Prostaglandins in Pregnancy
Authors
INTRODUCTION
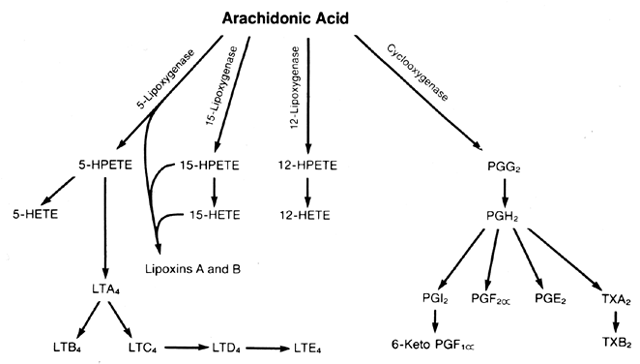
The chemical name for arachidonic acid is eicosatetraenoic acid; therefore, the metabolites of arachidonate often are referred to as “eicosanoids.” Arachidonate is metabolized by two major enzymatic pathways: cyclooxygenase and lipoxygenase (Fig. 1).1, 2 Metabolism by the cyclooxygenase pathway leads to the prostaglandins (PGs) and thromboxanes (TXs); metabolism by the lipoxygenase pathway leads to the hydroperoxyeicosatetraenoic acids (HPETEs), hydroxyeicosatetraenoic acids (HETEs), lipoxins, and leukotrienes (LTs). The HPETEs are hydroperoxidases of arachidonic acid, and the HETEs and LTB4 are hydroxylated forms. The leukotrienes C4, D4, and E4 are modifications of arachidonate that contain an amino acid attached to the fatty acid chain by a sulfide bond. Thus, they often are referred to as cysteine leukotrienes. The cysteine-containing leukotrienes are the compounds that were previously referred to as the “slow-reacting substance of anaphylaxis” (SRS-A).
There are two isoforms of the cyclooxygenase enzyme: COX-1, which is the constitutive form, and COX-2, which is the inducible form. Both isoforms convert free arachidonic acid into PGG2 and PGH2. There are several lipoxygenase enzymes. The 5-, 12- and 15-lipoxygenase enzymes lead to biologically active compounds. Arachidonic acid first is converted to the HPETEs and then to the HETEs, lipoxins, or leukotrienes.1, 2 The 5-lipoxygenase is the primary pathway to the leukotrienes. The lipoxins are formed by the actions of 5- and 15-lipoxygenases on arachidonic acid. Competition for arachidonic acid between the cyclooxygenase and lipoxygenase enzymes exists in cells and tissues containing both pathways.
Essentially, all cells produce eicosanoids. In general, they are considered intracellular or local mediators of physiologic responses, rather than circulating hormones. They exert their effects by autocrine mechanisms (within the same cell in which they are produced) or paracrine mechanisms (between cells or tissues in which they are produced). The fact that they exert their effects locally can complicate interpretation of results from studies evaluating blood levels or studies in which they are administered exogenously.
BLOOD LEVELS: SYSTEMIC VERSUS LOCAL MECHANISMS OF ACTION
Several investigators have attempted to measure circulating levels of prostaglandins during human pregnancy. Reported concentrations range from 3–10 pg/mL to several hundred picograms per milliliter of plasma, and there is little agreement among investigators as to the absolute plasma concentrations for any one prostaglandin. There are probably at least two reasons for the lack of agreement. First, solvent extraction, purification, and radioimmunoassays for plasma prostaglandins can be problematic, often resulting in nonspecifically high assay values for “blank” samples that do not contain any prostaglandin.3 Second, prostaglandins, like other arachidonic acid metabolites, function primarily as intracellular mediators of physiologic responses rather than as true circulating hormones. The prostaglandins that do leak into the circulation are metabolized rapidly by the lungs, liver, and kidneys.
The information available for plasma levels of PGE2, PGF2α, and their metabolites, 13, 14-dihydro-15-keto-PGE2 and 13,14-dihydro-15-keto-PGF2α, does not indicate convincing gestationally related changes except during parturition, when the plasma levels of PGF2α metabolite increase.4, 5, 6, 7, 8, 9, 10 Plasma concentrations of prostacyclin's stable metabolite, 6-keto-PGF1α, are reportedly higher during late pregnancy than during early pregnancy or the nonpregnant state,11, 12 and they increase further with labor.12, 13 However, there is considerable controversy as to whether prostacyclin and 6-keto-PGF1α are circulating hormones. Early reports of plasma 6-keto-PGF1α concentrations were in the range of 100–300 pg/mL, and it was believed that the lung acted as an endocrine organ, releasing prostacyclin into the systemic circulation.14, 15 Measurements with gas chromatography/mass spectrometry or with properly validated radioimmunoassays indicate that plasma prostacyclin levels are only approximately 2–40 pg/mL. Several references on both sides of this issue can be found in reviews.16, 17 It is probably more meaningful to determine and evaluate production rates from specific tissues rather than circulating levels when assessing the involvement of prostaglandins in physiologic functions during human pregnancy.
Circulating concentrations of thromboxane's stable metabolite, TXB2, also have been determined in human pregnancy. Plasma concentrations range from 100 to 300 pg/mL, and serum concentrations may reach 300 ng/mL.18, 19 The reason serum concentrations are so much higher than plasma concentrations is because platelets release thromboxane during the clotting process. Thromboxane levels are higher in pregnant women than in nonpregnant women, and they increase further with the onset of labor.
EFFECTS ON BLOOD PRESSURE, BLOOD FLOW, AND CARDIOVASCULAR FUNCTION
Maternal cardiovascular changes
Several cardiovascular changes typically occur during human pregnancy.20, 21, 22 Heart rate increases by 10–15%, cardiac output increases by 30–50%, and blood volume increases by up to 48%. Blood pressure changes are small in normotensive women, but generally there is a slight decrease of 2–3 mmHg in systolic pressure and 5–10 mmHg in diastolic pressure. Normal blood pressures during the third trimester average 95/50 mmHg in the lateral position and 106/65 mmHg in the supine position. Women with essential hypertension actually may show a significant decrease in blood pressure during the first two trimesters. These facts indicate that normal pregnancy is associated with an increase in the production of vasodilatory agents.
PROSTAGLANDINS
Prostaglandins are involved in the vasodilatation of pregnancy. Everett and colleagues23 showed that the dose of angiotensin II (AII) necessary to cause a 20-mmHg increase in diastolic blood pressure in pregnant women was significantly reduced in women treated with indomethacin. Thus, the decreased sensitivity to the vasoconstrictive effects of AII characteristic of healthy pregnant women was altered by prostaglandin synthesis inhibition to a state of increased sensitivity to vasoconstriction.
The prostaglandin most likely responsible for increased vasodilatation in normal pregnancy is prostacyclin (PGI2) because of its potent effect to relax the smooth muscle of blood vessels and lower systemic arterial blood pressure.24, 25, 26 Prostacyclin's actions on vascular tone are most likely exerted locally in the blood vessel wall through a paracrine mechanism between the endothelial cells, in which prostacyclin is primarily produced, and the vascular smooth muscle. Leukocytes also can form prostacyclin.27
If prostacyclin is not a circulating hormone, there must be some other compound that circulates to stimulate prostacyclin synthesis locally in blood vessels. The identity of such a compound is not known, but it seems reasonable to assume that the placenta is the source of a circulating vasodilatory agent. This idea is supported by the facts that blood pressure typically decreases during pregnancy and that simply turning a pregnant patient from the lateral to the supine position causes an increase in blood pressure despite a decrease in cardiac output.21, 22 Women in their third trimester (28–32 weeks' gestation) in whom preeclampsia is destined to develop have a greater increase in blood pressure than do healthy pregnant women when turned from the lateral to the supine position.28 One explanation for the increase in blood pressure in the supine position is that the gravid uterus compresses the vena cava and thereby reduces venous return to the heart, leading to decreased cardiac output. This would activate the aortic and carotid sinus baroreceptors to cause reflex neural vasoconstriction. An alternative explanation is that compression of the vena cava by the gravid uterus in the supine position restricts the amount of placentally derived vasodilatory hormone reaching the maternal systemic arterial blood vessels.
THROMBOXANE
The primary source of thromboxane in the adult circulation is the platelets, although leukocytes also produce it. Thromboxane is a potent stimulator of platelet aggregation and vasoconstriction,24, 25, 26 and as such, it has been implicated in several pathologic conditions that are associated with thrombosis and vascular congestion,25, 29 including preeclampsia, as discussed later in this chapter.
Under normal conditions, prostacyclin released by the vascular endothelial cells acts to stimulate an increase in adenosine 3',5'-cyclic phosphate (cAMP) levels within the platelets, thus inhibiting aggregation.30 Within the platelets, thromboxane can prevent prostacyclin-induced increases in cAMP, so under typical circumstances, thromboxane and prostacyclin counteract each other's actions to maintain homeostasis with respect to platelet function. If, however, the endothelial cells are damaged (e.g. injury, atherosclerosis) or prostacyclin synthesis is impaired, then the actions of thromboxane to produce platelet aggregation and vasoconstriction are manifested. This mechanism serves to protect the body at local sites of injury, but in pathologic states in which thromboxane is allowed to exert its effects throughout an organ or systemically throughout the body, this mechanism can be harmful.
During normal pregnancy, the production of thromboxane is increased. Maternal plasma levels of its stable metabolite, TXB2, as well as its urinary metabolites, are higher during late pregnancy than during midpregnancy or the nonpregnant state.18, 19, 31 The placenta, as well as platelets, is an important source of thromboxane during pregnancy (Fig. 2). Placentas obtained from healthy pregnant women produce approximately 92 μg of thromboxane daily in vitro,32 and trophoblastic cell cultures, uncontaminated with platelets or other blood cells, produce 50–100 pg of thromboxane per microgram of protein daily.33 After delivery of the placenta, maternal urinary metabolites of thromboxane decrease significantly,31 which is a further indication of the placenta as a source of thromboxane in pregnancy.
LIPOXYGENASE COMPOUNDS
Arachidonic acid is metabolized by lipoxygenase enzymes in several tissues, organs, and cells of the body, most notably the lungs and leukocytes (in which they were first discovered and from which the leukotrienes derive their name). The human placenta also produces lipoxygenase compounds. 5-HETE, 12-HETE, 15-HETE, LTB4,16, 17, 34, 35 and LTC4 all have been identified.
The physiologic functions of lipoxygenase metabolites during pregnancy are not known, but the HETEs, HPETEs, lipoxins, and leukotrienes do exert biologic actions, some of which must be important to placental function and pregnancy. Both 5-HETE and LTB4 stimulate leukocytic chemotaxis and chemokinesis, increase aggregation of leukocytes, stimulate the uptake of calcium and d-glucose by neutrophils and eosinophils, increase bronchial constriction and mucus secretion, increase vasodilatation, and enhance vascular permeability.36, 37, 38
LTC4 and LTD4 exert biologic actions in various tissues of the body as reviewed in several studies.2, 37, 38, 39, 40, 41 They produce contraction of a number of smooth muscle tissues, including the small airways of the lung, the ileum, the stomach, the gallbladder, and the uterus.
In the cardiovascular system, leukotrienes often produce opposite effects in different vascular beds and in different species.2, 40, 42 For example, LTC4 and LTD4 produce vasoconstriction and cause plasma leakage in guinea pig skin and the hamster cheek pouch, but they are potent vasodilators in human skin. LTC4 and LTD4 are potent vasoconstrictors in the coronary circulation of several species. Systemically, LTC4 and LTD4 lower arterial blood pressure in most adult nonpregnant species,2, 40, 42, 43, 44 including humans.45 In pregnant and postpartum rhesus monkeys,16, 17, 46 injection of LTB4, LTC4, or LTD4 (0.5 μg/kg) into the lower vena cava to mimic the systemic route of placentally produced hormones lowers both systolic and diastolic blood pressures.
