Medical Treatment of Male Infertility
Authors
INTRODUCTION
Infertility is a major medical problem in the United States, affecting approximately 2.4 million, or an estimated 1 in 6, couples.1 Infertility is described as the inability to conceive after 1 year of unprotected, adequately timed intercourse. An individual who has never conceived before has primary infertility, whereas a person who has had a prior pregnancy and then fails to conceive has secondary infertility. However, in general, the evaluation and treatment of male infertility in either condition is nearly identical. Infertility seems to have increased in the past decade, yet this may be related to the following factors: voluntary delay in childbearing with an age-correlated decline in fertility status, use of contraceptive techniques, increased number of sexual partners with an increased risk of acquiring sexually transmitted diseases, and an increasing willingness to obtain medical assistance for infertility.
Normal fertile couples of reproductive age have a conception rate of 20% to 25% per month, with more than 90% conceiving within 1 year. Male factor infertility is involved in approximately 50% of infertile couples, and in 30% of the cases, an abnormality is discovered solely in the man.2 Despite the enormity of these numbers, the medical treatment of male infertility is continually frustrating, in light of the numerous etiologies but few which are truly amenable to effective medical management. With most male infertility attributable to idiopathic oligospermia and other sperm anomalies, empirical interventions have failed to resolve this condition. However, with the improvement in technology in the past few decades, intrauterine insemination (IUI), in vitro fertilization (IVF), micro-assisted fertilization such as intracytoplasmic sperm injection (ICSI), as well as improved techniques in surgical manipulation, have revolutionized the treatment of male infertility. In this chapter, the evaluation and medical treatment of male infertility are reviewed, beginning with the physiology and diagnostic workup of the various etiologies of the infertile male, followed by the presentation of the empirical interventions and finally the introduction of assisted reproduction techniques.
PHYSIOLOGY OF MALE REPRODUCTION
The testis has two primary functions: spermatogenesis and production of testosterone. Sperm production and development take place on the Sertoli cells within the seminiferous tubules. Testosterone is synthesized in the Leydig cells, which are interspersed in the connective tissue surrounding the seminiferous tubules. These processes are dependent on the pituitary gonadotropins: luteinizing hormone (LH) and follicle-stimulating hormone (FSH).
Endocrine Control
Luteinizing hormone and FSH both bind to the Leydig cells and promote the synthesis of testosterone—LH directly and FSH by enhancing the production of LH receptors. Increased levels of testosterone feed back negatively at both the pituitary and the hypothalamic levels, decreasing secretion of LH and gonadotropin-Releasing hormone (GnRH), respectively.3
FSH acts at the level of the seminiferous tubules to stimulate spermatogenesis. It appears that FSH, together with testosterone, acts directly on the Sertoli cells, and that they influence the germ cells only indirectly. It is clear that FSH and testosterone influence the differentiation of germ cells, but the exact mechanism is not known. However, it has been determined that the Sertoli cells, in response to FSH, produce various proteins that affect the germ cell or its immediate environment. One of these is androgen-binding protein, which is secreted into the lumen of the seminiferous tubule. When testosterone and dihydrotestosterone diffuse from the Leydig cells into the tubules, they are bound by androgen-binding protein, greatly increasing the local intraluminal concentration of androgen. This high local androgen concentration (relative to the plasma) is necessary for effective spermatogenesis and maturation.4 FSH is also under negative feedback control, but not primarily by testosterone or other steroid hormones; rather, the Sertoli cells, in response to FSH, secrete a glycoprotein (inhibin) that suppresses FSH secretion in the pituitary.5
Thus, under the control of LH, the Leydig cells produce the testosterone-rich environment in the seminiferous tubules necessary for spermatogenesis. FSH, acting together with testosterone, indirectly promotes spermatogenesis at the level of the seminiferous tubules, by stimulating the production of protein hormones by the Sertoli cells. These glycoproteins act on the germ cells or their local environment to promote spermatogenesis.
Spermatogenesis
Spermatogenesis involves differentiation of spermatogonia into spermatocytes, spermatids, and finally, spermatozoa. Through mitotic division, the number of germ cells is expanded greatly during the progression from spermatogonia to primary spermatocytes.
Through meiotic division, the primary spermatocyte (4N) becomes the diploid secondary spermatocyte (2N), which then sequentially matures through the spermatid stage to form the haploid spermatozoa (N). Once formed, spermatozoa are released into the seminiferous tubules and are transported to the epididymis, where they develop the potential for progressive motility and fertilization. The process of differentiation from spermatogonia to spermatozoa requires 74 ± 5 days.6
DIAGNOSTIC EVALUATION OF MALE INFERTILITY
The semen analysis is the starting point in the evaluation of male infertility, and when found to be normal, endocrine abnormalities are extremely rare, and further hormonal evaluation usually is unnecessary. However, when seminal abnormalities are encountered, thorough historical, physical, and laboratory evaluations need to be performed, with the goal of making a specific diagnosis (Fig. 1).
Semen Analysis
After 2 to 3 days of abstinence, the specimen is collected by masturbation and brought to the laboratory within 1 to 2 hours. At least two to three semen analyses should be performed before establishing a firm diagnosis in the infertile male because there is a 10% false-negative diagnostic rate. In addition, the analyses should be spaced 2 to 3 months apart to ensure that any abnormalities encountered do not reflect the same previous temporary testicular insult.7
As a general guide to the diagnosis of male subfertility, the World Health Organization has published normal values for semen analysis (Table 1).8 Because the coefficients of variation for all of these parameters are large (30%–50%), the importance of testing multiple samples again is underscored. Furthermore, it is difficult to predict with certainty that patients with semen values below these traditional values indeed have subfertility. Not until semen abnormalities become extreme (e.g., density < 15 million/mL) is predictive ability satisfactory. An attempt to get more helpful information from the semen analysis involves multiplying sperm density and percent motility to obtain a measure of motile sperm per milliliter. This value may be more useful than density or motility alone because it reflects the concentration of active sperm potentially capable of fertilization. Even patients with motile sperm concentrations of less than 5 million had pregnancy rates of 38%, but when the counts were in the 60 to 100 million range, the pregnancy rate was 79%.4
TABLE 1. Semen Analysis
Parameters | Normal Values |
Volume | >2ml |
Density | >20 million/ml |
Motility | >50% forward progression |
Morphology | >30% normal forms |
Round cells | <5 million/ml |
White cells | <1 million/ml |
(World Health Organization: Laboratory Manual for the Examination of Human Semen and Sperm—Cervical Mucus Interaction. Cambridge University Press, Cambridge.1992)
Badenoch and colleagues,9 in a study comparing the sperm density of 104 proven fertile men with 51 infertile men, found that 11.5% of the fertile men had sperm densities of less than 20 million/mL, and 33% of the infertile population had sperm densities greater than 20 million/mL. When discriminant analysis was applied, sperm concentration could predict fertile status with only 68% accuracy. They concluded that sperm density was of no value in distinguishing fertile and infertile men and suggested that it was of no value in diagnosis or monitoring of male infertility.9 Although there are differing opinions as to the value of density measurements and semen analysis, generally it does identify those with extreme abnormalities and serves as a starting point for the evaluation of male infertility. Although differing values for semen abnormalities are used, the World Health Organization guidelines are reasonable and can be used to trigger consideration of a male-factor etiology and to direct further evaluation. In an attempt to further define the significance of morphology as a predictor of fertilization potential, Kruger and co-workers10 devised a system of “strict morphology,” whereby many more sperm are classified as abnormal. They suggest that morphologic assessment, by this approach, is the best predictor of fertilization at IVF. IVF studies using “strict criteria” had normal fertilization rates if normal forms were greater than 14%. Fertilization decreased thereafter along with the percent normal forms until at less than 4%, fertilization was only 7% to 8%.11 Once semen abnormalities are encountered, careful evaluation must be undertaken to diagnose a specific etiology. Although in most the cause is not found, some are diagnosed with specific etiologies that are amenable to medical or surgical therapy.
History
A complete medical and sexual history, including determination of primary or secondary infertility status, is essential to identify those few patients who have a treatable specific etiology.
Are there endocrine abnormalities? Did puberty and virilization proceed normally? Have there been changes in libido, potency, or hair loss suggestive of hypogonadism?
Are there anatomic abnormalities (e.g., hypospadias, microphallus)? Are there any voiding difficulties or recent changes in voiding pattern? Is there a history of diabetes mellitus or neuropathologic injury? Is there a history of testicular torsion, mumps, infection (e.g., orchitis, epididymitis, prostatitis, seminal vesiculitis), hernia repair, undescended testicles, genitourinary surgery, vasectomy, or trauma?
Has there been exposure to drugs or environmental toxins? Was there in utero exposure to diethylstilbestrol, which has been considered a potential cause of male infertility? Is the patient using marijuana, excessive alcohol, or cocaine, or smoking heavily? Alcohol and marijuana can lower sperm counts and testosterone production, and smoking and cocaine use have been associated with abnormal semen analyses. Is the patient suffering hypogonadotropic hypogonadism from abuse of anabolic steroids? Has there been occupational exposure to toxins, such as ethylene glycol, which recently has been linked to spermatogenic disorders?12 Chemotherapy, particularly alkylating agents, can cause irreversible germ cell damage, as can irradiation. There are commonly used medications (e.g., allopurinol, spironolactone, valproic acid, ketoconazole, cimetidine, sulfasalazine, cyclosporine, diethylstilbestrol, colchicine, nitrofurantoin, minocycline, and calcium-channel blockers) that can adversely affect semen parameters, requiring that the clinician carefully review all medications used by the patient.13
Is there chronic testicular exposure to excessive heat? The extracorporeal location of the testes is required to maintain the slightly lower temperature optimal for spermatogenesis. Chronic elevation of scrotal temperature caused by saunas, frequent use of hot tubs, or occupational exposure to excessive heat may adversely affect semen parameters and fertility, but a cause-and-effect relationship has been difficult to prove.
Are there sexual problems affecting fertility? Is there a change in ejaculatory composition, volume, retrograde status, or hematospermia? Ejaculatory and erectile dysfunction are not rare and can be caused by medications commonly used for hypertension, depression, and other common illnesses. In patients with borderline sperm density, daily or more frequent coitus can result in worsened sperm concentration and possibly, reduced fertility potential. If coitus is infrequent (abstinence > 10 days), fertility can be diminished due to both the decreased motility of aging sperm and the reduced probability of coitus occurring near the time of ovulation.
