transfer (TC-TEST). J In Vitro Fert Embryo Transf. 1990;7:137-140. |
|
Risquez F, Boyer P, Rolet F, Magnanai M, Guichard A et. al.: Retrograde tubal transfer of human embryos. Hum Reprod 1990;5:185-188. |
|
The American Fertility Society, Society for Reproductive Technology, Assisted Reproductive Technology in the United States and Canada: 1992 results generated from the American Fertility Society/Society for Assisted Reproductive Technology Registry. Fertil Steril 1994;62:1121-1128. |
|
Society for Reproductive Technology, American Society for Reproductive Medicine. Assisted reproductive technology in the United States and Canada: 1993 results generated from the American Society for Reproductive Medicine/Society for Assisted Reproductive Technology Registry. Fertil Steril 1995;64:13-21. |
|
National Summary: 2004 ART Report, CDC Reproductive Health Notes, CDC Org. |
|
Murdoch AP, Harris M, Mahroo M. Williams M. et. al.: Gamete intrafallopian transfer (GIFT) compared with intrauterine insemination in the treatment of unexplained infertility. Br J Obstet Gynaecol 1991;98:1107-1111. |
|
Dawood MY: In vitro fertilization, gamete intrafallopian transfer, and superovulation with intrauterine inseminations: efficacy and potential hazards on babies delivered. Am J Obstet Gynecol 1996;174:1208-1217. |
|
Leeton J, Roger P, Caro C, Healy D, Yates C: A controlled study between the use of gamete intrafallopian transfer (GIFT) and in vitro fertilization and embryo transfer in the management of idiopathic and male infertility. Fertil Steril 1987;48:605-607. |
|
Mills MS, Eddowes HA, Cahill HJ et. al.: A prospective controlled study of in - vitro fertilization, gamete intra-fallopian transfer and intrauterine insemination, combined with superovulation. Hum Reprod 1992;7:490-494. |
|
Ranieri M, Beckett VA, Marchant S, Kinis A, Serphal P: Gamete intra-fallopian transfer or in-vitro fertilization after failed ovarian stimulation and intrauterine insemination in unexplained infertility? Hum Reprod 1995;10:2023-2026. |
|
Rombauts L, Dear M, Breheny S, Healy DL: Cumulative pregnancy and live birth rates after gamete intra-fallopian transfer. Hum Reprod 1997;12:1338-1342. |
|
Pandian Z, Bhattacharya S, Nikolaou D, Vale L, Templeton A: The effectiveness of IVF in unexplained infertility: a systematic Cochrane Review. Hum Reprod 2003;18:2001-2007. |
|
Peterson CM, Hatasaka HH, Jones KP et. al.: Ovulation induction with gonadotropins and intrauterine insemination compared with an in vitro fertilization and no therapy: a prospective, nonrandomized, cohort study and meta-analysis. Fertil Steril 1994;62:535-544. |