The mechanisms of action through which systematically administered leukotrienes lower blood pressure are not known, but it is likely that they decrease cardiac output and stimulate prostacyclin production by endothelial cells in the lungs or locally in the systemic vasculature. LTC4 promotes prostacyclin synthesis by human endothelial cells,47, 48 and both LTC4 and LTD4 increase prostacyclin release by bovine aortic endothelial cells.49 Endothelial cells metabolize leukotrienes, which indicates that they have receptors for them but do not synthesize leukotrienes.48 Although it might be argued that leukotrienes are local mediators of physiologic responses and do not function as circulating hormones, this may not be entirely true during pregnancy because of supplemental production by the placenta.
The lipoxins share some of the same biologic effects of the prostaglandins, HETEs, and leukotrienes, but chemically they are distinct molecules. Lipoxin A induces arteriolar dilation but does not affect microvascular permeability, as does LTC4, or leukocyte adherence to venular endothelium, as does LTB4.1, 2 Lipoxin A stimulates superoxide anion generation without provoking aggregation, causes chemotaxis, and possesses spasmogenic activities, as do the HETEs and leukotrienes. Lipoxin A elicits long-lasting contractions of guinea pig lung strips, but, unlike LTC4, it does not stimulate contraction of the guinea pig ileum. Lipoxins A and B inhibit human natural killer cell activity, whereas HETEs and leukotrienes do not affect natural killer cell cytotoxicity. Furthermore, lipoxins may serve as important intracellular mediators. Lipoxin A is a potent activator of protein kinase C, even more potent than diacylglycerol or arachidonic acid. Thus, lipoxins display patterns of physiologic effects that are distinct from prostaglandins, thromboxanes, HETEs, or leukotrienes. The importance of lipoxins in normal and abnormal pregnancy is not known, but the fact that lipoxin A dilates arterioles implies that they may serve to augment vasodilatation during normal pregnancy.
Uterine blood flow
The effects of prostaglandins on uterine blood flow have been evaluated in vivo in a number of species, including pregnant monkeys and pregnant and nonpregnant chronically catheterized sheep. Radioactive microspheres and uterine artery flow probes are the two techniques most commonly used for evaluating uterine blood flow. When assessing the effects on uterine blood flow of uterotonic agents, such as the prostaglandins, their simultaneous effects on uterine contractility also must be determined. A compound that stimulates uterine contractions also reduces the amount of blood perfusing the uterus by myometrial compression of the blood vessels.
The prostaglandins that decrease uterine blood flow are PGE2, PGF2α, 6-keto-PGF1α, TXB2, and analogs of the prostaglandin endoperoxides PGG2 and PGH2.50, 51, 52, 53, 54 PGE2 and PGF2α are potent stimulators of uterine contractility. The effect of PGE2 on uterine blood flow is primarily due to its uterotonic action, whereas PGF2α exerts vasoconstrictive actions in addition to its uterine contractile effect.
The prostaglandins that increase uterine blood flow are PGI2, PGD2, PGA2, PGE1, and 6-keto-PGE1. PGE2 is a vasodilator in nonpregnant sheep, provided it does not stimulate uterine contractions.51, 52, 53, 54, 55, 56, 57, 58 PGD2 and PGA2 stimulate uterine contractions, whereas PGI2 inhibits uterine activity.
Placental blood flow
IN VITRO STUDIES
Several in vitro studies have been published concerning the vasoactive effects of thromboxane and prostaglandins on human placental vessels. These studies demonstrate that thromboxane is one of the most potent vasoconstrictors of the human placental vasculature.59, 60, 61, 62, 63 Because the active metabolite of thromboxane (TXA2) is extremely labile, it is necessary to use a stable analog for experimental studies. Most investigators use the thromboxane mimic U46619. In vitro studies demonstrate that thromboxane is 10- to 1000-times more active as a vasoconstrictor than is PGE2, PGF2α, AII, 5-hydroxytryptamine (5-HT, or serotonin), norepinephrine (NE), or bradykinin.
With respect to the vasoconstrictive effects of prostaglandins in the human placenta, there is agreement among all investigators that PGF2α and PGE2 are vasoconstrictors.60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71 PGD2 reportedly causes vasoconstriction when given alone,61, 64 but attenuates vasoconstriction when given with AII.61 PGA1 causes vasoconstriction equivalent to PGF2α.71 The stable metabolite of thromboxane, TXB2, is without effect on the placental vasculature.71
In the human placenta, prostacyclin consistently is the most potent vasodilator.61, 62, 63, 67, 68, 71 PGE1 was found to be a weak vasodilator in two studies,61, 62 but a weak vasoconstrictor in another study.71 One of the stable metabolites of prostacyclin, 6-keto-PGE1, is a weak vasodilator in the human placenta.62 One of prostacyclin's other stable metabolites, 6-keto-PGF1α, is a weak vasodilator.25
There is little information available regarding the vascular effects of leukotrienes in the placenta. When comparing the vasoconstrictive effect of LTB4, LTC4, and LTD4 with that of thromboxane in the perfused human placental cotyledon,59 we found that the leukotrienes cause vasoconstriction, but only at high doses. Their potency is considerably less than that of thromboxane.
Based on in vitro studies using isolated human placental vascular strips or isolated perfused human placental cotyledons, one can rank the vasoactive potencies of thromboxane, prostaglandins, and other vasoactive compounds as follows: vasoconstriction – TX (U46619) greater than PGF2α equal to PGA1 greater than PGE2 greater than AII greater than 5-HT greater than NE; vasodilation – PGI2 greater than PGE1 greater than 6-keto-PGE1.
IN VIVO STUDIES
Several in vivo studies have been reported concerning the vasoactive effects of prostaglandins in the placenta. The most commonly used animal model is the chronically catheterized sheep, but other species have been used, including nonhuman primates.
Vasoconstrictors
There is agreement among investigators that fetal administration of PGE2 or PGF2α causes umbilical–placental vasoconstriction in the sheep.72, 73, 74, 75, 76 The thromboxane mimic U46619 is a potent vasoconstrictor in the ovine fetal systemic circulation and in the umbilical–placental circulation.77, 78 Furthermore, fetal infusion of thromboxane can result in respiratory acidosis in the fetus, a further indication of its detrimental vasoconstrictive effects on the fetoplacental vasculature. Thus, there is agreement between in vitro and in vivo studies that thromboxane, PGE2, and PGF2α are vasoconstrictors in the fetoplacental vascular bed.
Does prostacyclin vasodilate the placental vasculature in vivo?
IN VITRO VERSUS IN VIVO RESULTS
With respect to vasodilatation in the fetoplacental vasculature, there is controversy and disagreement between in vitro and in vivo studies. Prostacyclin is a potent vasodilator,24, 25, 26 and it consistently relaxes human placental blood vessels when tested in vitro, as discussed previously. However, when tested in vivo, infusion of prostacyclin into the ovine fetus decreases fetoplacental blood flow and results in an increase or no change in the calculated placental vascular resistance.77, 78, 79 Furthermore, fetal infusion of prostacyclin into the systemic circulation in conjunction with either thromboxane or AII reverses systemic and renal vasoconstriction induced by thromboxane or AII but further decreases fetoplacental blood flow.77
The in vivo placental vascular results obtained for prostacyclin in the sheep are disturbing for at least two reasons. First, they contradict the consistently potent vasodilatory effect of prostacyclin obtained in human in vitro placental studies. Second, how can a compound that is such a potent vasodilator in all other vascular beds be a vasoconstrictor in the placenta, a vascular bed that must remain dilated if the fetus is to grow and mature properly?
The aberrant effects observed for prostacyclin in vivo can be explained by Poiseuille's law as it applies to the fetal–placental circulation. The placental vascular effects observed for prostacyclin in vivo (or for other vasodilators) are passive changes caused by shunting of blood away from the placenta to other vasodilated vascular beds more proximal to the heart. We have previously described this concept in detail.80
The placental vascular actions are due to systemic alterations in blood flow and perfusion pressure in nonplacental vascular beds rather than to direct vasoconstrictive actions of prostacyclin on the placental vasculature. Indirect evidence suggests that prostacyclin is important for in vivo vasodilation in the human placenta. Doppler ultrasonographic measurements of umbilical blood flow in pregnant women are correlated positively with prostacyclin production in vitro by specimens obtained from the umbilical arteries.81
Umbilical and fetal blood flow
The arachidonic acid metabolites are present in abundance82 and exert potent vasoactive effects in the fetus and on the umbilical vessels. Thromboxane, PGE2, and PGF2α cause vasoconstriction, whereas prostacyclin causes vasodilatation of the umbilical vessels.73, 74, 83, 84, 85, 86 Thromboxane causes vasoconstriction of the ductus arteriosus and pulmonary vasculature, whereas prostacyclin, PGE2, and PGE1 cause vasodilatation.87, 88, 89 Inhibitors of prostaglandin synthesis, such as indomethacin and sodium salicylate (aspirin), result in vasoconstriction and closure of the ductus arteriosus,90, 91 demonstrating that prostaglandins are necessary to maintain patency of the ductus arteriosus during fetal life.
Thromboxane is a potent vasoconstrictor in the fetal systemic circulation, as evidenced by in vivo studies in chronically catheterized sheep fetuses. Thromboxane infusion results in a significant increase in mean fetal aortic blood pressure, renal vasoconstriction, and fetal acidosis.77, 78 Prostacyclin, however, vasodilates the ovine fetal systemic circulation, as evidenced by its ability to significantly lower mean fetal aortic blood pressure77, 78, 79 and its ability to antagonize the vasoconstrictive effects of thromboxane and AII.77, 78 Prostacyclin increases blood flow to the fetal kidneys,77 adrenal glands,92 and lungs.78, 79
The leukotrienes are potent fetal pulmonary vasoconstrictors and may be the compounds primarily responsible for maintaining the high pulmonary vascular resistance during fetal life. In newborn lambs and piglets, LTD4 increases pulmonary and systemic vascular resistance,93, 94 whereas blockage of the leukotriene receptors with FPL 57231 decreases pulmonary vascular resistance, increases pulmonary blood flow, and decreases pulmonary and systemic arterial pressures in late gestational fetal lambs.95 Human newborns with pulmonary hypertension and persistent fetal circulation have LTC4 and LTD4 present in their tracheal lavage; healthy newborns do not.96 Leukotriene inhibition with the receptor blocker FPL 57231 prevents and reverses hypoxic pulmonary vasoconstriction in newborn lambs.97
PREECLAMPSIA
Preeclampsia is considered one of the most significant health problems in human pregnancy.20, 98, 99, 100, 101 It complicates approximately 5–7% of pregnancies and is a leading cause of fetal growth retardation, indicated premature delivery, and maternal death. It is characterized primarily by proteinuria and increased vasoconstriction leading to maternal hypertension and reduced uteroplacental blood flow. Platelet aggregation, thrombocytopenia, edema, and hyperreflexia, occasionally manifested as convulsions (eclampsia), may be associated with the disease process. Because preeclampsia occurs only during pregnancy or in the presence of placental tissue (i.e. hydatidiform mole),20 the causative factors of preeclampsia most logically originate in the placenta and any effective treatment must correct or prevent abnormalities in the placenta, as well as in the mother. The cause of preeclampsia is not known, but in recent years, a considerable amount of evidence has accrued to indicate that it is associated with an imbalance of increased thromboxane and decreased prostacyclin production, as discussed subsequently.