Attention also should be directed to the family medical history as a possible key in establishing the etiology of male infertility. Is there a family history of systemic diseases, such as diabetes mellitus or cystic fibrosis? What is the fertility status of the patient's siblings? In a similar fashion, an interview of the patient's partner must be performed for the possible missed diagnosis of female infertility.
Physical Examination
During the physical examination, attention must be directed to a search for systemic disease; an assessment of overall virilization; and a careful examination of the genitourinary system. Hypogonadal disorders, such as Klinefelter's and Kallmann's syndromes, manifest incomplete virilization: altered hair distribution, body habitus, and musculature; or gynecomastia. In addition, Kallmann's syndrome also is associated with anosmia, a lack of sense of smell, which should be evaluated during the physical examination. Examination of the genitourinary system should include evaluation of the penile urethra for hypospadias, which can interfere with vaginal deposition of sperm. Testicular size should be measured, and the epididymis and prostate palpated to detect tenderness or swelling, indicative of possible infection or obstruction. Identification of bilateral vasa deferentia should be performed to exclude or confirm a history of bilateral vasectomy or congenital bilateral absence of the vas deferens. Also, an examination of the spermatic cord structures in the supine and the standing positions, with and without Valsalva's maneuvers, can detect the presence of varicocele. Doppler instrumentation and scrotal ultrasound also can assist in confirming the diagnosis of varicocele.
Additional Laboratory Evaluation
Additional laboratory evaluation is directed by the results of the semen analysis and the historical and physical findings. For example, if the semen volume is low (< 2 mL), a fructose test may be indicated to rule out obstruction. If fructose is negative and the semen fails to coagulate, then an obstruction may be present at the level of the seminal vesicles. In contrast, if the volume is low and fructose is positive, this should direct further evaluation to determine the presence of retrograde ejaculation. In these situations, a postejaculatory urine and transrectal ultrasound can assist in diagnosing retrograde ejaculation and ejaculatory duct obstruction, respectively.
In the presence of oligospermia or unexplained infertility, the clinician may wish to further assess fertilization potential by employing the sperm penetration assay (SPA), particularly if IVF is contemplated. In the SPA, the patient's sperm are incubated with zona-free hamster oocytes and their ability to fuse, penetrate, and then de-condense within the ooplasm is compared with that of known fertile sperm. An abnormal SPA does not correlate perfectly with poor fertilization potential, but normal penetration correlates well with normal fertilization. A poor SPA can be used as a predictor of possible fertilization failure at IVF and can allow the modification of the insemination technique or preinsemination counseling for consideration of the use of donor sperm for a portion of the oocytes. However, one recent study looking at pregnancy outcome and fecundity rates in relation to the results of the SPA found it not to be predictive, although the numbers were small.14
In patients who have undergone vasectomy, testicular trauma, malignancy, or infection, or if there is significant sperm agglutination in the semen analysis, further evaluation for antisperm antibodies would be indicated. The immunobead test (IBT) and mixed antiglobulin reaction test (SpermMAR) are the tests of choice for detecting sperm surface antibodies. The IBT involves using anti-immunoglobulin G (IgG)-labeled beads to bind antibody-coated sperm. Conversely, the SpermMar test involves mixing fresh sperm, IgG-coated latex particles, and anti-IgG. These tests are positive for antisperm antibodies when greater than 10% of motile sperm are bound, which may occur in approximately one in eight infertile men, as well as in 1% of fertile men.15
Most men with oligospermia have a normal endocrine profile; nevertheless, when significant seminal abnormalities or evidence of hypogonadism are encountered, assays for testosterone, FSH, LH, prolactin, and thyroid-stimulating hormone (thyrotropin, TSH) should be obtained to identify those men who may benefit from medical therapy. Because abnormalities of prolactin or LH are rare in the presence of normal testosterone or FSH, some clinicians recommend screening with FSH and testosterone alone, obtaining prolactin and LH only when indicated by FSH or testosterone results.
SPECIFIC CAUSES AND TREATMENT OF MALE INFERTILITY
Although idiopathic oligospermia and other abnormalities of motility (asthenozoospermia), form (teratozoospermia), penetration, and fusion constitute the most frequent cause of male infertility, there are specific causes (Table 2) that should be diagnosed because specific treatments are available. Causes of male infertility can be subdivided based on the status of the gonadotropins into at least three categories: hypergonadotropic hypogonadism, hypogonadotropic hypogonadism, and eugonadotropic oligospermia (see Fig. 1).
TABLE 2. Relative Frequency of Conditions Associated with Male Infertility
Condition | Frequency (%) |
Idiopathic | 38.9 |
Varicocele (medium to large) | 25 |
Varicocele (small) | 15.3 |
Cryptorchidism | 6.4 |
Possible obstruction | 4.5 |
Epididymal or vas obstruction | 4.1 |
Klinefelter's syndrome | 1.9 |
Mumps orchitis | 1.6 |
Hypogonadotropic hypogonadism | 0.6 |
Nonmotile sperm | 0.6 |
Irradiation/chemotherapy | 0.5 |
Coital disorders | 0.5 |
Androgen resistance | 0.1 |
Total | 100 |
(Adapted from Baker HWG, Burger HG, de Kretser DM et al: Relative incidence of etiological disorders in male infertility. In Santen RJ, Swerdloff RS (eds): Male Reproductive Dysfunction, p. 341. Marcel Dekker, 1986)
Hypergonadotropic Hypogonadism (Testicular Failure)
There is little evidence that any form of medical therapy improves spermatogenesis in the patient with an FSH level more than two times normal. The level of FSH is a reflection of the degree of testicular damage. The FSH elevation results from decreased production of inhibin by the injured Sertoli cells. With the decrease in negative feedback by inhibin, FSH rises. In classic hypergonadotropic hypogonadism, the patient presents with a low testosterone level, elevated gonadotropins, and azoospermia or severe oligospermia (Table 3). To maintain secondary sexual characteristics and function and to prevent osteoporosis, these hypogonadal patients require testosterone replacement (testosterone enanthate, 200 mg intramuscularly every 2 to 3 weeks).16 Testicular failure can be classified according to whether it was acquired or congenital. The sex chromosome aneuploidy (47 XXY), known as Klinefelter's syndrome, is the most common cause of primary testicular failure, associated with an elevated FSH level. The incidence is about 1 in 1,000 male live births, and 1 in 300 spontaneous abortions.17 The syndrome is characterized by a relatively tall, thin male with small testes, and gynecomastia. These men usually are azoospermic, but in the presence of genetic mosaicism, they occasionally may be oligospermic.
TABLE 3. Categorization of Oligosphermic Men by Endocrine Profile
Oligospermia | T | FSH | LH | PRL |
Eugonadotropic | NI | NI | NI | NI |
Hypergonadotropic hypogonadism | | | | NI |
Injury to germinal epithelium | NI | | NI | NI |
Hypogonadotropic hypogonadism | | | | NI |
Partial androgen insensitivity | | NI | | NI |
Hyperprolactinemia | | | | |
T = testosterone; FSH = follicle-stimulating hormone; LH = luteinizing hormone; PRL = prolactin; NI = normal.
There is also a subgroup of patients who have spermatogenic failure with elevated FSH but normal LH and testosterone levels (see Table 3). This group does not require testosterone replacement therapy. With complete spermatogenic failure, the only treatment options available are donor insemination or adoption. If the spermatogenic failure is incomplete, micro-assisted fertilization can be considered.
Spermatogenic impairment or failure can be acquired from injury to the testes from toxins, drugs, irradiation, infection (mumps orchitis), or cryptorchidism, and, if severe, can lead to FSH elevation. If the FSH level is significantly elevated, there is no effective therapy other than the possibility of micro-assisted fertilization. Because few therapeutic options exist for these patients, prevention, where possible, is the only opportunity for intervention. For example, mumps orchitis can be prevented by timely immunization, and if cryptorchidism is corrected before 2 years of age, the prognosis is improved significantly compared with those repaired later in life. Systemic illnesses, such as chronic renal failure, cirrhosis, and thyrotoxicosis, also can lead to testicular failure. However, if the illness can be treated successfully (e.g., renal transplantation), recovery may occur.
Another small group of infertility patients with normal masculinization and severe oligospermia have elevated LH and testosterone but normal FSH levels (see Table 3). The diagnosis of partial androgen insensitivity is suspected and can be confirmed by measuring androgen receptor function in genital skin fibroblast culture. These patients represent the least affected of a spectrum of abnormality of the androgen receptor, which ranges from complete insensitivity (testicular feminization), through partial insensitivity (Reifenstein's syndrome: azoospermia and incomplete masculinization), to near total sensitivity (complete masculinization with oligospermia).18 This X-linked recessive condition is diagnosed infrequently, but in the subpopulation with idiopathic azoospermia, one study found 40% to have androgen receptor defects.19 High-dose androgen therapy may be of some benefit, but generally there is no effective medical treatment, although micro-assisted fertilization may be an option.
Hypogonadotropic Hypogonadism
Hypogonadotropic hypogonadism is a rare cause of male infertility. In contrast to hypergonadotropic hypogonadism, patients with low testosterone and hypogonadotropism levels (see Table 3) often are treatable. Hypogonadotropic hypogonadism can be caused by mass lesions or inflammatory processes (tumors, infiltrative disorders, infection, or autoimmune hypophysitis) in the pituitary or hypothalamus, congenital conditions such as Kallmann's or adrenogenital syndromes, or idiopathic deficiency of FSH/LH or GnRH.
MASS LESION.
A mass lesion in this area of the brain is the most common cause of hypogonadotropic hypogonadism, and prolactinomas are the most common tumors. Men with prolactinomas often present with impotence and androgen deficiency. Contrary to the microadenomas found in women, those found in men are usually large. The hypogonadism produced by these tumors is caused by prolactin's interference with GnRH secretion, by direct tumor effect on the pituitary, and by a direct antiandrogen effect (see Table 3). The antiandrogen effect is demonstrated by the restoration of libido and sexual function with normalization of prolactin, but not with replacement of testosterone alone. Surgical removal or treatment with bromocriptine has been shown to increase sperm density and serum testosterone.20,21 In a study by Murray and associates,22 five men with prolactinomas had semen analyses performed before surgery and bromocriptine therapy. All analyses were abnormal before intervention, and three normalized subsequent to treatment. The mean sperm count rose from 5.6 to 15.8 million/mL.