The first evidence that prostaglandins were involved in preeclampsia came from studies involving vascular sensitivity of pregnant women to AII. Although AII levels are not higher in women with preeclampsia than in healthy pregnant women,99, 102 the vascular responsiveness to AII is increased greatly in preeclamptic women. Gant and associates103 demonstrated that the dose of AII required to elicit a pressor response of 20 mmHg in diastolic blood pressure was significantly less than normal as early as 23–26 weeks of pregnancy in women destined to develop pregnancy-induced hypertension. This study demonstrated the increased vascular responsiveness of preeclamptic women, and it showed that this increased responsiveness was present as early as the second trimester of pregnancy, long before clinical symptoms were manifest.
The mechanism for the increased vascular responsiveness is not known, but defective prostaglandin production or a loss of response to prostaglandins contributes to the development of pregnancy-induced hypertension.23, 99, 104 Everett and colleagues23 showed that the dose of AII necessary to cause a 20-mmHg rise in diastolic blood pressure in pregnant women is reduced significantly in women treated with indomethacin. In other words, by inhibiting prostaglandin synthesis, the investigators mimicked the increased vascular responsiveness of preeclampsia.
Prostacyclin
Prostacyclin is a potent vasodilator, an inhibitor of platelet aggregation,24, 25, 26 and an inhibitor of uterine contractility;105, 106, 107 thus, its combined effects favor prevention of maternal hypertension, prevention of platelet aggregation, and promotion of increased uteroplacental blood flow. A deficiency in its production during pregnancy would contribute to the clinical manifestations of preeclampsia. The role of prostacyclin in pregnancy has been reviewed extensively.108 Various tissues of human pregnancy are known to produce prostacyclin, such as the placenta; umbilical, placental, and uterine vessels; ductus arteriosus; amnion, chorion, and decidua; and myometrium.16, 17
A significant amount of data indicate that prostacyclin production is decreased in preeclampsia, as reviewed comprehensively.16, 17 The first evidence came in 1980 from studies of vascular tissues.109, 110, 111 Additional studies confirmed the decreased vascular production of prostacyclin in hypertensive pregnancies. Prostacyclin production is, therefore, decreased in umbilical arteries, placental veins, uterine vessels, and subcutaneous vessels obtained from preeclamptic women as compared with healthy pregnant women.
Other studies also confirm decreased prostacyclin production in preeclampsia. For example, urinary metabolite concentrations are depressed,112 as are amniotic fluid concentrations. Plasma levels of prostacyclin's stable metabolite 6-keto-PGF1α in preeclampsia have been reported to be decreased, unchanged, or increased compared with normal pregnancy. Studies suggest that prostacyclin itself does not circulate in concentrations sufficient to produce physiologic effects, but rather that it exerts its effects locally by a paracrine mechanism between the endothelial cells and the vascular smooth muscle to relax the blood vessels in which it is produced.
Placental production of prostacyclin is decreased significantly in preeclampsia (Fig. 3),32, 113 as well as in normotensive pregnancies complicated by fetal growth retardation.114, 115 Prostacyclin is produced primarily by the endothelial cells of the placental vessels, but the trophoblast also produces some prostacyclin.16, 17, 33, 115, 116, 117 Trophoblastic production of prostacyclin might function to prevent platelet clumping in the intervillous space. Although the production rate of prostacyclin is small when expressed per milligram of wet tissue per hour, the large placental mass makes it a formidable endocrine organ during pregnancy. A placental production rate of 6.7–7.2 pg/mg per hour is equivalent to approximately 94–97 μg per placenta per day.32, 113
With less prostacyclin being produced in preeclampsia, the vasoconstrictor effects of AII, thromboxane, and catecholamines would not be opposed efficiently, leading to hypertension. The renin-angiotensin system is suppressed paradoxically in preeclampsia, but AII levels still are elevated over the nonpregnant state.99, 102 Prostacyclin stimulates this system,118, 119 so defective prostacyclin production also might explain why AII levels are lower in preeclampsia than in normal pregnancy.
Deficiency in prostacyclin is present before the onset of clinical symptoms. Mills et al. reported in a large multicenter prospective study that urinary concentrations of prostacyclin metabolite were significantly reduced as early as 13–16 weeks of gestation in women who went on to develop preeclampsia.120 Klockenbusch et al. reported a similar finding based on longitudinal measurements.121 These findings that prostacyclin changes predate the clinical onset of preeclampsia are consistent with the findings of Gant et al.122 and suggest that increased vascular responsiveness early in pregnancy in women who go on to develop preeclampsia may be due to deficient prostacyclin production.
Thromboxane
Thromboxane opposes the actions of prostacyclin. Thromboxane is a potent vasoconstrictor, a stimulator of platelet aggregation,24, 25, 26 and a stimulator of uterine contractility.106 Its combined actions, if unopposed, lead to maternal hypertension, increased platelet aggregation, and decreased uteroplacental blood flow.
The deficiency in prostacyclin production by preeclamptic placentas is associated with enhanced production of thromboxane similar to the imbalances in these eicosanoids that have been suggested for other pathologic states that favor the development of thrombosis, such as arterial thrombosis, venous thrombosis, myocardial infarction, diabetes, and thrombocytopenia purpura.25, 29 The preeclamptic placenta produces more than three times as much thromboxane as the normal placenta, but less than half as much prostacyclin (Fig. 4; also see Fig. 3). The placental imbalance of increased thromboxane and decreased prostacyclin in preeclampsia is even more striking when one compares their production rate ratios between normal and preeclamptic pregnancies (Fig. 5). The normal placenta produces approximately equivalent amounts of thromboxane and prostacyclin; thus, their biologic actions on vascular tone, platelet aggregation, and uterine contractility are balanced. The preeclamptic placenta, however, produces more than seven times as much thromboxane as prostacyclin, so the balance of biologic actions would be tipped heavily in favor of thromboxane (Fig. 6).32 This imbalance explains some of the clinical symptoms of preeclampsia.
Simultaneous measurements of prostacyclin and thromboxane in preeclampsia have been reported for amniotic fluid levels; umbilical, uterine venous, and peripheral plasma levels; and placental release.16, 17 Although not all investigators have found decreased prostacyclin coincident with increased thromboxane, in each case, the data show an imbalance in the ratio of thromboxane to prostacyclin that favors thromboxane.
Maternal circulating levels of thromboxane are not increased in mild preeclampsia,123, 124, 125 but they are increased significantly in severe preeclampsia125 and in hypertensive pregnancies with insufficient fetal growth.126 Prostacyclin is decreased significantly in both mild and severe preeclampsia, so the concept of an imbalance between thromboxane and prostacyclin is valid for both mild and severe forms of the disorder.
The thromboxane/prostacyclin imbalance of preeclampsia could reduce the blood flow between the placenta and fetus because thromboxane constricts and prostacyclin dilates the umbilical artery.83, 84, 85 Additionally, the distribution of blood flow within the fetus could be altered because the increased thromboxane or the decreased prostacyclin could cause vasoconstriction of the ductus arteriosus and pulmonary vasculature.87, 88, 89 The thromboxane/prostacyclin imbalance also would cause vasoconstriction in other fetal vessels and vascular beds because thromboxane is a potent vasoconstrictor in the fetal circulation.77, 78 Vasoconstriction coupled with altered distribution of blood flow presumably would have an adverse effect on fetal growth and development.
Increased oxidative stress and the imbalance between thromboxane and prostacyclin
The cause of the imbalance of increased thromboxane and decreased prostacyclin production in women with preeclampsia is not known, but it could be related to a second significant biochemical imbalance. Women with preeclampsia have an increase in oxidative stress and lipid peroxidation; simultaneously, they have a deficiency in several important antioxidants.127, 128 The abnormally increased levels of lipid peroxides could be responsible for the imbalance of increased thromboxane and decreased prostacyclin because lipid peroxides can increase thromboxane synthesis by stimulating cyclooxygenase,129, 130, 131 but at the same time, they can inhibit prostacyclin synthesis by inhibiting prostacyclin synthase.131, 132 Prolonged exposure of endothelial cells to lipid peroxides both in vivo and in vitro results in decreased prostacyclin synthesis.132, 133 In vitamin E-deficient rats in which lipid peroxide levels are increased, the ratio of thromboxane to prostacyclin is increased many-fold.134 Therefore, abnormally elevated levels of lipid peroxides in preeclampsia could result in the stimulation of thromboxane synthesis and the inhibition of prostacyclin synthesis resulting in their imbalance.
Low-dose aspirin: selective inhibition of thromboxane
Low doses of aspirin (60–150 mg/day) preferentially inhibit thromboxane production without significantly affecting prostacyclin production. Maternal serum thromboxane B2 concentrations are decreased 47–98% by low-dose aspirin and maternal plasma concentrations by 83%.124, 135, 136 Consistent with inhibition of maternal platelet thromboxane synthesis, maternal platelet aggregation is reduced significantly.136 Maternal plasma or serum concentrations of prostacyclin metabolite either are unchanged or are decreased only slightly by low-dose aspirin; thus, the ratio of thromboxane to prostacyclin is decreased. In one study of women in whom preeclampsia was at risk of developing, the ratio of thromboxane to prostacyclin decreased 35% after 3 weeks of low-dose aspirin therapy but increased 51% during the same period in the placebo-treated control group.124
Low doses of aspirin also preferentially inhibit thromboxane production in the placenta. In vitro studies show that placental arterial production of thromboxane is decreased 84% by low-dose aspirin, but production of prostacyclin is not inhibited significantly.137 Similarly, incubation of whole placental tissue with aspirin can result in significant inhibition of thromboxane but not prostacyclin production.138 Clinical studies with low-dose aspirin suggest that placental thromboxane production is preferentially inhibited in vivo, as indicated by increased umbilical–placental blood flow and perfusion, increased fetal growth, increased neonatal weight, and increased placental weight.123, 124, 139, 140, 141, 142, 143, 144, 145, 146
Analysis of maternal urinary metabolites of thromboxane and prostacyclin gives an indication of renal production (TXB2 and 6-keto-PGF1α) or overall production (2,3-dinor-TXB2 and 2,3-dinor-6-keto-PGF1α). Low-dose aspirin decreases the urinary metabolites of thromboxane by 61–87% but has no effect on the urinary metabolites of prostacyclin.31, 123
The ratio of thromboxane to prostacyclin is altered by low-dose aspirin in favor of restoring a balance between thromboxane and prostacyclin. In each of the studies cited previously, the ratio of thromboxane to prostacyclin was decreased by low-dose aspirin. Therefore, the adverse effects of unopposed thromboxane are attenuated or eliminated.
Low-dose aspirin therapy for prevention of preeclampsia
Early studies evaluating low-dose aspirin therapy for the prevention of preeclampsia reported dramatic decreases in the incidence of preeclampsia.147 However, two large multicenter studies, the NICHD Maternal–Fetal Medicine Unit Network trial of low-dose aspirin and the Collaborative Low-dose Aspirin Study in Pregnancy (CLASP), reported only modest decreases in the incidence of preeclampsia.148, 149 The reason for the discrepancy between the earlier studies and the large multicenter studies is not known, but compliance could be an important factor. The initial report based on intent to treat of the NICHD Maternal–Fetal Medicine Unit Network trial of low-dose aspirin to prevent preeclampsia in nulliparous women found a modest reduction in the incidence of preeclampsia from 6.3% for the placebo group to 4.6% for the aspirin group (p = 0.05); however, the intent to treat analysis included noncompliant women and women who discontinued the use of aspirin.149 Reanalysis based on compliance150 revealed a significant decrease in the incidence of preeclampsia from 5.7% for patients who were less than 25% compliant, to 2.7% for patients who were greater than 75% compliant (Fig. 7).151 Furthermore, there was a significant decrease in the incidence of low-birth weight <2500 g from 8.8% to 4.3%, a significant decrease in preterm birth <37 weeks from 10.3% to 5.6%, and a significant decrease in “bad outcome” (defined as preeclampsia, eclampsia, severe preeclampsia, or perinatal mortality) from 8.8% to 4.3%. Of those women in the aspirin group, only 53% had a compliance rate greater than 75%, which raises a concern for interpretation of studies based solely on intent to treat. If only one-half of the patients in the treatment group are compliant in taking the test compound, the effectiveness of the compound is not being adequately evaluated. Meta-analysis of low-dose aspirin trials demonstrates that aspirin is effective in preventing preeclampsia.152, 153 Emphasis on educating the patient regarding the importance of compliance and making it easy for the patient to comply should be used for low-dose aspirin therapy for the prevention of preeclampsia.
DECREASED MATERNAL BLOOD PRESSURE AND SENSITIVITY TO PRESSORS
Low-dose aspirin given to pregnant women is beneficial with respect to blood pressure and vascular sensitivity to pressors. Women in whom hypertension is destined to develop in pregnancy are more responsive to the vasoconstrictive effects of AII.103 Low-dose aspirin therapy decreases the maternal systemic arterial pressor response to infused AII, and most test results that are positive for AII sensitivity (i.e. abnormally increased vascular sensitivity to AII) become negative after low-dose aspirin therapy.135, 154, 155, 156 These studies show that the maternal vasculature becomes less sensitive to vasopressors after administration of low-dose aspirin.
The ratio of thromboxane to prostacyclin is positively correlated with maternal mean blood pressure:124 the higher the ratio, the higher the blood pressure. Low-dose aspirin therapy to lower the ratio of thromboxane to prostacyclin results in lower blood pressure. In women with preeclampsia, low-dose aspirin therapy decreases systolic, diastolic, and mean blood pressures.155, 157
PARTURITION
Prostaglandins (PGE2, PGF2α) have been firmly established as important compounds in the parturitional process. Several comprehensive reviews have been published on this subject. These should be consulted for more detailed descriptions of the role of prostaglandins in labor.4, 5, 82, 158, 159, 160, 161, 162 This section presents an overview of the most salient points.
Evidence for prostaglandins in labor
Three primary lines of evidence indicate the importance of prostaglandins to the process of labor:
- Inhibition of prostaglandin synthesis with cyclooxygenase inhibitors, such as indomethacin, flufenamic acid, or aspirin, inhibits uterine contractility, prolongs the length of gestation, and prolongs the duration of labor in monkeys and women.163, 164, 165
- Exogenous administration of prostaglandins (PGE2 or PGF2α) stimulates uterine contractility in women and monkeys50, 166 and is used to augment labor and for therapeutic termination of pregnancy. Unlike the uterine responsiveness to oxytocin that is manifest at term, the human uterus contracts to prostaglandins at any stage of pregnancy and also during the nonfertile cycle.
- Prostaglandin production is increased at the time of labor (but not before). Peripheral plasma levels of PGF2α metabolite (PGFM) and TXB2 increase during labor in women and monkeys,6, 7, 8, 18, 167 as do amniotic fluid concentrations of PGFM, PGE2, PGF2α, 6-keto-PGF1α, TX, and arachidonate.6, 167, 168, 169, 170, 171, 172, 173, 174, 175 PGE2 production by amnion and PGE and PGF production by decidua are increased significantly in tissues obtained from women at term in spontaneous labor compared with elective cesarean section.176
Stimulators of prostaglandin synthesis
The first step in the synthesis of prostaglandins is the release of arachidonic acid from glycerophospholipids in the cell membranes. The amnion is a rich source of phosphatidylethanolamine and phosphatidylinositol,177 and these phospholipids appear to be the major source of arachidonate for human parturition. Phospholipase A2 acts on phosphatidylethanolamine to release arachidonate, and phospholipase C acts on phosphatidylinositol to release arachidonate-rich diacylglycerol, which then is further metabolized by diacylglycerol and monoacylglycerol lipases to yield arachidonic acid.
The specific activities of phospholipase A2 and phospholipase C are increased in amnion at term compared with during midgestation.178 The activity of diacylglycerol lipase does not change during the latter part of gestation, but prostaglandin synthase (cyclooxygenase) activity in amnion is greater in tissue collected after spontaneous labor at term than in tissue collected after cesarean section without labor at term.179 Free intracellular calcium is necessary for activation of phospholipase A2 and C and for cyclooxygenase.
In sheep, the fetus is the initiator of parturition. In a beautiful set of classic ablation and endocrine experiments, Liggins and colleagues162 established the sequence of events initiated by the ovine fetus that results in parturition. A summary diagram is presented in Fig. 8. Briefly, the sequence of events is as follows: (1) corticotropin-releasing factor (CRF) is released from the fetal hypothalamus; (2) CRF stimulates adrenocorticotropic hormone (ACTH) release from the fetal pituitary gland; (3) ACTH stimulates cortisol release from the fetal adrenal glands; (4) increased circulating cortisol levels stimulate fetal lung maturation and induce development of 17α-hydroxylase in the placenta; (5) 17α-hydroxylase converts placental progesterone to estradiol, resulting in a decline in progesterone levels and a rise in estradiol levels; (6) the increase in the ratio of estradiol to progesterone results in progesterone withdrawal concomitant with estradiol stimulation of prostaglandin synthesis; (7) increased prostaglandin synthesis stimulates uterine contractility and the onset of parturition. Fetal hypophysectomy or adrenalectomy results in a prolongation of gestation in sheep, whereas fetal ACTH or glucocorticoid infusion results in premature labor.
In primates, the role of the fetus in parturition is less clear. Human anencephalic fetuses180 and monkey experimental anencephalic fetuses181 are born at an average gestational length that is not significantly different from healthy intact fetuses. There is, however, a significant disruption in the timing of birth, with more fetuses born prematurely or postmaturely. Glucocorticoid administration to pregnant women or monkeys does not induce premature labor as it does in sheep.161, 182 Quite the contrary, dexamethasone administered to late gestational pregnant monkeys increases the percentage of animals delivering postmaturely.
The fetus of primates does not play the key role in initiating labor as it does in sheep. However, the primate fetus does play an important role in influencing the timing of birth to ensure that it is not born too early or too late. The mechanisms through which the primate fetus influences the timing of birth are not known, but they may involve nocturnal activity of the fetal adrenal glands.
In the monkey, fetal adrenal activity increases at night because of the release of negative feedback inhibition from transplacental passage of maternal cortisol.183 The nocturnal increase in fetal adrenal activity correlates with a nocturnal increase in uterine contractility. Dexamethasone administration not only suppresses fetal adrenal activity, but also abolishes the nocturnal increase in uterine activity, as does death of the fetus. Women, if allowed to deliver naturally, also have a nocturnal rhythm in uterine activity, with the onset of labor contractions being most frequent at night.184
The fetal adrenal glands appear to control the nocturnal increase in uterine contractility by supplying precursors for estrogen biosynthesis. Studies in nonhuman primates demonstrate that the increase in fetal adrenal activity at night results in an increase in the circulating levels of dehydroepiandrosterone sulfate (DHEAS)185 and androstenedione, which then are converted to estrogens by the placenta.186 A nocturnal rhythm in uterine contractility resulting in premature labor can be induced by the infusion of these estrogen precursors.187 Estrogen stimulates several processes that lead to contraction of the uterine muscle. For example, estrogen stimulates the maternal secretion of oxytocin and increases the number of receptors for oxytocin in the myometrium. Estrogen also stimulates the production of prostaglandins and the formation of gap junctions in the myometrium. Uterine muscle becomes more irritable under the influence of estrogen. The nocturnal rhythm in uterine contractility is under the influence of a nocturnal increase in the maternal circulating levels of oxytocin.188 Although there are no marked changes in the levels of oxytocin during the day, there is a progressive increase in the nocturnal rise of maternal oxytocin before the onset of labor.
Several other stimulators of prostaglandin synthesis have been identified. Epidermal growth factor (EGF) stimulates PGE2 output by amniotic cells,189 increases the rate of synthesis of cyclooxygenase in amnion,190 and stimulates uterine contractility in estrogen-primed rats.191 Human platelet-derived growth factor also stimulates prostaglandin synthesis,192 as does platelet activating factor (PAF).177, 193 The amnion can synthesize and metabolize PAF, and PAF is present in human amniotic fluid, fetal membranes, and fetal urine.
Neutrophils are also a source of prostaglandins at the time of labor. Labor is associated with extensive infiltration of neutrophils into the decidua and myometrium.194 Statistical modeling of gene expression at the time of labor found that the most likely initiating event was inflammation. Interleukin-8, a potent neutrophil chemokine, and COX-2, the inducible form of cyclooxygenase, were two key inflammatory genes with increased expression early in the labor process.195 The early increase in interleukin-8 would activate and attract neutrophils to infiltrate reproductive tissues and increased activity of COX-2 would increase prostaglandin production.
Inhibitors of prostaglandin synthesis
An alternative hypothesis to parturition being initiated by stimulators of prostaglandin synthesis is that pregnancy is maintained until the time for delivery by inhibitors of prostaglandin synthesis. At the time of delivery, the inhibitors are removed or overwhelmed by the appearance of prostaglandin stimulators.
Inhibitors of prostaglandin synthesis have been identified in human amniotic fluid.196, 197, 198 Their inhibitory activity is present at midgestation, but the activity decreases significantly by term and declines further with labor. This corresponds to a simultaneous increase in the activity of stimulators of prostaglandin synthesis in the amniotic fluid. The inhibitory actions appear to be exerted either at the cyclooxygenase enzyme or at the phospholipase A2 enzyme. The chemical identities of the inhibitors are not known, but at least one appears to be similar to lipomodulin (lipocortin, macrocortin), the inhibitory protein active against phospholipase A2 that is stimulated by the glucocorticoids. The pregnancy factors responsible for regulating the prostaglandin synthesis inhibitors are not known.
Steroids
In many nonprimate species (e.g. sheep, rabbits, goats), there is a significant decrease in maternal circulating levels of progesterone coincident with a significant increase in estrogen levels that precedes the onset of parturition. This results in “progesterone withdrawal,” allowing the uterus to contract, and simultaneously results in estrogen stimulation of prostaglandin production, causing the uterus to contract. Overt changes such as these in maternal peripheral blood do not occur in human or nonhuman primate pregnancy, so the concept that progesterone withdrawal precedes labor has been questioned for primates.
Progesterone withdrawal may, however, occur locally within the intrauterine tissues.4, 158 Progesterone inhibits PGF2α production, whereas estrogen stimulates PGE and PGF2α production by endometrial tissue. Progesterone is formed from pregnenolone by the action of the 3β-hydroxysteroid dehydrogenase/isomerase enzyme system (3βHSD) in amnion, chorion, decidua, and placenta. Estrogens also are formed in these tissues, and they inhibit this enzyme system. At the time of labor, there is a decrease in the formation of progesterone from pregnenolone within the decidua and chorion. At the same time, there is an increase in the activity of the estrone sulfatase enzyme in decidua and chorion. Therefore, the possibility exists that there is a local progesterone withdrawal within the intrauterine tissues brought about by inhibition of the activity of 3βHSD induced by rising intracellular concentrations of unconjugated estrogens.