However, pregnancy rates remain low even with correction of the hyperprolactinemia. Other tumors or conditions in the pituitary or adjacent areas, such as gonadotropin-secreting adenomas, sarcoidosis, or the metabolic condition of hemochromatosis, also can lead to hypogonadotropic hypogonadism. These conditions can lead to direct pituitary destruction with resultant deficiencies in gonadotropins, as well as other pituitary tropic hormones. Thus, any patient with hypogonadotropic hypogonadism should undergo hypothalamic/pituitary imaging (magnetic resonance imaging or computed tomography); if a mass lesion is suspected, these patients also should have thyroid and adrenal function evaluated.
ADRENOGENITAL SYNDROME.
The adrenogenital syndrome is a rare congenital cause of gonadotropin deficiency, in which an enzyme defect in the steroid pathway for synthesis of cortisol (most commonly 21-hydroxylase) results in increased adrenocorticotropic hormone, with stimulation of the adrenals and overproduction of androgen. The excess androgens suppress pituitary gonadotropin secretion, and the testes are not stimulated. When testosterone levels are normal to elevated in the presence of decreased FSH and LH, the diagnosis of adrenogenital syndrome is suspected, and it may be confirmed by high serum 17-hydroxyprogesterone and androstenedione levels. Administration of glucocorticoids in slightly higher than physiologic doses suppresses adrenal androgen production by negative feedback on adrenocorticotropic hormone secretion and enable gonadotropin levels to rise. This results in testicular growth, spermatogenesis, and a high rate of fertility.
CONGENITAL GONADOTROPIN DEFICIENCY.
Patients with congenital gonadotropin deficiency usually are hypogonadotropic on the basis of deficient secretion of GnRH. Isolated GnRH deficiency can be idiopathic or caused by genetic maldevelopment of the GnRH neurons (Kallmann's syndrome); it also can appear after puberty and can be incomplete (fertile eunuch syndrome). Patients with postpubertal onset of isolated GnRH deficiency have undergone normal puberty and virilization but hypogonadism with impotence and azoospermia have subsequently developed. This group of patients is important because they represent one group in which azoospermia is reversible. However, most patients with isolated GnRH deficiency present with a history of abnormal puberty.23 Kallmann's syndrome (hypogonadism and anosmia), and the syndrome of postpubertal loss of GnRH are indistinguishable from a neuroendocrine point of view; both are characterized by low testosterone, apulsatile secretion of GnRH, absence of a mass lesion, and normal functioning of the remainder of the pituitary.
GONADOTROPIN THERAPY.
Both FSH and LH are deficient in hypogonadotropic hypogonadism, and both steroidogenesis and spermatogenesis are impaired. Most of these patients have required prolonged testosterone treatment because of failure to undergo normal puberty, but this does not appear to adversely affect subsequent response to gonadotropin therapy. When patients are treated with exogenous gonadotropins or a pulsatile infusion of GnRH, initiation of spermatogenesis and pregnancy occur. The success of any treatment regimen depends on the severity of the defect. Human chorionic gonadotropin (hCG) has been used alone for treating hypogonadotropic hypogonadism but is most effective in gonadotropin deficiency acquired postpuberty. hCG is functionally comparable to LH and stimulates the Leydig cells to produce testosterone and estradiol. Increased testosterone facilitates the spermatogenic effect of FSH,24 and in patients with partial gonadotropin deficiency, it can result in improved spermatogenesis and pregnancies. Unfortunately, as the levels of the sex steroids rise, they inhibit FSH secretion by negative feedback at the level of the hypothalamus and pituitary. The usual treatment regimen is 6,000 to 8,000 IU of hCG three times a week for 24 weeks.16 Most patients, and especially those with congenital hypogonadotropic hypogonadism, require the addition of human menopausal gonadotropin (hMG).25 Patients are treated with hCG, as described previously, until testosterone becomes normal and no further improvements are obtained in spermatogenesis or testicular size. Then, hMG, which contains both FSH and LH, is administered two times per week until the sperm density is greater than 5 million/mL. Thereafter, sperm counts usually can be maintained with the continuation of hCG alone. An alternate regimen consists of starting with daily injections of hMG (65 IU FSH, 75 IU LH) and continuing for 90 to 120 days, doubling the dose at 120 days if the response is not adequate. Simultaneously, hCG (5,000 IU) is given every 5 days. Lunenfeld and colleagues treated 62 patients with this regimen, and 29 had improved sperm density; 22 of the 29 produced 39 children.26 There are conflicting reports as to how often and for how long spermatogenesis can be maintained by hCG alone after discontinuation of combined therapy.25,26 Other investigators have used urofollitropin (“pure” FSH) in place of hMG with significant improvements in counts and motility.27 Overall, treatment of hypogonadotropic hypogonadism with gonadotropins is quite successful; sperm production is obtained in 50% to 80% of patients, and the pregnancy rates are high (40%–80%).28
GONADOTROPIN-RELEASING HORMONE THERAPY.
In the presence of an intact pituitary, the pulsatile administration of GnRH to patients with idiopathic hypogonadotropic hypogonadism can result in the normalization of gonadotropin levels, spermatogenesis, and testosterone production. Although this form of therapy may seem ideal because it specifically replaces the deficient GnRH, it suffers from its mode of delivery and the long duration of therapy. Administering GnRH by subcutaneous infusion pump in pulsatile doses to five patients, Hoffman and Crowley were able to obtain normal gonadotropin levels by 1 week and spermatogenesis in three of five patients by 43 weeks.29 In a subsequent study, they determined that patients who had failed to respond to the usual dose of GnRH responded when the dose was increased gradually, indicating that there is an individual threshold dose. There have been reports of pregnancies following treatment with GnRH,30,31 but extensive clinical experience is not yet available; results to date do not appear to be better than with gonadotropins.
Eugonadotropic Oligospermia
Most eugonadotropic men with oligospermia are virile and have normal sexual function (see Table 3). The etiology in most cases is idiopathic, although it appears that the abnormalities are intrinsic to the testis. There are, however, some eugonadotropic men with specific abnormalities that require individualized therapies.
OBSTRUCTION OF THE EPIDIDYMIS OR VAS DEFERENS.
The diagnosis of obstruction of the epididymis or vas deferens is suggested by normal volume azoospermia, normal sized testicles, and a normal FSH. There may be a history of infection, noted by induration and tenderness on examination of the testicles, epididymis, or prostate. The possibility of obstructive complications from prior genitourinary, such as vasectomy, or hernia surgery also needs to be considered. Diagnosis and treatment for these conditions are surgical, such as vasovasostomy or vasoepididymostomy, and potentially may allow for future consecutive pregnancies. Conversely, in situations such as congenital bilateral absence of the vas deferens, micro-assisted fertilization techniques such as ICSI combined with sperm aspiration or testicular biopsy have produced significant success with fertilization and pregnancy.32
RETROGRADE EJACULATION.
In the presence of normal sized testes, oligospermia, and low semen volume, suspected retrograde ejaculation can be confirmed by finding large numbers of sperm in the centrifuged urine specimen obtained immediately after ejaculation. This condition should be suspected in any patient with a history significant for any neuropathologic condition, such as diabetes mellitus. Treatment can be directed at control of the internal sphincter by the use of sympathomimetics (pseudoephedrine hydrochloride 60 mg four times daily, ephedrine 25 mg to 50 mg four times daily, phenylpropanolamine 50 mg to 75 mg twice daily) or imipramine (25 mg twice daily or 50 mg every night). In addition, the urine can be alkalinized (sodium bicarbonate 650 mg four times daily or baking soda 1 to 2 tablespoons in water starting 1 to 2 days before sperm retrieval) and the osmolality regulated by increasing or restricting fluid (300 to 380 mOsm/L), and then after ejaculation, the urine can be collected and centrifuged to recover the retroejaculated semen. It is then washed (Percoll) along with any normally ejaculated specimen, and used for intrauterine or in vitro insemination.33 If the patient is unable to regulate the pH and osmolality of the urine, the bladder can be drained and filled with buffered medium (100 mL) before ejaculation.
FREQUENT EJACULATION.
A potentially reversible cause of eugonadotropic oligospermia is ejaculation more than one time per day. Reduction of sexual frequency may result in improvements in semen parameters. In general, successful conception involves intercourse every other day around the time of ovulation, which can be determined by LH-predictor kits.
GENITOURINARY TRACT INFECTION.
According to the World Health Organization, leukocytospermia is defined as greater than 1 million leukocytes per milliliter of semen. An association between high concentrations of leukocytes in semen and a decline in sperm parameters, probably secondary to the products of these activated cells, has been noted. In the presence of symptoms, sperm agglutination, or more than 5 million leukocytes/mL, some believe that these patients should be treated, including coverage for chlamydia. However, on routine semen analysis, distinguishing between immature germ cells and leukocytes is difficult. In fact, it has been shown that antibiotic therapy for asymptomatic leukocytospermia does not improve semen parameters and in some cases may cause deterioration secondary to the effects of the antibiotics themselves.34 In addition, a prospective study by Tomlinson and co-workers found that the measurement of seminal leukocytes in routine semen analyses was of little prognostic value in predicting male fertilization potential.35
REDUCED BIOLOGICALLY ACTIVE LUTEINIZING HORMONE.
Cases have been reported in which patients have normal levels of immunologic LH but decreased LH biological activity. They have been treated successfully with gonadotropins. A case has been reported of a biologically inactive LH (caused by a single amino acid substitution) in a patient with hypogonadism, a normal FSH level, and an elevated LH level.36
SPERM ANTIBODIES.