Another possibility for progesterone withdrawal involves an alteration in progesterone receptors in the myometrium. Progesterone maintains uterine quiescence by binding to the progesterone receptor (PR)-B isoform. Presence of the PR-A isoform antagonizes the relaxing action of progesterone. At the time of parturition there is an increase in the ratio of PR-A to PR-B.199, 200, 201, 202 The ability of progesterone to maintain uterine quiescence is thus reduced resulting in a functional progesterone withdrawal despite no decrease in production or plasma levels of progesterone. Evidence suggests that the change in progesterone receptors is mediated by prostaglandins. PGE2 and PGF2α acting via the PKC pathway increase the myometrial PR-A/PR-B expression ratio.203
Evidence for lipoxygenase compounds (HETEs, LTs) in labor
Most of the work relating arachidonic acid metabolites to parturition has focused on the cyclooxygenase metabolites, the prostaglandins. Arachidonic acid also can be metabolized by the lipoxygenase enzymes in the human amnion, chorion, decidua, and placenta to form HETEs and leukotrienes.16, 17, 34, 35, 82 There is evidence that these compounds also may be involved in the parturitional process. Walsh and colleagues204 presented evidence for this in 1986 by demonstrating that 5-HETE was present in significantly higher concentrations than PGF2α in the amniotic fluid of chronically catheterized rhesus monkeys (Fig. 9). Furthermore, serial measurements of amniotic fluid 5-HETE demonstrated that its concentrations were altered in association with uterine contractions and labor. In 1987, Romero and others205 reported that 12-HETE, 15-HETE, and LTB4 all were present in human amniotic fluid at term and that all were significantly higher in amniotic fluid collected from women in labor compared with women not in labor at term. These investigators also reported that 15-HETE and LTB4 were increased in association with intra-amniotic infection and preterm labor.206 Also in 1987, Bennett and associates207 reported that group B streptococcus stimulated the release of PGE2 and of dihydroxylated and monohydroxylated HETEs from term human amnion cells in culture.
The concentrations of LTC4 in amniotic fluid of monkeys also increase significantly with the onset of labor (Fig. 8).208 As was true for 5-HETE, in this study, the concentrations of LTC4 were significantly higher than those of PGF2α. Furthermore, one animal went into premature labor and delivered despite treatment with indomethacin and suppressed PGF2α levels. Amniotic fluid levels of 5-HETE and LTC4, however, increased progressively until the onset of labor and rupture of the amniotic fluid sac. This demonstrates that labor can occur in the absence of prostaglandins with the presence of HETEs and leukotrienes.
There are so few data available for the lipoxygenase compounds in association with pregnancy and labor that one can only speculate as to their physiologic functions. The HETEs are best known for their chemokinetic and chemotaxic actions on leukocytes.1, 2 Mononuclear phagocytes, especially the immature ones, increase in human peripheral blood in association with labor,209 and the cytotoxic activity of human lymphocytes increases significantly at the time of labor.210 The intrauterine production of HETEs can act as a signal to recruit leukocytes to the uterus and to activate them, there to augment prostaglandin and leukotriene production and act as a first line of defense against any infection that might enter the uterus from the vagina during or after delivery.
LTC4 is best known for its potent smooth-muscle stimulating activity. LTC4 is equal in potency to PGF2α, but weaker than PGE2, in stimulating contractions of the guinea pig uterus.211 The uterus contains receptors for LTC4.212 It is likely that both 5-HETE and LTC4 act in concert with PGF and PGE to stimulate uterine contractility and parturition because 5-HETE also stimulates human myometrial contractility.
Liggins213 has likened cervical ripening at the time of labor to an inflammatory reaction. Considerable evidence now exists that the entire parturitional process is an inflammatory reaction.214, 215, 216, 217
SUMMARY
The role of prostaglandins in pregnancy, including the roles of thromboxane and the lipoxygenase metabolites, the HETEs and leukotrienes, has been discussed in this chapter. The arachidonic acid metabolites are important compounds in pregnancy. They affect maternal blood pressure and blood flow to the uterus, placenta and umbilical–fetal circulation. In preeclampsia, there is an imbalance of increased thromboxane and decreased prostacyclin production that helps explain many of the clinical symptoms of this disorder, such as hypertension, platelet aggregation, and reduced uteroplacental blood flow. Low doses of aspirin selectively inhibit thromboxane without affecting prostacyclin, and all clinical trials with low-dose aspirin for the prevention of preeclampsia show a decreased incidence. The arachidonic acid metabolites also are important compounds for parturition. Prostaglandins stimulate uterine contractility, and there is considerable evidence about their role in the process of labor. The lipoxygenase metabolites, the HETEs and leukotrienes, also play an important role in the parturitional process.
REFERENCES
Samuelsson B. Leukotrienes and related compounds. Kyoto Conference on Prostaglandins. In: Hayaishi O, Yamamoto S (eds), Advances in Prostaglandin, Thromboxane, Leukotriene Research, vol 15, p 1. New York: Raven Press; 1985 |
|
Samuelsson B, Dahlen S-E, Lindgren JA et al. Leukotrienes and lipoxins: Structures, biosynthesis, and biological effects. Science 1987;237: 1171 |
|
Gaström E, Kindahl H. Radioimmunoassay of prostaglandins and thromboxanes. In: Frolich JC (ed), Methods of Prostaglandin Research, p 119. New York: Raven Press; 1978 |
|
Challis JRG. Characteristics of parturition. In: Creasy RK, Resnik R (eds), Maternal–Fetal Medicine: Principles and Practice, 4th ed, p. 463. Philadelphia: WB Saunders; 1989 |
|
Novy MJ, Liggins GC. Role of prostaglandins, prostacyclin, and thromboxane in the physiologic control of the uterus and in parturition. Semin Perinatol 1980;4:45 |
|
Satoh K, Yasumizu T, Fukuoka H et al. Prostaglandin F2&b.alpha; metabolite levels in plasma, amniotic fluid, and urine during pregnancy and labor. Am J Obstet Gynecol 1979;133:886 |
|
Mitchell MD, Flint AP, Bibby J et al. Plasma concentrations of prostaglandins during late human pregnancy: Influence of normal and preterm labor. J Clin Endocrinol Metab 1978;46:947 |
|
Green K, Bygdeman M, Toppozada M et al. The role of prostaglandin F2&b.alpha; in human parturition: Endogenous plasma levels of 15-keto-13, 14-dihydroprostaglandin F2&b.alpha; during labor. Am J Obstet Gynecol 1974;120:25 |
|
Johnson DA, Manning PA, Hennam JF et al. The concentration of prostaglandin F2&b.alpha; in maternal plasma, foetal plasma and amniotic fluid during pregnancy in women. Acta Endocrinol 1975;79:589 |
|
Whalen JB, Clancey CJ, Farley DB et al. Plasma prostaglandins in pregnancy. Obstet Gynecol 1978;51:52 |
|
Lewis PJ, Boylan P, Friedman LA et al. Prostacyclin in pregnancy. Br Med J 1980;280:1581 |
|
Barrow SE, Blair IA, Waddell KA et al. Prostacyclin in late pregnancy: Analysis of 6-oxo-prostaglandin F1&b.alpha; in maternal plasma. In: Lewis PJ, Moncada S, O'Grady J (eds), Prostacyclin in Pregnancy, p 79. New York: Raven Press; 1983 |
|
Ylikorkala O, Mäkärinen L, Viinikka L. Prostacyclin production increases during human parturition. Br J Obstet Gynaecol 81981;8:513 |
|
Gryglewski RJ, Korbut R, Ocetkiewicz A. Generation of prostacyclin by lungs in vivo and its release into the arterial circulation. Nature 1978;273:765 |
|
Moncada S, Korbut R, Bunting S et al. Prostacyclin is a circulating hormone. Nature 1978;273:767 |
|
Walsh SW. Eicosanoids and pregnancy-related hypertension. In: Hillier K (ed), Eicosanoids and Reproduction, p 128. Lancaster, UK: MTP Press; 1987 |
|
Walsh SW, Parisi VM. The role of arachidonic acid metabolites in preeclampsia. Semin Perinatol 1986;10:334 |
|
Mitchell MD, Bibby JG, Hicks BR et al. Thromboxane B2 and human parturition: Concentrations in the plasma and production in vitro. J Endocrinol 1978;78:435 |
|
Ylikorkala O, Viinikka L. Thromboxane A2 in pregnancy and puerperium. Br Med J 1980;281:1601 |
|
Pritchard JA, MacDonald PC, Gant NF. Williams Obstetrics, 17th ed. East Norwalk, CT: Appleton-Century-Crofts; 1985 |
|
Quilligan EJ. Maternal physiology. In: Danforth DN (ed), Obstetrics and Gynecology, 4th ed, p 326. Philadelphia: JB Lippincott; 1982 |
|
Brinkman CR III. Maternal cardiovascular and renal disorders—biologic adaptation to pregnancy. In: Creasy RK, Resnik R (eds), Maternal–Fetal Medicine: Principles and Practice, p 679. Philadelphia: WB Saunders, 1984 |
|
Everett RB, Worley RJ, MacDonald PC et al. Effect of prostaglandin synthetase inhibitors on pressor response to angiotensin II in human pregnancy. J Clin Endocrinol Metab 1978;46:1007 |
|
Moncada S, Vane JR. Arachidonic acid metabolites and the interactions between platelets and blood-vessel walls. N Engl J Med 1979;300:1142 |
|
Moncada S, Vane JR. Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2, and prostacyclin. Pharmacol Rev 1979;30:293 |
|
Moncada S, Vane JR. The role of prostacyclin in vascular tissue. Fed Proc 1979;38:66 |
|
Flower RJ, Cardinal DC. Use of a novel platelet aggregometer to study the generation by, and action of, prostacyclin in whole blood. In: Vane JF (ed), Prostacyclin, p 211. New York: Raven Press; 1979 |
|
Gant NF, Chand S, Worley RJ et al. A clinical test useful for predicting the development of acute hypertension in pregnancy. Am J Obstet Gynecol 1974;120:1 |
|
Dusting GJ, Moncada S, Vane JR. Prostacyclin: Its biosynthesis, actions, and clinical potential. In Oates JA (ed), Prostaglandins and the Cardiovascular System, Advances in Prostaglandin, Thromboxane, Leukotriene Research, vol 10, p 59. New York: Raven Press; 1982 |
|
Gorman RR. Modulation of human platelet function by prostacyclin and thromboxane A2. Fed Proc 1979;38:83 |
|
Fitzgerald DJ, Mayo G, Catella F et al. Increased thromboxane biosynthesis in normal pregnancy is mainly derived from platelets. Am J Obstet Gynecol 1987;157:325 |
|
Walsh SW. Preeclampsia: An imbalance in placental prostacyclin and thromboxane production. Am J Obstet Gynecol 1985;152:335 |
|
Nelson DM, Walsh SW. Thromboxane and prostacyclin production by different compartments of the human placental villus. J Clin Endocrinol Metab 1989;68:676 |
|
Saeed SA, Mitchell MD. Formation of arachidonate lipoxygenase metabolites by human fetal membranes, uterine decidua vera and placenta. Prostaglandins Leukotrienes Med 1982;8:635 |
|
Myatt L, Rose MP, Elder MG. Lipoxygenase products of arachidonic acid in human fetal membranes (abstract 160). Presented at the 32nd Annual Meeting of the Society for Gynecologic Investigation, Phoenix, March 20–23, 1985 |
|
Goetzl EJ, Goldman DW, Naccache PH et al. Mediation of leukocyte components of inflammatory reactions by lipoxygenase products of arachidonic acid. In: Samuelsson B, Paoletti R (eds), Leukotrienes and Other Lipoxygenase Products, Advances in Prostaglandin, Thromboxane, Leukotriene Research, vol 9, p 273. New York: Raven Press; 1982 |
|
Samuelsson B. The leukotrienes: An introduction. In: Samuelsson B, Paoletti R (eds), Leukotrienes and Other Lipoxygenase Products, Advances in Prostaglandin, Thromboxane, Leukotriene Research, vol 9, p 1. New York: Raven Press; 1982 |
|
Samuelsson B. Leukotrienes: Mediators of immediate hypersensitivity reactions and inflammation. Science 1983;220:568 |
|
Bach MK. Current Concepts: The Leukotrienes: Their Structure, Actions, and Role in Diseases. Kalamazoo: Scope Publishers; 1983 |
|
Piper PJ. Formation and actions of leukotrienes. Physiol Rev 1984;64:744 |
|
Feuerstein G, Hallenbeck JM. Leukotrienes in health and disease. FASEB J 1987;1:186 |
|
Letts LG, Cirino M. Vascular actions of leukotrienes. In: Lefer AM, Gee MH (eds), Leukotrienes in Cardiovascular and Pulmonary Function, p 47. New York: Alan R. Liss; 1985 |
|
Smedegard G, Hedqvist P, Dahlen S-E et al. Leukotriene C4 affects pulmonary and cardiovascular dynamics in monkey. Nature 1982;295:327 |
|
Casey L, Clarke J, Fletcher J et al. Cardiovascular, respiratory, and hematologic effects of leukotriene D4 in primates. In: Samuelsson B, Paoletti R (eds), Leukotrienes and Other Lipoxygenase Products, Advances in Prostaglandin, Thromboxane, Leukotriene Research, vol 9, p 201. New York: Raven Press; 1982 |
|
Kaijser L. Cardiovascular and pulmonary effects of leucotriene C4 in man. Eur J Respir Dis 1982;63(suppl 124):76 |
|
Walsh SW, Parisi VM. Leukotrienes, but not hydroxyeicosatetraenoic acids, lower blood pressure in pregnant and postpartum rhesus monkeys. Clin Exp Hypertens [B], Hypertens Pregn 1989;B8:305 |
|
Cramer EB, Pologe L, Pawlowski NA et al. Leukotriene C promotes prostacyclin synthesis by human endothelial cells. Proc Natl Acad Sci USA 1983;80:4109 |
|
Johnson AR, Revtyak GE, Ibe BO et al. Endothelial cells metabolize but do not synthesize leukotrienes. In: Lefer AM, Gee MH (eds), Leukotrienes in Cardiovascular and Pulmonary Function, p 185. New York: Alan R. Liss |
|
Clark MA, Littlejohn D, Mong S et al. Effect of leukotrienes, bradykinin and calcium ionophore (A 23187) on bovine endothelial cells: Release of prostacyclin. Prostaglandins 1986;31:157 |
|
Novy MJ, Thomas CL, Lees MH. Uterine contractility and regional blood flow responses to oxytocin and prostaglandin E2 in pregnant rhesus monkeys. Am J Obstet Gynecol 1975;22:419 |
|
Still JG, Greiss FC. The effect of prostaglandins and other vasoactive substances on uterine blood flow and myometrial activity. Am J Obstet Gynecol 1978;130:1 |
|
Clark KE, Austin JE, Stys SJ. Effect of bisenoic prostaglandins on the uterine vasculature of the nonpregnant sheep. Prostaglandins 1981;22:333 |
|
Clark KE, Austin JE, Seeds AE. Effect of bisenoic prostaglandins and arachidonic acid on the uterine vasculature of pregnant sheep. Am J Obstet Gynecol 1982;142:261 |
|
Schramm W, Einer-Jensen N, Brown MB et al. Effect of four primary prostaglandins and relaxin on blood flow in the ovine endometrium and myometrium. Biol Reprod 1984;30:523 |
|
Rankin JHG, Phernetton TM, Stock MK et al. Effect of PGI2 on the response of the ovine placenta to norepinephrine. Prostaglandins 1982;24:207 |
|
Landauer M, Phernetton TM, Parisi VM et al. Ovine placental vascular response to the local application of prostacyclin. Am J Obstet Gynecol 1985;151:460 |
|
Schwartz DB, Phernetton TM, Stock MK et al. Placental vascular responses to 6-keto-prostaglandin E1 in the near-term sheep. Am J Obstet Gynecol 1983;145:406 |
|
Parisi VM, Rankin JHG. The effect of prostacyclin on angiotensin II-induced placental vasoconstriction. Am J Obstet Gynecol 1095;151:444 |
|
Thorp JA, Walsh SW, Brath PC. Comparison of the vasoactive effects of leukotrienes with thromboxane mimic in the perfused human placenta. Am J Obstet Gynecol 1988;159:1376 |
|
Bjoro K, Stray-Pedersen S. Effects of vasoactive autacoids on different segments of human umbilicoplacental vessels. Gynecol Obstet Invest 1986;22:1 |
|
Glance DG, Elder MG, Myatt L. The actions of prostaglandins and their interactions with angiotensin II in the isolated perfused human placental cotyledon. Br J Obstet Gynaecol 1986;93:488 |
|
Howard RB, Hosokawa T, Maquire MH. Pressor and depressor actions of prostanoids in the intact human fetoplacental vascular bed. Prostaglandins Leukotrienes Med 1986;21:323 |
|
Mak KKW, Gude NM, Walters WAW et al. Effects of vasoactive autacoids on the human umbilical-fetal placental vasculature. Br J Obstet Gynaecol 1984;91:99 |
|
Abramovich DR, Page KR, Parkin AML. The effect of prostaglandin D2 on the blood vessels of the perfused isolated cotyledon of the human placenta. Br J Pharmacol 1984;81:19 |
|
Czekanowski R, Muzyczuk Z, Czekanowska M. The influence of prostaglandins upon the umbilical and placental circulation in humans in vitro. Mater Med Pol 1984;52:108 |
|
Gonzalez-Panizza VH, Alvarez H, Benedetti WL. The in vitro contractility of the human placental chorial vessels. J Reprod Med 1981;26:478 |
|
Maigaard S, Forman A, Andersson K-E. Digoxin inhibition of relaxation induced by prostacyclin and vasoactive intestinal polypeptide in small human placental arteries. Placenta 1985;6:435 |
|
Maigaard S, Forman A, Andersson K-E. Relaxant and contractile effects of some amines and prostanoids in myometrial and vascular smooth muscle within the human uteroplacental unit. Acta Physiol Scand 1986;128:33 |
|
Maigaard S, Forman A, Broggard-Hansen KP et al. Inhibitory effects of nitrendipine on myometrial and vascular smooth muscle in human pregnant uterus and placenta. Acta Pharmacol Toxicol 1986;59:1 |
|
Nielsen-Kudsk JE. Effects of theophylline on small human placental arteries in vitro. Acta Pharmacol Toxicol 1985;56:14 |
|
Tulenko TN. The actions of prostaglandins and cyclooxygenase inhibition on the resistance vessels supplying the human fetal placenta. Prostaglandins 1981;21:1033 |
|
Berman W Jr, Goodlin RC, Heymann MA et al. Effects of pharmacologic agents on umbilical blood flow in fetal lambs in utero. Biol Neonate 1978;33:225 |
|
McLaughlin MK, Brennan SC, Chez RA. Vasoconstrictive effects of prostaglandins in sheep placental circulations. Am J Obstet Gynecol 1978;130:408 |
|
Novy MJ, Piasecki G, Jackson BT. Effect of prostaglandins E2 and F2&b.alpha; on umbilical blood flow and fetal hemodynamics. Prostaglandins 1974;5:543 |
|
Rankin JHG. A role of prostaglandins in the regulation of the placental blood flow. Prostaglandins 1976;11:343 |
|
Rankin JHG. Role of prostaglandins in the maintenance of the placental circulation. In: Coceani F, Olley PM (eds), Prostaglandins and Perinatal Medicine, Advances in Prostaglandin and Thromboxane Research, vol 4, p 261. New York: Raven Press; 1978 |
|
Parisi VM, Walsh SW. Fetoplacental vascular responses to prostacyclin after thromboxane-induced vasoconstriction. Am J Obstet Gynecol 1989;160:502 |
|
Trudinger B, Connelly A, Giles W et al. The effects of prostacyclin and thromboxane analogue (U46619) on the fetal circulation and umbilical flow velocity waveforms. J Dev Physiol 1989;11:179 |
|
Rankin JHG, Phernetton TM, Anderson DF et al. Effect of prostaglandin I2 on ovine placental vasculature. J Dev Physiol 1979;1:151 |
|
Walsh SW, Parisi VM. The role of prostanoids and thromboxane in the regulation of placental blood flow. In: Rosenfeld CR (ed), The Uterine Circulation, p 273. Ithaca: Perinatology Press; 1989 |
|
Mäkilä U-M, Jouppila P, Kirkinen P et al. Relation between umbilical prostacyclin production and blood-flow in the fetus. Lancet 1983;1:728 |
|
Mitchell MD, Brennecke SP, Saeed SA et al. Arachidonic acid metabolism in the fetus and neonate. In: Cohen MM (ed), Biological Protection with Prostaglandins, vol 1, p 27. Boca Raton, FL: CRC Press; 1985 |
|
Pomerantz K, Sintetos A, Ramwell P. The effect of prostacyclin on the human umbilical artery. Prostaglandins 1978;15:1035 |
|
Tuvemo T. Action of prostaglandins and blockers of prostaglandin synthesis on the isolated human umbilical artery. In: Coceani F, Olley PM (eds), Prostaglandins and Perinatal Medicine, Advances in Prostaglandin and Thromboxane Research, vol 4, p 271. New York: Raven Press; 1978 |
|
Tuvemo T. Role of prostaglandins, prostacyclin, and thromboxanes in the control of the umbilical-placental circulation. Semin Perinatol 1980;4:91 |
|
Rankin JHG, Phernetton TM. Circulatory responses of the near-term sheep fetus to prostaglandin E2. Am J Physiol 1976;231:760 |
|
Terragno NA, Terragno A, McGiff JC. Role of prostaglandins in blood vessels. Semin Perinatol 1980;4:85 |
|
Cassin S. Role of prostaglandins and thromboxanes in the control of the pulmonary circulation in the fetus and newborn. Semin Perinatol 1980;4:101 |
|
Coceani F, Olley PM. Role of prostaglandins, prostacyclin, and thromboxanes in the control of prenatal patency and postnatal closure of the ductus arteriosus. Semin Perinatol 1980;4:109 |
|
Sharpe GL, Larsson KS, Thalme B. Studies on closure of the ductus arteriosus: XII. In utero effect of indomethacin and sodium salicylate in rats and rabbits. Prostaglandins 1975;9:585 |
|
Heymann MA, Rudolph AM. Effects of prostaglandins and blockers of prostaglandin synthesis on the ductus arteriosus: Animal and human studies. In: Coceani F, Olley PM (eds), Prostaglandins and Perinatal Medicine. Advances in Prostaglandin and Thromboxane Research, vol 4, p 363. New York: Raven Press; 1978 |
|
Phernetton TM, Rankin JHG. Effect of prostaglandin I2 on ovine maternal and fetal adrenal blood flows. Proc Soc Exp Biol Med 1979;162:324 |
|
Yokocki K, Olley PM, Sideris E et al. Leukotriene D4: A potent vasoconstrictor of the pulmonary and systemic circulations in the newborn lamb. In: Samuelsson B, Paoletti R (eds), Leukotrienes and Other Lipoxygenase Products, Advances in Prostaglandin, Thromboxane, Leukotriene Research, vol 9, p 211. New York: Raven Press; 1982 |
|