Although there is not complete consensus as to the exact role and significance of antisperm antibodies,35 there is general acceptance that at least in some cases, seminal fluid antibodies do cause infertility. Sperm are very antigenic, and when their relative isolation from the vascular compartment is disrupted by injury, torsion, infection, or surgery, such as vasectomy, autoantibodies can develop. The incidence of antisperm antibodies varies between 8% to 21% among infertile men.37 Antisperm antibodies may affect fertilization by inhibiting cervical mucus penetration, facilitate destruction of coated sperm by uterine leukocytes, inhibit capacitation, impair the acrosome reaction, or inhibit the binding of spermatozoa to the zona pellucida.15 It is less likely that serum antisperm antibodies, in either men or women, are of significance.38,39 If agglutination is found at routine semen analysis or antibodies are otherwise suspected, the IBT can be employed to establish the diagnosis. In one study, if more than 50% of the sperm were antibody bound, the pregnancy rate was 15.3%, but if less than 50% were bound, the pregnancy rate was 66.7%.40
The treatment of antisperm antibodies has included the use of condoms, steroids, sperm washing, and IVF. The use of condoms has not been effective and is no longer commonly used. The use of glucocorticoids to suppress antibody levels and increase pregnancy rates has been successful in some studies,41,42 but others have not had success.43 Immunosuppression by steroids occasionally can result in aseptic necrosis of the femoral head, peptic ulceration, diabetes, and cardiovascular and psychiatric conditions, which should be explained to the patient before institution of therapy.15 Hendry and co-workers41 had some success with cyclic prednisolone taken during the first 12 days of their partner's cycle (20 mg twice daily, days 1 to 10, and 5 mg, days 11 and 12). Omu and co-workers42 have had success with continuous prednisolone, 5 mg given daily for 3 to 6 months. An alternate approach is to attempt to remove the antibodies from the sperm and then perform IUI.44 Various techniques are used to remove unattached antibody from the seminal fluid, such as ejaculation into medium-containing protein and multiple sperm washes. One unique approach has been to use antibody-labeled beads and Percoll gradients to identify and separate out a fraction of antibody-free sperm for insemination.45 Because the aforementioned series noted success with corticosteroid treatments in less than one third of patients, assisted reproductive techniques ultimately provide the greatest chance for conception. Studies have shown that corticosteroid therapy coupled with either IUI or IVF does not significantly enhance pregnancy rates compared with IUI or IVF alone.46,47 When these techniques fail to provide conception, micro-assisted fertilization techniques such as ICSI have provided pregnancy rates of greater than 50%.48
TESTICULAR INJURY.
Exposure to a number of agents can cause testicular injury, including environmental toxins, chemotherapeutic drugs, irradiation, and ethanol. Mumps orchitis and cryptorchidism also can result in injury and altered spermatogenesis. Gonadotropin levels are normal or elevated, depending on the degree of injury. There is no medical treatment except prevention or withdrawal of the offending agent. If there are any remaining sperm, assisted fertilization can be considered.
VARICOCELE.
Varicocele is defined as a dilation of the veins of the pampiniform plexus allowing for excessive blood flow, secondary to deficient venous valves, leading to increased scrotal temperatures and thus an adverse influence on spermatogenesis. Varicocele is seen in approximately 15% to 20% of adult men, whereas in infertility patients, the estimation is double.49 Varicocele repair, varicocelectomy, has been shown to improve semen parameters, and it appears the patients who benefit from surgery are those with grade 2 or 3 varicoceles and mild semen abnormalities.50 Some have suggested the use of assisted reproduction rather than varicocelectomy in male infertility; however, this significantly proves to be less cost-effective and does not allow for consecutive pregnancies.51
TREATMENT OF IDIOPATHIC OLIGOSPERMIA, ASTHENOSPERMIA, AND TERATOSPERMIA TERATOSPERMIA
Most men with infertility are eugonadotropic, normally virile, and otherwise healthy, but they have low sperm density (< 15 million/mL) or other seminal abnormalities. No specific etiology is known, but a variety of fundamental defects in spermatogenesis is suspected. As one might expect when the etiology is unknown, a multitude of empiric therapies have been tried, unfortunately with limited success. Whereas in the past many of these men would be destined to be childless and would eventually be obliged to resort to donor insemination or adoption, IVF and the new micro-assisted fertilization techniques offer the opportunity to father children even in the most severe cases.
Empiric Therapy
Empiric therapies have included: androgens, gonadotropins, antiestrogens, GnRH, and antiprostaglandins and pentoxifylline. Success has been limited, and placebo-controlled studies have been few.
ANDROGENS.
Androgens have been administered in a variety of ways to enhance spermatogenesis. However, when comparative studies have been performed using placebo controls, androgens have not been shown to be effective. One method was to deliver, over a period of at least 3 months, low doses of androgen (methyltestosterone 10 to 50 mg/day), with the idea of replacing a supposed testosterone deficiency. Androgens administered in this fashion did not improve fertility.52 High-dose androgen therapy (testosterone enanthate, 200 mg to 500 mg intramuscularly every 2 weeks) is given to suppress spermatogenesis for a time, with the hope that after discontinuation sperm production will rebound to levels higher than presuppression levels. The high levels of testosterone suppress LH and thus spermatogenesis. Although spermatogenesis usually rebounds somewhat after pituitary suppression with testosterone, fertility is not increased.53 Two recent placebo-controlled studies of men with oligospermia, asthenospermia, or teratospermia also found no benefit of androgens compared with placebo with respect to either improvements in seminal parameters or pregnancy rates.54,55
GONADOTROPINS AND GONADOTROPIN-RELEASING HORMONE.
The administration of exogenous gonadotropins (hCG, hMG) to men with idiopathic oligospermia has not been demonstrated to be beneficial.26 In a placebo-controlled study by Knuth and associates, using a combination of hCG and hMG, neither semen parameters nor pregnancy rates were enhanced compared with placebo.56 Furthermore, in a recent study, it was noted that treatment of men with FSH did not significantly improve pregnancy rates.57 Likewise, GnRH treatment of idiopathic oligospermia has not been shown to be effective.58
ANTIESTROGENS.
Clomiphene citrate and tamoxifen are antiestrogens that exert their action by competing with estrogen for the estrogen receptors. They enhance gonadal function by reducing the negative feedback of estrogen at hypothalamic and pituitary levels, resulting in increased GnRH activity, gonadotropin secretion, production of testosterone, and hopefully, improved spermatogenesis. Estradiol is known to have a direct inhibitory effect on the Leydig cells, and the antiestrogens may enhance testicular function by this mechanism as well. Another antiestrogen, testolactone, similarly promotes testicular function both centrally and directly by decreasing the influence of estradiol. However, it does not function by competition for the estrogen receptor but by directly decreasing the conversion of androgen to E2 through aromatase inhibition.
Uncontrolled studies have reported improvement in sperm density and pregnancy rates after clomiphene citrate treatment, whereas others have failed to show benefit. However, when investigations have been well controlled, efficacy has not been demonstrated.59,60 Sokol and colleagues61 prospectively compared clomiphene citrate (50 mg/day) and placebo, each given for a 12-month period, in a group of 21 oligospermic men. They found no differences between the clomiphene and placebo groups with respect to either sperm counts or pregnancy rates. Interestingly, over the time of the study, both groups experienced a gradual increase in sperm density. However, a randomized, double-blind study of nearly 200 couples by the World Health Organization showed no effect of clomiphene treatment.62
Investigation of tamoxifen and testolactone as treatment for idiopathic oligospermia has shown mixed results. There have been studies using tamoxifen (e.g., 20 mg/day for 4 to 12 months) that have shown improvement in sperm counts and increased pregnancy rates, but they were not placebo-controlled.63 In contrast, a placebo-controlled study by AinMelk and colleagues demonstrated no difference in pregnancy rates.64 Likewise, testolactone (e.g., 1 g/day for 6 to 12 months) has shown some promise in uncontrolled studies,65 but in a placebo-controlled crossover study, no efficacy for testolactone was found.66
ANTIPROSTAGLANDINS.
It has been suggested that antiprostaglandins improve spermatogenesis. In a controlled study by Barkay and co-workers, indomethacin (50 to 75 mg/day for 60 days) was found to improve significantly both semen parameters and pregnancy rates compared with no improvement in the placebo group.67 The best results (pregnancy rate of 36%) were obtained at the 75-mg dose. These results need to be confirmed by additional controlled studies.
PENTOXIFYLLINE.
A methylxanthine, pentoxifylline, is a phosphodiesterase inhibitor and, as such, results in an increase in intracellular cyclic adenosine monophosphate (cAMP) levels, which are involved with sperm motility hyperactivation and the acrosome reaction. Merino and associates68 noted that men receiving 1200 mg of pentoxifylline over 6 months demonstrated a significant increase in sperm motility, which is in contrast to the observations by Tesarik and colleagues.69
Thus, the empiric treatment of idiopathic oligospermia has met with limited success, and when therapeutic regimens have been tested against placebo controls, they generally have failed to demonstrate efficacy.
Intrauterine Insemination
Artificial insemination with husband semen has been used to treat infertile couples for almost 200 years. It is an accepted form of treatment for men with severe hypospadias, retrograde ejaculation, neurologic impotence, and sexual dysfunction refractory to counseling. It also has been used in the presence of oligospermia, asthenozoospermia, low or high ejaculate volumes, and antisperm antibodies; however, the success rate in these settings is low and the utility is open to review. The technique also has been used in the presence of abnormal cervical mucus, stenotic cervical canal, or antisperm antibodies in the female. The desire to bypass poor cervical mucus, or to assist sperm transit in male-factor infertility has led to the technique of IUI. Abandoned because of serious allergic reactions, IUI with unprepared semen has been replaced with IUI with “washed sperm.” There are a variety of techniques designed to separate sperm from the other seminal fluids, remove antibodies or debris, and isolate a fraction of sperm with optimal motility and morphology. These include simple washing, where semen is diluted into a volume of culture medium and centrifuged, the supernatant decanted, and then the process repeated. The final product has the seminal fluid portion removed but all sperm and cellular components remain. The swim-up and Percoll techniques are designed to isolate a population of sperm with better motility and morphology and thus higher pregnancy rates.70 In the swim-up procedure, after washing and centrifugation, the specimen is incubated, allowing the most motile sperm to swim up from the pellet into the medium. The specimen for insemination is obtained from the supernatant, rather than the pellet. It contains fewer sperm, but the motility is enhanced. With the Percoll procedure, motile sperm are separated differentially by means of their ability to traverse progressively more dense layers of a viscous medium.71 The number of sperm for insemination should not be less than 1 million, and pregnancy rates are not increased to greater than 15 million, although the multiple pregnancy rate is increased to more than 20 million.72 However, others have not been able to demonstrate increased pregnancy rates with IUI over timed intercourse in oligoasthenospermic infertility.73
Recently, IUI in conjunction with superovulation has become a popular treatment for male-factor infertility, as well as unexplained infertility. The combination of IUI and superovulation with gonadotropins has the theoretical advantage of placing a greater number of motile and morphologically normal sperm in close proximity to multiple mature oocytes. In a group of patients with male-factor infertility or abnormal postcoital tests, IUI plus superovulation resulted in a fourfold increase in the pregnancy rate compared with IUI alone.74 In a study by Serhal and co-workers, the pregnancy rate/cycle was significantly greater for the combination of IUI and gonadotropin superovulation (26.4%) than for IUI alone (2.7%) or superovulation alone (6.1%).75 In a review of several studies, Dodson and Haney found the fecundity rate with IUI and superovulation in male-factor infertility was 8.7%; for unexplained infertility, it was 17%.76 It appears that IUI alone or superovulation alone is not effective, but the combination may offer some limited benefit to patients with male-factor infertility.77
Donor Insemination
For couples who have failed to conceive despite therapy or those with extreme idiopathic oligospermia, donor insemination offers the couple an alternative to adoption. In the absence of contributing female-factor infertility, donor insemination is extremely successful (70% with 5 to 6 cycles). If donor insemination is unacceptable and adoption is not desired, the male who wants to pursue having his own genetic offspring can avail himself of the new reproductive technologies: IVF and micro-manipulation.
Sperm Retrieval Techniques for In Vitro Fertilization and Micro-Assisted Fertilization
There are a variety of techniques involved in the retrieval of spermatozoa for utilization in IVF and micro-assisted fertilization, such as ICSI. This includes: masturbation, electroejaculation/vibratory stimulation, percutaneous epididymal sperm aspiration (PESA), microsurgical epididymal sperm aspiration (MESA), and testicular sperm extraction (TESE). Masturbation requires 2 to 3 days of abstinence before production of the sample, which subsequently undergoes sperm preparation before use. Electroejaculation/vibratory stimulation requires the use of a device and urinary alkalization in patients with ejaculatory dysfunction to produce an adequate sample. PESA and MESA are used in men with azoospermia secondary to obstructive and nonobstructive disorders. PESA involves local anesthesia and multiple blind passages with a 21- to 23-gauge needle through the epididymis, with the advantage of avoiding a skin incision. However, the disadvantage is the “blind” nature of the procedure, which may cause significant damage to the epididymis, making future attempts at conception difficult. Conversely, MESA provides retrieval of many sperm, with less epididymal damage, which then may be cryopreserved and used for future procedures.78 TESE involves an open surgical biopsy of the testicle when epididymal sperm retrieval is unable to occur and provides similar pregnancy rates when using micro-assisted fertilization.79
In Vitro Fertilization
In vitro fertilization and its micromanipulative innovations show promise of being an advance for male infertility on the level that traditional IVF has been for female tubal infertility. The concept of IVF originated with the desire to treat infertility caused by failed gamete union secondary to tubal obstruction. The first successful in vitro manipulation of gametes by conventional IVF occurred with the birth of Louise Brown in England in 1978. Although the methodology of processing and storing sperm had been available for many years, the ability to recruit, collect, and culture oocytes was required before the possibility of enhancing fertilization by the in vitro incubation of gametes became possible. Since the delivery of the first baby some 21 years ago, the technique has spread worldwide, and consistent results are now obtained. Moreover, the indications soon expanded to include unexplained infertility and male factor. Although it was logical that inseminating oocytes in vitro would facilitate fertilization, the number of motile sperm originally used for insemination (2 to 6 million/oocyte) limited application of the technique in oligospermic infertility. With time and experience, the number of motile sperm used for insemination has declined (50,000 to 100,000/oocyte) with maintenance of fertilization rates and tolerable levels of polyspermic fertilization.80 This development opened the door for treatment of the oligospermic male using IVF. Additionally, controlled ovarian hyperstimulation with hMG allows the recruitment of multiple ovarian follicles and retrieval of multiple oocytes, which then can be inseminated using processed sperm from the male partner.
CONVENTIONAL IN VITRO FERTILIZATION AND THE OLIGOSPERMIC MALE.
Male-factor infertility is the indication for approximately 25% of IVF cycles. Oligospermia, abnormal morphology, and motility adversely influence fertilization rates.81 However, once fertilization has occurred, the implantation rate per transfer is equal to that of other IVF patients.82,83 Fertilization rates for male-factor IVF have been reported by Tournave83 and Cohen84 to be 30% and 53%, respectively. However, the overall pregnancy rate for IVF in the United States as reported by the Society for Assisted Reproductive Technology is 18% for male infertility.85 The national IVF registry has demonstrated consistently an increased chance of failed fertilization in male-factor infertility (50%) compared with tubal infertility (15%). These low fertilization rates result in fewer embryos for transfer and a lower overall success rate (deliveries/retrieval).86
Failure of fertilization at IVF is devastating to the infertile couple. The financial and emotional investment is of such magnitude that even when it is anticipated because of known severe andrological infertility, the disappointment is great. What can the patient be offered after failed fertilization, and what of the patient with such extreme semen abnormalities that even conventional IVF is not a consideration? Several new techniques have been developed to assist the fertilization process, some of which promise to make conceiving a pregnancy a reality for even the most severe oligospermic, asthenospermic, and teratospermic males. When there is failure of fertilization at IVF or when a male factor is anticipated or known, options include: gamete intrafallopian transfer (GIFT); repeat conventional IVF; modification of sperm concentration; and micro-assisted fertilization. Although recommended by some, the use of a second sperm sample (husband/partner or donor) to reinseminate (rescue) oocytes that have failed to undergo fertilization has not proven efficacious.
INTRAFALLOPIAN TRANSFER PROCEDURES.
In nontubal infertility, intrafallopian transfer procedures have gained popularity because of modestly increased clinical pregnancy rates compared with IVF (25% vs. 10%). It is not clear whether these results are due to the benefits of the in vivo tubal environment or whether they only reflect differences in patient selection. The rationale for GIFT is that the oviduct, being the natural location for fertilization, offers the optimal environment. In nonmale-factor patients, the usual number of sperm placed with the oocytes in the oviduct is 100,000. In male-factor cases, some investigators suggest the use of increased sperm numbers (325,000 sperm/oviduct),82 whereas others report using reduced numbers (2,500/oviduct).87 It is likely in these cases that the criteria used to define a male factor are different. Indeed, in retrospect, a male factor may not even exist because functional sperm are clearly present. A drawback with GIFT is that in the absence of pregnancy, fertilization cannot be confirmed. Thus, some investigators perform fertilization in vitro and then transfer the zygote to the fallopian tube. In one study of 78 planned zygote intrafallopian transfer (ZIFT) cycles, failure of fertilization was observed in 34 (43.6%). These results would not have been available if GIFT was the planned procedure, and perhaps 34 patients would have undergone unnecessary laparoscopy.88
Although some retrospective studies have shown advantages of ZIFT over IVF, a prospective, randomized study comparing IVF and ZIFT found no advantage of ZIFT over IVF for the treatment of male-factor infertility.88 In a comparison of IVF, GIFT and ZIFT, Tournaye and associates reported take-home baby rates of 13.5%, 7%, and 20%, respectively.89 These results were not different statistically. Furthermore, with ZIFT, one-cell zygotes are transferred before determination of pronuclear stage arrest or cleavage failure, both of which are more common in male-factor infertility83; thus, not permitting embryo preselection, as has been shown advantageous in IVF.90 Both ZIFT and GIFT have the added disadvantage of requiring laparoscopy and general anesthesia for transfer with consequent increased cost. The possibility of transcervical-intrafallopian transfer91 of cleavage-stage embryos could impact the success and cost equation, but the efficacy of these procedures has yet to be determined.
Thus, conventional IVF provides advantages in cost and convenience when compared with GIFT or ZIFT, and success rates probably are not significantly different. If GIFT is considered, it should be limited to cases of mild to moderate male-factor infertility, where fertilization potential is known or expected to be good.
REPEAT CONVENTIONAL IN VITRO INSEMINATION.
Studies have noted that of male-factor patients who had one IVF cycle with failed fertilization, 81% had achieved successful fertilization after another cycle. However, with the new micro-assisted fertilization techniques such as ICSI, with more effective fertilization and pregnancy rates yet slightly higher costs, many clinicians have recommended going directly to these procedures rather than IVF.92
HEAVY CONVENTIONAL INSEMINATION.
Although concentrations of 50,000 to 100,000 sperm/mL are customary for conventional IVF, much higher concentrations are appropriate for male-factor infertility. Several groups recommend that in the presence of severe male-factor infertility or conventional IVF fertilization failures, patients undergo an IVF cycle with “heavy” conventional insemination. The effective sperm concentration can be as high as 1 to 5 million/mL.93 Tucker and colleagues93 reported 53% fertilization and 36% viable pregnancy rates with heavy conventional insemination in a series of patients with severe male-factor infertility or previous fertilization failure. They recommend that all but the most extreme oligospermia undergo heavy insemination before proceeding to micromanipulation. When oligospermia is severe and insufficient sperm are available, these concentrations frequently can be obtained using the microdrop technique. Sperm concentrations of 1 million/mL can be obtained by placing as few as 25,000 cells in 25-mL droplets of medium under oil. Bayer and co-workers have had success with this technique in the presence of severe male-factor infertility.94 Another technique that can be employed when sperm numbers are limited is to inseminate multiple oocytes in a single dish. Fertilization can be enhanced further in male-factor cases by inseminating with not only a high concentration of sperm but with a highly motile population as well. Special procedures (e.g., mini-Percoll), designed to separate a highly motile subpopulation of sperm have evolved for this purpose.95
Micro-Assisted Fertilization
Although conventional IVF has been very helpful in treating couples with long-term andrological infertility, there are patients with severe male-factor infertility (extreme oligospermia, absence of normal morphology, and motility), and fertilization failure patients with no currently detectable semen abnormalities, who are appropriately considered for treatment by more aggressive techniques. Philosophical extremes still exist in deciding which patients should be considered for micromanipulation: a last resort procedure or one appropriate for all suspected or diagnosed male-factor infertility. These techniques involve the use of micromanipulators to mechanically assist the sperm-egg interaction process.