Leffler CW, Mitchell JA, Green RS. Cardiovascular effects of leukotrienes in neonatal piglets: Role in hypoxic pulmonary vasoconstriction? Circ Res 1984;55:780 |
|
Soifer SJ, Loitz RD, Roman C et al. Leukotriene end organ antagonists increase pulmonary blood flow in fetal lambs. Am J Physiol 1985;249:H570 |
|
Stenmark KR, James SL, Voelkel NF et al. Leukotriene C4 and D4 in neonates with hypoxemia and pulmonary hypertension. N Engl J Med 1983;309:77 |
|
Schreiber MD, Heymann MA, Soifer SJ. Leukotriene inhibition prevents and reverses hypoxic pulmonary vasoconstriction in newborn lambs. Pediatr Res 1985;19:437 |
|
Chesley LC. Hypertensive Disorders in Pregnancy. New York: Appleton-Century-Crofts; 1978 |
|
Dennis EJ III, McFarland KF, Hester LL Jr. The pre-eclampsia-eclampsia syndrome. In: Danforth DN (ed), Obstetrics and Gynecology, 4th ed, p 455. Philadelphia: JB Lippincott; 1982 |
|
MacGillivray I. Preeclampsia: The Hypertensive Disease of Pregnancy. Philadelphia: WB Saunders; 1983 |
|
Roberts JM. Pregnancy-related hypertension. In: Creasy RK, Resnik R (eds), Maternal–Fetal Medicine: Principles and Practice, p 703. Philadelphia: WB Saunders; 1984 |
|
Pedersen EB, Christensen NJ, Christensen P et al. Preeclampsia—a state of prostaglandin deficiency? Urinary prostaglandin excretion, the renin–aldosterone system, and circulating catecholamines in preeclampsia. Hypertension 1983;5:105 |
|
Gant NF, Daley GL, Chand S et al. A study of angiotensin II pressor responses throughout primigravid pregnancy. J Clin Invest 1973;52:2682 |
|
Speroff L. Toxemia of pregnancy: Mechanism and therapeutic management. Am J Cardiol 1973;32:582 |
|
Omini C, Folco GC, Pasargiklian R et al. Prostacyclin (PGI2) in pregnant human uterus. Prostaglandins 1979;17:113 |
|
Wilhelmsson L, Wikland M, Wiqvist N. PGH2, TXA2, and PGI2 have potent and differentiated actions on human uterine contractility. Prostaglandins 1981;21:277 |
|
Lye SJ, Challis JRG. Inhibition by PGI2 of myometrial activity in vivo in nonpregnant ovariectomized sheep. J Reprod Fertil 1982;66:311 |
|
Lewis PJ, Moncada S, O'Grady J (eds). Prostacyclin in Pregnancy. New York: Raven Press; 1983 |
|
Remuzzi G, Marchesi D, Zoja C et al. Reduced umbilical and placental vascular prostacyclin in severe preeclampsia. Prostaglandins 1980;20:105 |
|
Bussolino F, Benedetto C, Massobrio M et al. Maternal vascular prostacyclin activity in preeclampsia. Lancet 1980;2:702 |
|
Downing I, Shepherd GL, Lewis PJ. Reduced prostacyclin production in preeclampsia. Lancet 1980;2:1374 |
|
Goodman RP, Killam AP, Brash AR et al. Prostacyclin production during pregnancy: Comparison of production during normal pregnancy and pregnancy complicated by hypertension. Am J Obstet Gynecol 1982;142:817 |
|
Walsh SW, Behr MJ, Allen NH. Placental prostacyclin production in normal and toxemic pregnancies. Am J Obstet Gynecol 1985;151:110 |
|
Stuart MJ, Clark DA, Sunderji SG et al. Decreased prostacyclin production: A characteristic of chronic placental insufficiency syndromes. Lancet 1981;1:1126 |
|
Jogee M, Myatt L, Elder MG. Decreased prostacyclin production by placental cells in culture from pregnancies complicated by fetal growth retardation. Br J Obstet Gynaecol 1983;90:247 |
|
Jogee M, Myatt L, Moore P et al. Prostacyclin production by human placental cells in short-term culture. Placenta 1983;4:219 |
|
Rakoczi I, Tihanyi K, Falkay G et al. Prostacyclin production in trophoblast. In: Lewis PJ, Moncada S, O'Grady J (eds), Prostacyclin in Pregnancy, p 15. New York: Raven Press; 1983 |
|
Miyamori I, Fitzgerald GA, Brown MJ et al. Prostacyclin stimulates the renin angiotensin aldosterone system in man. J Clin Endocrinol Metab 1979;49:943 |
|
Oates JA, Whorton AR, Gerber J et al. Prostacyclin and the kidney. In: Vane JR, Bergstrom S (eds), Prostacyclin, p 195. New York: Raven Press; 1979 |
|
Mills JL, DerSimonian R, Raymond E, Morrow JD, Roberts LJ, 2nd, Clemens JD, Hauth JC, Catalano P, Sibai B, Curet LB, Levine RJ. Prostacyclin and thromboxane changes predating clinical onset of preeclampsia: a multicenter prospective study. JAMA. 1999;282:356-362. |
|
Klockenbusch W, Somville T, Hafner D, Strobach H, Schror K. Excretion of prostacyclin and thromboxane metabolites before, during, and after pregnancy-induced hypertension. Eur J Obstet Gynecol Reprod Biol. 1994;57:47-50. |
|
Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest. 1973;52:2682-2689. |
|
Benigni A, Gregorini G, Frusca T et al. Effect of low-dose aspirin on fetal and maternal generation of thromboxane by platelets in women at risk for pregnancy-induced hypertension. N Engl J Med 1989;321:357 |
|
Schiff E, Peleg E, Goldenberg M et al. The use of aspirin to prevent pregnancy-induced hypertension and lower the ratio of thromboxane A2 to prostacyclin in relatively high risk pregnancies. N Engl J Med 1989;321:351 |
|
Wang Y, Walsh SW, Guo J et al. The imbalance between thromboxane and prostacyclin in preeclampsia is associated with an imbalance between lipid peroxides and vitamin E in maternal blood. Am J Obstet Gynecol 1991;165:1695 |
|
Wallenburg HCS, Rotmans N. Enhanced reactivity of the platelet thromboxane pathway in normotensive and hypertensive pregnancies with insufficient fetal growth. Am J Obstet Gynecol 1982;144:523 |
|
Hubel CA, Roberts JM, Taylor RN et al. Lipid peroxidation in pregnancy: New perspectives on preeclampsia. Am J Obstet Gynecol 1989;161:1025 |
|
Walsh SW. Lipid peroxidation in pregnancy. Hypertens Pregn 1994;13:1 |
|
Walsh SW, Wang Y, Jesse R. Peroxide induces vasoconstriction in the human placenta by stimulating thromboxane. Am J Obstet Gynecol 1993;169:1007 |
|
Hemler ME, Cook HW, Lands WEM. Prostaglandin biosynthesis can be triggered by lipid peroxides. Arch Biochem Biophys 1979;193:340 |
|
Warso MA, Lands WEM. Lipid peroxidation in relation to prostacyclin and thromboxane physiology and pathophysiology. Br Med Bull 1983;39:277 |
|
Wang J, Zhen E, Guo Z, Lu Y. Effect of hyperlipidemic serum on lipid peroxidation, synthesis of prostacyclin and thromboxane by cultured endothelial cells: Protective effect of antioxidants. Free Radic Biol Med 1989;7:243 |
|
Lorentzen B, Endresen MJ, Hovig T et al. Sera from preeclamptic women increase the content of triglycerides and reduce the release of prostacyclin in cultured endothelial cells. Thromb Res 1991;63:363 |
|
Falanga A, Doni MG, Delaini F et al. Unbalanced plasma control of TXA2 and PGI2 synthesis in vitamin E-deficient rats. Am J Physiol 1983;245:H867 |
|
Spitz B, Magness RR, Cox SM et al. Low-dose aspirin. I. Effect on angiotensin II pressor responses and blood prostaglandin concentrations in pregnant women sensitive to angiotensin II. Am J Obstet Gynecol 1988;159:1035 |
|
Sibai BM, Mirro R, Chesney CM et al. Low-dose aspirin in pregnancy. Obstet Gynecol 1989;74:551 |
|
Thorp JA, Walsh SW, Brath PC. Low-dose aspirin inhibits thromboxane, but not prostacyclin production by human placental arteries. Am J Obstet Gynecol 1988;159:1381 |
|
Nelson DM, Walsh SW. Aspirin differentially affects thromboxane and prostacyclin production by trophoblast and villous core compartments of human placental villi. Am J Obstet Gynecol 1989;161:1593 |
|
Gastaldi A, Frusca T, Gregorini G: Prevention of preeclampsia and possible correlation with physiopathology. Clin Exp Hypertens [B] 1988;B7:139 |
|
Frusca T, Gregorini G, Ballerini S et al: Low dose aspirin in preventing preeclampsia and IUGR (abstr). Clin Exp Hypertens [B] 1989;B8:218 |
|
Ballerini S, Valcamonico A, Gregorini G et al: Low dose aspirin (ASA) given to prevent preeclampsia only partially inhibits fetal platelet cyclooxygenase activity (abstr). Clin Exp Hypertens [B] 1989;B8:219 |
|
Trudinger BJ, Cook CM, Thompson RS et al: Low-dose aspirin therapy improves fetal weight in umbilical placental insufficiency. Am J Obstet Gynecol 1988;159:681 |
|
Trudinger BJ, Cook CM, Thompson RS et al: Low-dose aspirin improves fetal weight in umbilical placental insufficiency. Lancet 1988;2:214 |
|
Elder MG, DeSwiet M, Robertson A et al: Letter to the editor: Low-dose aspirin in pregnancy. Lancet 1988;1:410 |
|
Wallenburg HCS, Rotmans N: Letter to the editor: Prophylactic low-dose aspirin and dipyridamole in pregnancy. Lancet 1988;1:939 |
|
Wallenburg HCS, Rotmans N: Prevention of recurrent idiopathic fetal growth retardation by low-dose aspirin and dipyridamole. Am J Obstet Gynecol 1987;157:1230 |
|
Hauth JC and Cunningham FG. Low-Dose Aspirin During Pregnancy. In: Williams Obstetrics Supplement No. 14 (19 ed.), Cunningham FG, MacDonald PC, Gant NF, Leveno KJ and Gilstrap LC, III (eds). Norwalk, Connecticut: Appleton & Lange, 1995, p. S1-S14 |
|
CLASP. a randomised trial of low-dose aspirin for the prevention and treatment of pre-eclampsia among 9364 pregnant women. CLASP (Collaborative Low-dose Aspirin Study in Pregnancy) Collaborative Group. Lancet 1994;343:619 |
|
Sibai BM, Caritis SN, Thom E et al. Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med 1993;329:1213 |
|
Hauth J, Owen J, Thom E et al. Prevention of preeclampsia with low-dose aspirin in nulliparas: A compliance analysis [Abstract #56]. In: Scientific Program and Abstracts 43rd Meeting, Philadelphia, Pennsylvania, March 20-23, 1996. J Soc Gynecol Invest 1996;3(2):86A |
|
Walsh SW. Eicosanoids in preeclampsia. Prostaglandins Leukot Essent Fatty Acids 2004;70:223 |
|
Askie LM, Duley L, Henderson-Smart DJ, Stewart LA. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet 2007;369:1791 |
|
Bujold E, Roberge S, Lacasse Y, Bureau M, Audibert F, Marcoux S, Forest JC, Giguere Y. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116:402-414. |
|
Sanchez-Ramos L, O'Sullivan MJ, Carrido-Calderon J. Effect of low-dose aspirin on angiotensin II pressor response in human pregnancy. Am J Obstet Gynecol 1987;156:193 |
|
Caruso A, Ferrazzani S, DeCarolis S et al. Effects of low-dose aspirin on vascular sensitivity to angiotensin II and on 24 hours arterial blood pressure in pregnancy. Clin Exp Hypertens [B] 1988;B7:171 |
|
Wallenburg HCS, Dekker GA, Makovitz JW et al. Effect of low-dose aspirin on vascular refractoriness in angiotensin-sensitive primigravid women. Am J Obstet Gynecol 1991;164:1169 |
|
Toppozada MK. Treatment of severe preeclampsia by prostaglandins or their synthesis inhibitors (abstr 132). Presented at the First European Congress on Prostaglandins in Reproduction (ECPR), July 6–9, 1988, Vienna |
|
Challis JRG, Mitchell BF, Power SGA et al. Steroid and prostaglandin production by intrauterine tissues in the sheep and human in relation to parturition. In: Albrecht E, Pepe GJ (eds), Perinatal Endocrinology, p 263. Ithaca: Perinatology Press; 1985 |
|
MacDonald PC, Porter J (eds). Initiation of Parturition: Prevention of Prematurity: Report on the Fourth Ross Conference on Obstetric Research, p 1. Columbus: Ross Laboratories; 1983 |
|
Thorburn GD. Prostaglandins and the regulation of myometrial activity: A working model. In: Jones CT, Nathanielsz PW (eds), The Physiological Development of the Fetus and Newborn, p 381. New York: Academic Press; 1985 |
|
Liggins GC, Forster CS, Grieves SA et al. Control of parturition in man. Biol Reprod 1977;16:39 |
|
Liggins GC, Fairclough RJ, Grieves SA et al. The mechanism of initiation of parturition in the ewe. Recent Prog Horm Res 1973;29:111 |
|
Novy MJ, Cook MJ, Manaugh L. Indomethacin block of normal onset of parturition in primates. Am J Obstet Gynecol 1974;118:412 |
|
Johnson WL, Harbert GM, Martin CB. Pharmacologic control of uterine contractility: In vitro human and in vivo monkey studies. Am J Obstet Gynecol 1975;123:364 |
|
Schwartz A, Brook I, Insler V et al. Effect of flufenamic acid on uterine contractions and plasma levels of 15-keto-13,14,dihydroprostaglandin F2&b.alpha; in preterm labor. Gynecol Obstet Invest 1978;9:139 |
|
Karim SMM. Physiological role of prostaglandins in the control of parturition and menstruation. J Reprod Fertil 1972;16(Suppl):105 |
|
Mitchell MD, Patrick JE, Robinson JS et al. Prostaglandins in the plasma and amniotic fluid of rhesus monkeys during pregnancy and after intrauterine foetal death. J Endocrinol 1976;71:67 |
|
Keirse MJNC, Turnbull AC. E prostaglandins in amniotic fluid during late pregnancy and labor. J Obstet Gynaecol Br Commonw 1973;80:970 |
|
Keirse MJNC, Turnbull AC. The fetal membranes as a possible source of amniotic fluid prostaglandins. Br J Obstet Gynaecol 1976;83:146 |
|
Novy MJ. Endocrine and pharmacological factors which influence the onset of labour in rhesus monkeys. In: O'Connor M, Knight J (eds), The Fetus and Birth, p 259. New York: Elsevier/Exerpta Medica; 1977 |
|
Challis JRG, Robinson JS, Thornburn GD. Fetal and maternal endocrine changes during pregnancy and parturition in the rhesus monkey. In: O'Connor M, Knight J (eds), The Fetus and Birth, p 211. New York: Elsevier/Excerpta Medica; 1977 |
|
Walsh SW, Stanczyk FZ, Novy MJ. Daily hormonal changes in the maternal, fetal and amniotic fluid compartments before parturition in a primate species. J Clin Endocrinol Metab 1984;58:629 |
|
Robinson JS, Natale R, Clover L et al. Prostaglandin E, thromboxane B2 and 6-oxo-prostaglandin F1&b.alpha; in amniotic fluid and maternal plasma of rhesus monkeys (Macaca mulatta) during the latter third of gestation. J Endocrinol 1979;81:345 |
|
Mitchell MD, Keirse MJNC, Brunt JD et al. Concentrations of the prostacyclin metabolite, 6-keto-prostaglandin F1&b.alpha; in amniotic fluid during late pregnancy and labour. Br J Obstet Gynaecol 1979;86:350 |
|
MacDonald PC, Schultz FM, Duenhoelter JH et al. Initiation of human parturition: I. Mechanism of action of arachidonic acid. Obstet Gynecol 1974;44:629 |
|
Skinner KA, Challis JRG. Changes in the synthesis and metabolism of prostaglandins by human fetal membranes and decidua at labor. Am J Obstet Gynecol 1985;151:519 |
|
Bleasdale JE, Johnston JM. Prostaglandins and human parturition: Regulation of arachidonic acid mobilization. In: Scarpelli EM, Cosmi EV (eds), Reviews in Perinatal Medicine, vol 5, p 151. New York: Alan R Liss; 1984 |
|
Bleasdale JE, Okazaki T, Sagawa N et al. The mobilization of arachidonic acid for prostaglandin production during parturition. In: MacDonald PC, Porter J (eds), Initiation of Parturition: Prevention of Prematurity: Report on the Fourth Ross Conference on Obstetric Research, p 129. Columbus: Ross Laboratories; 1983 |
|
Okazaki T, Casey ML, Okita JR et al. Initiation of human parturition: XII. Biosynthesis and metabolism of prostaglandins in human fetal membranes and uterine decidua. Am J Obstet Gynecol 1981;139:373 |
|
Honnebier WJ, Swaab DF. The influence of anencephaly upon intrauterine growth of fetus and placenta and upon gestation length. J Obstet Gynaecol Br Commonw 1973;80:577 |
|
Novy MJ, Walsh SW, Kittinger GW. Experimental fetal anencephaly in the rhesus monkey: Effect on gestational length and fetal and maternal palsma steroids. J Clin Endocrinol Metab 1977;45:1031 |
|
Novy MJ, Walsh SW. Dexamethasone and estradiol treatment in pregnant rhesus macaques: Effects on gestational length, maternal plasma hormones, and fetal growth. Am J Obstet Gynecol 1983;145:920 |
|
Walsh SW. Regulation of progesterone and estrogen production during rhesus monkey pregnancy. In: Albrecht ED, Pepe GJ (eds), Research in Perinatal Medicine, vol IV, Perinatal Endocrinology, p 219. Ithaca: Perinatology Press; 1985 |
|
Smolensky MH. Aspects of human chronopathology. In: Reinberg A, Smolensky MH (eds), Biological Rhythms and Medicine: Cellular, Metabolic, Physiopathologic, and Pharmacologic Aspects, p 131. New York: Springer-Verlag; 1983 |
|
Walsh SW, Ducsay CA, Novy MJ. Circadian hormonal interactions among the mother, fetus, and amniotic fluid. Am J Obstet Gynecol 1984;150:745 |
|
Walsh SW, Resko JA, Grumbach MM, Novy MJ. In utero evidence for a functional fetoplacental unit in rhesus monkeys. Biol Reprod 1980;23:264 |
|
Nathanielsz PW. The timing of birth. Am Scientist 1996;84:562 |
|
Hirst JJ, Haluska GJ, Cook M, Novy MJ. Plasma oxytocin and nocturnal uterine activity: Maternal but not fetal concentrations increase progressively during late pregnancy and delivery in rhesus monkeys. Am J Obstet Gynecol 1993;169:415 |
|
Mitchell MD. Epidermal growth factor actions on arachidonic acid metabolism in human amnion cells. Biochim Biophys Acta 1987;928:240 |
|
Casey ML, Korte K, MacDonald PC. Epidermal growth factor stimulation of prostaglandin E2 biosynthesis in amnion cells. Induction of prostaglandin H2 synthase. J Biol Chem 1988;263:7846 |
|
Gardner RM, Lingham RB, Stancel GM. Contractions of the isolated uterus stimulated by epidermal growth factor. FASEB J 1987;1:224 |
|
Habenicht AJR, Goerig M, Grulich J et al. Human platelet-derived growth factor stimulates prostaglandin synthesis by activation and by rapid de novo synthesis of cyclooxygenase. J Clin Invest 1985;75:1381 |
|
Billah MM, Johnston JM. Identification of phospholipid platelet-activating factor (1-0-alkyl-2-acetyl-glycero-3-phosphocholine) in human amniotic fluid and urine. Biochem Biophys Res Commun 1983;113:51 |
|
Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, 3rd, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16:206-215. |
|
Bisits AM, Smith R, Mesiano S, Yeo G, Kwek K, MacIntyre D, Chan EC. Inflammatory aetiology of human myometrial activation tested using directed graphs. PLoS Comput Biol. 2005;1:132-136. |
|
Saeed SA, Strickland DM, Young DC et al. Inhibition of prostaglandin synthesis by human amniotic fluid: Acute reduction in inhibitory activity of amniotic fluid obtained during labor. J Clin Endocrinol Metab 1982;55:801 |
|
Cohen DK, Craig DA, Strickland DM et al. Prostaglandin biosynthesis stimulatory and inhibitory substances in human amniotic fluid during pregnancy and labor. Prostaglandins 1985;30:13 |
|
Wilson T, Liggins GC, Aimer GP et al. Partial purification and characterization of two compounds from amniotic fluid which inhibit phospholipase activity in human endometrial cells. Biochem Biophys Res Comm 1985;131 22 |
|
Haluska GJ, Wells TR, Hirst JJ et al. Progesterone receptor localization and isoforms in myometrium, decidua, and fetal membranes from rhesus macaques: evidence for functional progesterone withdrawal at parturition. J Soc Gynecol Investig 2003;9:125 |
|
Mesiano S. Myometrial progesterone responsiveness and the control of human parturition. J Soc Gynecol Investig 2004;11:193 |
|
Mesiano S, Chan EC, Fitter JT et al. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab 2002;87:2924 |
|
Zakar T, Hertelendy F. Progesterone withdrawal: key to parturition. Am J Obstet Gynecol 2007;196:289 |
|
Madsen G, Zakar T, Ku CY et al. Prostaglandins differentially modulate progesterone receptor-A and -B expression in human myometrial cells: evidence for prostaglandin-induced functional progesterone withdrawal. J Clin Endocrinol Metab 2004;89:1010 |
|
Walsh SW, Young SR, Stockmar EJ. Increased 5-lipoxygenase activity precedes labor (abstract 31). Presented at the 33rd Annual Meeting of the Society for Gynecologic Investigation, Toronto, March 19–22, 1986 |
|
Romero R, Emamian M, Wan M et al. Increased concentrations of arachidonic acid lipoxygenase metabolites in amniotic fluid during parturition. Obstet Gynecol 1987;70:849 |
|
Romero R, Quintero R, Emamian M et al. Arachidonate lipoxygenase metabolites in amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol 1987;157:1454 |
|
Bennett PR, Rose MP, Myatt L et al. Preterm labor: Stimulation of arachidonic acid metabolism in human amnion cells by bacterial products. Am J Obstet Gynecol 1987;156:649 |
|
Walsh SW. 5-Hydroxyeicosatetraenoic acid, leukotriene C4, and prostaglandin F2&b.alpha; in amniotic fluid before and during term and preterm labor. Am J Obstet Gynecol 1989;161:1352 |
|
Buchan GS, Gibbins BL, Griffin JFT. The influence of parturition on peripheral blood mononuclear phagocyte subpopulations in pregnant women. J Leukocyte Biol 1985;37:231 |
|
Szekeres-Bartho J, Varga P, Pacsa AS. Immunologic factors contributing to the initiation of labor-lymphocyte reactivity in term labor and threatened preterm delivery. Am J Obstet Gynecol 1986;155:108 |
|
Weichman BM, Tucker SS. Contraction of guinea pig uterus by synthetic leukotrienes. Prostaglandins 1982;24:245 |
|
Bray MA. Leukotriene receptors. In: Lefer AM, Gee MH (eds). Leukotrienes in Cardiovascular and Pulmonary Function, p 17. New York: Alan R Liss; 1985 |
|
Liggins GC. Cervical ripening as an inflammatory reaction. In: Ellwood DA, Anderson ABM (eds), The Cervix in Pregnancy and Labour, p 1. Edinburgh: Churchill Livingstone; 1981 |
|
Andrews WW, Goldenberg RL, Faye-Petersen O et al. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol 2006;195:803 |
|
Gervasi MT, Chaiworapongsa T, Naccasha N et al. Phenotypic and metabolic characteristics of maternal monocytes and granulocytes in preterm labor with intact membranes. Am J Obstet Gynecol 2001;185:1124 |
|
Haddad R, Tromp G, Kuivaniemi H et al. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol 2006;195:394, e391 |
|
Molloy EJ, O'Neill A J, Grantham JJ et al.. Labor induces a maternal inflammatory response syndrome. Am J Obstet Gynecol 2004;190:448 |