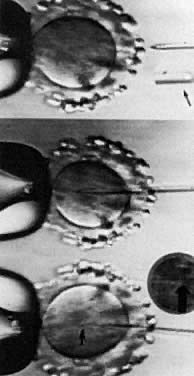
THE MICROMANIPULATION TECHNIQUE.
Under visualization on the stage of an inverted microscope, oocytes in culture medium droplets under oil are held and micromanipulated with specially prepared holding and injection pipettes. The oocyte is immobilized with gentle suction applied by the holding pipette, and the injection pipette is used to incise the zona pellucida (ZP), deposit several sperm in the perivitelline space, or inject a single sperm directly into the ooplasm. These techniques can be classified as: zonal, subzonal, and intracytoplasmic procedures. Although known by many names such as zona drilling, subzonal insertion, and direct egg injection, the following terms are used in this chapter: partial zona dissection (PZD), subzonal injection (SUZI), and intracytoplasmic sperm injection (ICSI) (Fig. 2).
PARTIAL ZONA DISSECTION.
Before fertilization can occur, sperm must penetrate the ZP, traverse the perivitelline space, and fuse with the oolemma in the process of entering the ooplasm. The ZP, an acellular glycoprotein coat surrounding the oocyte, plays several roles in fertilization and early development, including the provision of a species-specific barrier to fertilization, a barrier to polyspermia, and a protective shell for the preimplantation embryo. Because ZP passage represents a prerequisite step for the fertilizing sperm, the creation of gaps or slits in the zona should facilitate this process (see Fig. 2). Oocytes dissected in this manner are inseminated subsequently with standard concentrations of capacitated sperm. Chemical, mechanical, and laser techniques have been employed. The ZP was first opened using acidified Tyrode's solution delivered through a microneedle (zona drilling). This process was successful in mice but not when applied in human IVF because of induced oocyte damage. In 1988, the first human pregnancy was produced from microsurgical fertilization using a micropipette to mechanically open the ZP (PZD).96 To minimize damage to the oocyte during this process, limited shrinkage is induced by conducting the procedure in sucrose. The technique usually involves placing the micropipette through the ZP into the perivitelline space and then continuing out through the opposite side of the ZP without entering the ooplasm. Releasing the oocyte from the holding pipette, the ZP overlying the micropipette is rubbed against the side of the holding pipette until a slit is created in the ZP. Other investigators have advocated using laser for PZD because of its simplicity and precision.97
However, the overall success of PZD has been limited. It is estimated that only 100 to 150 live births have resulted from the method.98 In a comparative study involving 130 male-factor cycles, no differences were found between IVF and PZD with respect to fertilization (12% vs. 13%); the cleavage rate was higher for the IVF group (89% vs. 73%); and the pregnancy rate for IVF was 33% compared with 12% for PZD.99 The conclusion was drawn that PZD was no better than conventional IVF. Furthermore, PZD is complicated by excessive rates of polyspermia, variously reported at 30% to 47%. Although rates of polyspermia may be modulated by patient selection, gap size, and sperm concentration, results have been inconsistent. In most centers, the implantation capacity also is reduced, most likely because of oocyte damage during the procedure (2%–30%), or improper culture conditions during the micromanipulation (temperature and pH). These problems and inconsistent results have led many investigators to abandon PZD.100
SUBZONAL INJECTION.
The second generation of micromanipulation techniques includes SUZI or the direct subzonal insertion of sperm through and under the zona pellucida into the perivitelline space (see Fig. 2). Depending on their morphologic appearance, several sperm (3 to 6) are aspirated into a sharp, bevelled microneedle and injected through the ZP. Because the ZP and its ZP3, the zona glycoprotein implicated in acrosome reaction induction, are bypassed in this process, strategies to prematurely induce the acrosome reaction have been conceived. Two methods designed for this purpose are: washed sperm incubated for 24 hours in Tyrode's medium + 50% follicular fluid, and electroporation + incubation in 3.5 mmol/L pentoxifylline.101 Although these procedures increase the acrosome-free sperm to as high as 54%, their efficacy remains unproven. Indeed, motile sperm in the perivitelline space still may be exposed to ZP3 present on the inner aspects of the zona. In theory, increasing the number of reacted sperm should allow the use of fewer sperm (3 vs. 6), thereby decreasing the risk of polyspermic fertilization.
Unfortunately, the clinical success with SUZI has been less than spectacular, with fertilization rates hovering around 20% and pregnancy rates around 10%.102,103 The drawbacks of SUZI are the same as those for PZD: polyspermic fertilization and impaired implantation. Because the oolemma cannot block multiple sperm fusion, SUZI is inherently complicated by polyspermic fertilization. The fertilization rate with SUZI can be increased by increasing the number of sperm placed in the perivitelline space, but the proportion of polyspermic embryos may become unacceptably high. However, polyspermia may be lower than with PZD103 and can be influenced by the number of sperm inserted. Implantation rates with SUZI embryos also may be better than for PZD.84 Likewise, the risk of blastomere or cytoplasm extrusion and contamination of the perivitelline space with medium is reduced with SUZI (smaller gap). However, this technique recently has been superseded by ICSI.
INTRACYTOPLASMIC SPERM INJECTION.
The next logical step was to use micromanipulation to inject a single sperm through the ZP and oolemma directly into the ooplasm (see Fig. 2). Although the most invasive technique, the inherent advantage to ICSI is avoidance of polyspermic fertilization because only one sperm is injected. Initial concerns with ICSI centered on whether the cytoskeleton would be disrupted, causing defective cell division (minimized by injecting distal to the meiotic spindle); whether fertilization would occur with genetically abnormal sperm; and whether oocyte activation (completion of the fertilization process, initiation of cleavage, and development), if triggered, would be normal. In animals, the ICSI procedure has not always resulted in activation, and various treatment regimens have been developed to overcome this limitation. For instance, oocytes can be activated by exposure to ethanol, calcium ionophore, or electrical fields (electroporation). The common mediator with these methods is most likely elevation of intracellular calcium ion concentration, either by extracellular calcium influx or by intracytoplasmic mobilization of calcium stores.104 It has become apparent that injection of a single sperm into the cytoplasm is sufficient stimulus in humans to trigger activation. In addition, the increased intracellular calcium associated with the injection process does not, in and of itself, provoke egg activation,105 but rather, the release of a sperm factor appears to be required. Interestingly, an intact acrosome is not a deterrent to ICSI success.
Although preclinical trials of ICSI established that fertilization could be obtained,106 clinical pregnancies were not reported until 1992.107 High fertilization and pregnancy rates with ICSI currently are routine in several centers around the world.98 The most extensive experience has been realized in a program in Brussels.108 In their ICSI technique, sperm either were incubated overnight, treated with pentoxifylline and deoxyadenosine, electroporation, or were exposed transiently to elevated calcium levels to increase the chance for fertilization. These recipes for success have been largely abandoned. Percoll-gradient processed sperm are dispersed in polyvinylpyrrolidone, a viscous solution that allows separation of motile sperm from immotile cells, specifically in conditions of severe oligoasthenozoospermia.109 Subsequent immobilization of the spermatozoa by various techniques, such as crimping the tail of the spermatozoa at the tip, allows for delivery into the micropipette.110 Beveled, injection micropipettes (7 μm outer and 5 μm inner diameter) then are front-loaded tail first, with the resultant immobilized spermatozoa for injection into the ooplasm (Fig. 3).108 In men with complete asthenozoospermia, there are no motile sperm and less than 50% of the sperm are viable. As a result, the hypo-osmotic swelling test was developed and used to enhance fertilization and pregnancy in these patients, as well as in previously cryopreserved sperm that usually have poor motility. When sperm are incubated in a hypo-osmolar medium, those that are viable and functional undergo swelling of the cytoplasmic space and the sperm tail fibers curl. These sperm in turn may be used in ICSI and provide a better outcome.111 The indications for ICSI are the absence of fertilization, a failure to fertilize in a previous cycle of IVF, antisperm antibodies, teratozoospermia, sever oligoasthenozoospermia, obstructive azoospermia, such as with congenital bilateral absence of the vas deferens, nonobstructive azoospermia, and anejaculation.112
In the early developmental phase of the ICSI procedure, a comparison of 750 cycles of ICSI and SUZI was conducted, and a fertilization rate of 55% for ICSI versus 17% for SUZI was obtained.108 ICSI quickly was adopted as the standard assisted fertilization treatment for couples unable to benefit from conventional IVF. Palermo and associates have noted in their experience of 355 cycles with 2,970 injected oocytes that greater than 93% of the oocytes remained intact after the procedure, with a 69% fertilization rate and a 38% ongoing pregnancy rate achieved per oocyte retrieved.113 Another series by Ubaldi and co-workers114 from Brussels consisted of 2,820 cycles of ICSI; greater than 95% of the 36,425 oocytes remained intact, and the pregnancy rate approximated 38%. This study also detailed no significant difference between the pregnancy rates per cycle with ejaculated, epididymal, and testicular sperm. Others also have been successful with ICSI (fertilization rate > 50%) and have recommended using ICSI any time the predicted fertilization rate from heavy conventional insemination is less than 40% These authors reason that the majority of failed fertilization is because of specific severe defects, such as teratozoospermia, oligospermia, asthenozoospermia, or acrosomal defects, which affect sperm/ZP binding.115
Although concerns about disruption of chromosomal or cytoskeletal elements or the consequences of fertilization with genetically abnormal sperm continue, confidence is accumulating that clinical outcomes will be normal. In infertile men, approximately 7% have a major sex chromosome abnormality, half of which are accounted for by a mosaic Klinefelter condition. The incidence rises from 2% in men with normal sperm concentration to 20% in those with azoospermia.116 Despite these data and an apparently high risk for chromosome abnormalities in ICSI fetuses, Bonduelle and colleagues found the risk of chromosomal abnormalities to be approximately 1%, similar to the general newborn population.117 Furthermore, the early pregnancy loss rate has been approximately 19%, and fetal karyotypes performed on 151 patients for prenatal diagnosis (amniocentesis or chorionic villus sampling), and examination of 119 children after birth did not reveal an increase in congenital anomalies.108 Sperm employed for ICSI can be morphologically very abnormal; pregnancies have occurred with very low counts, 0% motility, 0% normal morphology, as well as with cryopreserved sperm. In fact, in situations of previously diagnosed primary testicular failure with FSH levels greater than 30 mIU/mL, nearly 50% of men had mature sperm on testicular biopsy suitable for ICSI.118 In addition, application of round spermatid nuclei injection (ROSNI) in humans is undergoing careful consideration, with fertilization rates of approximately 30%, two pregnancies, and resultant childbirth.119 Because ICSI allows fertilization by sperm, which under natural conditions are incapable of ZP penetration and oocyte-sperm fusion, concern also exists that germ-line mutations might be transmitted, resulting in heritable defects. The inheritance of susceptibility to infertility is another concern and would not be recognized until late in the next generation (the reproductive years). Once sexing of the spermatozoa becomes routinely available, prevention of sex-linked diseases may be preventable by selecting the healthier gender. Thus, careful evaluation, genetic consultation with the couples, and follow-up of the pregnancies resulting from ICSI are necessary.
CONCLUSION
When faced with infertility due to male-factor abnormalities, a diligent search should be made to determine the precise diagnosis. Although most patients fall into the category of idiopathic oligospermia, some patients have a specific treatable cause (see Fig. 1). For example, patients with hypogonadotropic hypogonadism, although rare, often are treatable with gonadotropins. When hypogonadotropic hypogonadism is secondary to a prolactinoma, bromocriptine may be effective, and a central mass lesion may require surgery. Even in the category of eugonadotropic oligoazoospermia, where most patients are classified as idiopathic, patients may have specific treatable etiologies, such as obstruction or retrograde ejaculation. Those patients with testicular failure must be identified so that hormone replacement therapy can be administered, and if total spermatogenic failure is present, the physician can counsel the patient as to adoption or donor insemination, and assist in dealing with the emotional aspects of nontreatable infertility. The empiric treatment of idiopathic oligospermia generally has not proved effective, and, in the absence of controlled studies demonstrating otherwise, should not be encouraged. Nevertheless, the advent of IVF and micro-assisted fertilization has given the infertile patient with idiopathic oligospermia new hope of having their own genetic offspring. Specifically, ICSI has revolutionized the treatment of male infertility, possibly one day allowing for gender selection as well as disease-free newborns.
The current authors wish to acknowledge Milo L. Hibbert, MD, for his extensive contributions to the original version of this manuscript published previously and for permission to use his original tables and figures.
REFERENCES
National Survey of Family Growth Study: In Infertility—Medical and Social Choices, pp 49–57. Washington DC, Congress of the United States, Office of Technology Assessment, 1988 |
|
Morell V: Basic infertility assessment. Prim Care 24: 195, 1997 |
|
Winters SJ, Janick JJ, Loriaux DL et al: Studies on the role of sex steroids in the feedback control of gonadotropin concentrations in men: II. Use of the estrogen antagonist, clomiphene citrate. J Clin Endocrinol Metab 48: 222, 1979 |
|
Speroff L, Glass RH, Kase NG: Clinical Gynecologic Endocrinology and Infertility, 5th ed, pp 873–878. Baltimore, Williams & Wilkins, 1994 |
|
Plymate SR, Paulsen CA, McLachlan RI: Relationship of serum inhibin levels to serum follicle stimulating hormone and sperm production in normal males and men with varicoceles. J Clin Endocrinol Metab 74: 859, 1992 |
|
Heller CG, Clermont Y: Kinetics of the germinal epithelium in man. Recent Prog Horm Res 20: 545, 1964 |
|
Opsahl MS, Dixon NG, Robins ER, Cunningham DS: Single vs. multiple semen specimens in screening for male infertility factors: A comparison. J Reprod Med 41: 313, 1996 |
|
World Health Organization: Laboratory Manual for the Examination of Human Semen and Sperm--Cervical Mucus Interaction. Cambridge, Cambridge University Press, 1992 |
|
Badenoch DF, Evan SJ, McCloskey DJ: Sperm density measurement: Should this be abandoned? Br J Urol 64: 521, 1993 |
|
Kruger TF, Acosta AA, Simmons KF et al: Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril 49: 112, 1988 |
|
Hargreave TB, Elton RA: Fecundability rates from an infertile population. Br J Urol 58: 194, 1986 |
|
Veulemans H, Steeno O, Masschelein R et al: Exposure to ethylene glycol ethers and spermatogenic disorders in man: A case-control study. Br J Ind Med 50: 71, 1993 |
|
Lipshultz LI, Howards SS: Infertility in the Male, 3rd ed, pp. 399–401. St. Louis, Mosby-Year Book, 1997 |
|
O'Shea DL, Odem RR, Cholewa C et al: Long-term follow-up of couples after hamster egg penetration testing. Fertile Steril 60: 1040, 1993 |
|
Bates CA: Antisperm antibodies and male subfertility. Br J Urol 80: 691, 1997 |
|
Sokol RZ: Pharmacologic treatment of infertility. In White R-V (ed): Problems in Urology, p 461. Philadelphia, JB Lippincott, 1987 |
|
Thompson MW, McInnes RR, Huntington FW: Genetics in Medicine, 5th ed, p 239. Philadelphia, WB Saunders Company, 1991 |
|
McPhaul MJ, Marcelli M, Soppi S et al: Genetic basis of endocrine disease 4: The spectrum of mutations in the androgen receptor gene that causes androgen resistance. J Clin Endocrinol Metab 76: 17, 1993 |
|
Aiman J, Griffin JE: The frequency of androgen receptor deficiency in infertile men. J Clin Endocrinol Metab 54: 725, 1982 |
|
Arafah BM, Manni A, Brodey JS et al: Cure of hypogonadism after removal of prolactin-secreting adenomas in men. J Clin Endocrinol Metab 52: 91, 1981 |
|
Winters SJ, Troen P: Altered pulsatile secretion of luteinizing hormone in hypogonadal men with hyperprolactinemia. Clin Endocrinol 21: 257, 1984 |
|
Murray FT, Cameron DF, Ketchum C: Return of gonadal function in men with prolactin-secreting pituitary tumors. J Clin Endocrinol Metab 59: 79, 1984 |
|
Whitcomb RW, Crowley WF: Diagnosis and treatment of isolated gonadotropin-releasing hormone deficiency in men. J Clin Endocrinol Metab 70, 1990 |
|
Matsumoto AM, Karpas AE, Bremmer WJ: Chronic human chorionic gonadotropin administration in normal men: Evidence that follicle-stimulating hormone is necessary for the maintenance of quantitatively normal spermatogenesis in man. J Clin Endocrinol Metab 62: 1184, 1986 |
|
Sherins RJ: Evaluation and management of men with hypogonadotropic hypogonadism. In Garcia C-R et al (eds): Current Therapy of Infertility, 1984–1985, p 640. Philadelphia, WB Saunders, 1986 |
|
Lunenfeld B, Mor A, Mani M: Treatment of male infertility. I. Human gonadotropins. Fertil Steril 18: 581, 1967 |
|
Menchini-Fabris GF, Turchi P, Canale D: Medical treatment of male infertility. Int J Fertil 37: 330, 1992 |
|
Finkle DM, Phillips JL, Snyder PJ: Stimulation of spermatogenesis by gonadotropins in men with hypogonadotropic hypogonadism. N Engl J Med 313: 651, 1985 |
|
Hoffman AR, Crowley WF Jr: Induction of puberty in men by long-term pulsatile administration of low-dose gonadotropin-releasing hormone. N Engl J Med 307: 1237, 1982 |
|
Donald RA, Wheeler M, Sonksen PH et al: Hypogonadotropic hypogonadism resistant to hCG and responsive to LHRH: Report of a case. Clin Endocrinol 18: 385, 1983 |
|
Skarin G, Nillius SJ, Wibell L: Chronic pulsatile low dose GnRH therapy for induction of testosterone production and spermatogenesis in a man with secondary hypogonadotropic hypogonadism. J Clin Endocrinol Metab 55: 723, 1982 |
|
Tournaye H, Devroey P, Liu J et al: Microsurgical epididymal sperm aspiration and intracytoplasmic sperm injection: A new effective approach to infertility as a result of congenital bilateral absence of the vas deferens. Fertil Steril 61: 1045, 1994 |
|
Hershlag A, Schiff SF, DeCherney AH: Retrograde ejaculation. Hum Reprod 6: 255, 1991 |
|
Yanushpolsky EH, Politch JA, Hill JA, Anderson DJ: Antibiotic therapy and leukocytospermia: A prospective, randomized, controlled study. Fertil Steril 63: 142, 1995 |
|
Tomlinson MJ, Barratt CLR, Cooke ID: Prospective study of leukocytes and leukocyte subpopulations in semen suggests they are not a cause of male infertility. Fertil Steril 60: 1069, 1993 |
|
Weiss J, Axelrod L, Whitcomb RW et al: Hypogonadism caused by a single amino acid substitution in the beta subunit of luteinizing hormone. N Engl J Med 326: 179, 1992 |
|
Vazquez-Levin MH, Notrica JA, Polak de Fried E: Male immunologic infertility: Sperm performance on in vitro fertilization. Fertil Steril 68: 675, 1997 |
|
Eggert-Kruse W, Christmann M, Gerhard I et al: Circulating antisperm antibodies and fertility prognosis: A prospective study. Hum reprod 4: 513, 1989 |
|
Collins JA, Burrows EA, Yeo J et al: Frequency and predictive value of antisperm antibodies among infertile couples. Hum Reprod 8: 592, 1993 |
|
Ayvaliotis B, Bronson R, Rosenfeld D et al: Conception rates in couples where autoimmunity to sperm is detected. Fertil Steril 44: 739, 1986 |
|
Hendry WF, Hughes L, Scammell G et al: Comparison of prednisone and placebo in subfertile men with antibodies to spermatozoa. Lancet 335: 85, 1990 |
|
Omu AE, Al-Qattan F, Abdul Hamada B: Effect of low dose continuous corticosteroid therapy in men with antisperm antibodies on spermatozoal quality and conception rate. Eur J Obstet Gynecol Reprod Biol 69: 129, 1996 |
|
Smarr SC, Wing R, Hammond MG: Effect of therapy on infertile couples with antisperm antibodies. Am J Obstet Gynecol 158: 969, 1988 |
|
Agarwal A: Treatment of immunological infertility by sperm washing and intrauterine insemination. Arch Androl 29: 207, 1992 |
|
Grundy CE, Robinson J, Gordon AG et al: Selection of an antibody-free population of spermatozoa from semen samples of men suffering from immunological infertility. Hum Reprod 6: 593, 1991 |
|
Grigoriou O, Konidaris S, Antonaki V, Papadias C, Antoniou G, Gargaropoulos A: Corticosteroid treatment does not improve the results of intrauterine insemination in male subfertility caused by antisperm antibodies. Eur J Obstet Gynecol Reprod Biol 65: 227, 1996 |
|
Lahteenmaki A, Rasanen M, Hovatta O: Low-dose prednisolone does not improve the outcome of in-vitro fertilization in male immunological infertility. Hum Reprod 10: 3124, 1995 |
|
Jun JH, Lim CK, Park YS et al: Efficacy of intracytoplasmic sperm injection (ICSI) treatment in the immunological infertile patients. Am J Reprod Immunol 37: 310, 1997 |
|
Jarow JP, Coburn M, Sigman M: Incidence of varicoceles in men with primary and secondary infertility. Urology 47: 73, 1996 |
|
Vazquez-Levin MH, Friedmann P, Goldberg SI et al: Response of routine semen analysis and critical assessment of sperm morphology by Kruger classification to therapeutic varicocelectomy. J Urol 158: 1804, 1997 |
|
Schlegel PN: Is assisted reproduction the optimal treatment for varicocele-associated male infertility? A cost-effectiveness analysis. Urology 49: 83, 1997 |
|
Steinberger A: Clinical assessment of treatment results in male infertility. In Santen RJ, Swerdloff RS (eds): Male Reproductive Dysfunction, p 370. New York, Marcel Dekker, 1986 |
|
Sokol RZ: Medical and endocrine therapy of male factor infertility. Infertil Reprod Med Clin North Am 3: 389, 1992 |
|
Gerris J, Comhaire F, Hellemans P et al: Placebo-controlled trial of high-dose mesterolone treatment of idiopathic male infertility. Fertil Steril 55: 603, 1991 |
|
Comhaire FH: Treatment of idiopathic testicular failure with high-dose testosterone undecanoate: A double-blind pilot study. Fertil Steril 54: 689, 1990 |
|
Knuth UA, Honigl W, Bals-Prastch M et al: Treatment of severe oligospermia with human chorionic gonadotropin/human menopausal gonadotropin: A placebo-controlled double blind trial. J Clin Endocrinol Metab 65: 1081, 1987 |
|
Matorras R, Perez C, Corcostegui B et al: Treatment of the male with follicle-stimulating hormone in intrauterine insemination with husband's spermatozoa: A randomized study. Hum Reprod 12: 24, 1997 |
|
Matsumoto AM, Gross KM, Sheckter CB et al: The luteinizing hormone-releasing hormone pulse generator in men: Abnormalities and clinical management. Am J Obstet Gynecol 163: 1743, 1990 |
|
Micic S, Dotlic R: Evaluation of sperm parameters in clinical trial with clomiphene citrate of oligospermic men. J Urol 133: 221, 1985 |
|
Weiland RG, Ansari AH, Klein DE et al: Idiopathic oligospermia: Control observations and response to cis-clomiphene. Fertil Steril 23: 471, 1972 |
|
Sokol RZ, Steiner BS, Bustillo M et al: A controlled trial of the efficacy of clomiphene citrate in male infertility. Fertil Steril 49: 865, 1988 |
|
World Health Organization: A double-blind trial of clomiphene citrate for the treatment of idiopathic male infertility. Int J Androl 15:299, 1992 |
|
Buvat J, Ardaens K, Lemaire A et al: Increased sperm count in 25 cases of idiopathic normogonadotropic oligospermia following treatment with tamoxifen. Fertil Steril 39: 700, 1983 |
|
AinMelk Y, Belisle S, Caimel M et al: Tamoxifen citrate therapy in male infertility. Fertil Steril 48: 113, 1987 |
|
Vigersky RA, Glass AR: Effects of delta-1-testolactone on the pituitary-testicular axis in oligospermic men. J Clin Endocrinol Metab 52: 897, 1981 |
|
Clark RV, Sherins RJ: Clinical trial of testolactone for treatment of idiopathic male infertility. J Androl 10: 240, 1989 |
|
Barkay J, Haysaz-Karpel S, Ben-Ezra et al: The prostaglandin inhibitor effect of anti-inflammatory drugs in the therapy of male infertility. Fertil Steril 42: 406, 1984 |
|
Merino G, Martinez Chequer JC, Barahona E et al: Effects of pentoxifylline on sperm motility in normagonadotropic asthenozoospermic men. Arch Androl 39: 65, 1997 |
|
Tesarik J, Thebault A, Testart J: Effect of pentoxifylline on sperm movement characteristics in normozoospermic and asthenozoospermic specimens. Hum Reprod 7: 1257, 1992 |
|
Carrell DT, Kuneck PH, Peterson CM et al: A randomized, prospective analysis of five sperm preparation techniques before intrauterine insemination of husband sperm. Fertil Steril 69: 122, 1998 |
|
Berger T, Marrs RP, Moyer DL: Comparison of techniques for selection of motile human spermatozoa. Fertil Steril 43: 268, 1985 |
|
Kerin J, Byrd W: Supracervical placement of spermatozoa. In Soules MR (ed): Controversies in Reproductive Endocrinology and Infertility. Amsterdam, Elsevier, 1989 |
|
Ho PC, Poon IML, Chan SYW et al: Intrauterine insemination is not useful in oligoasthenospermia. Fertil Steril 51: 682, 1989 |
|
Kemmann E, Bohrer M, Shelden R et al: Active ovulation management increases the monthly probability of pregnancy occurrence in ovulatory women who receive intrauterine insemination. Fertil Steril 48; 916, 1987 |
|
Serhal PF, Katz M, Little V et al: Unexplained infertility—The value of pergonal superovulation combined with intrauterine insemination. Fertil Steril 49: 602, 1988 |
|
Dodson WL, Haney AF: Controlled ovarian hyperstimulation and intrauterine insemination for treatment of infertility. Fertil Steril 55: 457, 1991 |
|
Ombelet W, Puttemans P, Bosmans E: Intrauterine insemination: a first-step procedure in the algorithm of male subfertility treatment. Hum Reprod 10 (Suppl 1): 90, 1995 |
|
Kim ED, Lipshultz LI: Male subfertility: Diagnostic and therapeutic advances. Br J Urol 80: 633, 1997 |
|
Ghazzawi IM, Sarraf MG, Taher MR, Khalifa FA: Comparison of the fertilizing capability of spermatozoa from ejaculates, epididymal aspirates and testicular biopsies using intracytoplasmic sperm injection. Hum Reprod 13: 348, 1998 |
|
Wolf DP, Byrd W, Dandekar P et al: Sperm concentration and the fertilization of human eggs in vitro. Biol Reprod 31: 837, 1984 |
|
Jeulin C, Feneux D, Serres C et al: Sperm factors related to failure of human in vitro fertilization. J Reprod Fertil 76: 735, 1986 |
|
Matson PL, Blackledge DG, Richardson PA et al: The role of gamete intrafallopian transfer (GIFT) in the treatment of oligospermia infertility. Fertil Steril 48: 608, 1987 |
|
Tournaye H, Devroey P, Camus M et al: Comparison of in vitro fertilization in male and tubal infertility: A 3 year survey. Hum Reprod 7: 218, 1992 |
|
Cohen J, Edwards R, Fehilly C et al: In vitro fertilization: A treatment for male infertility. Fertil Steril 43: 422, 1985 |
|
American Fertility Society/Society for Assisted Reproductive Technology: Assisted reproduction technology in the United States and Canada: 1992 results generated from the American Fertility Society/Society for Assisted Reproductive Technology Registry. Fertil Steril 62:6, 1994 |
|
Hughes EG, King C, Wood ED: A prospective study of prognostic factors in in vitro fertilization and embryo transfer. Fertil Steril 51: 838, 1989 |
|
Khan I, Camus M, Staessen CK et al: Success rate in gamete intrafallopian transfer using low concentrations of washed spermatozoa. Fertil Steril 50: 922, 1988 |
|
Tournaye H, Devroey P, Camus M et al: Zygote intrafallopian transfer or in vitro fertilization and embryo transfer for the treatment of male-factor infertility: A prospective randomized trial. Fertil Steril 58: 344, 1992 |
|
Tournaye H, Camus M, Khan I et al: In vitro fertilization, gamete- or zygote intrafallopian transfer for the treatment of male infertility. Hum Reprod 6: 263, 1991 |
|
Puissant F, Van Ryssekberge, Barlow P et al: Embryo scoring as a prognostic tool in IVF treatment. Hum Reprod 2: 705, 1987 |
|
Bustillo M, Schulman JD: Transcervical ultrasound guided intrafallopian placement of gametes, zygotes and embryos. J In Vitro Fert Embryo Transfer 6: 321, 1989 |
|
Ben-Shlomo I, Bider D, Dor J et al: Failure of fertilization in couples with male factor infertility: What next? Fertil Steril 58: 187, 1992 |
|
Tucker MJ, Wilker SR, Massey J: Rational approach to assisted fertilization. Hum Reprod 8: 1778, 1993 |
|
Bayer SR, Lee MA, McInnes DR et al: In vitro fertilization and male factor infertility: A critical review and update. Assisted Reprod Rev 3: 244, 1993 |
|
Ord T, Patrizio P, Marello E et al: MiniPercoll: A new method of semen preparation for IVF in severe male factor infertility. Hum Reprod 5: 987, 1990 |
|
Malter HE, Cohen J: Partial zona dissection of the human oocyte: A non-traumatic method using micromanipulation to assist zona pellucida penetration. Fertil Steril 52: 139, 1989 |
|
El-Danasouri I, Westphal LM, Neeve Y et al: Zona opening with 308 nm XeC1 excimer laser improves fertilization by spermatozoa from long-term vasectomized mice. Hum Reprod 8: 464, 1993 |
|
Cohen J, Wiemer KE, Alikani M: Advanced laboratory techniques for assisted reproduction. Assisted Reprod Technol 4: 733, 1993 |
|
Calderon G, Veiga A, Penella J et al: Two years of assisted fertilization by using partial zona dissection. Fertil Steril 60: 105, 1993 |
|
Tucker MJ, Bishop FM, Cohen J et al: Routine application of partial zona dissection for male factor infertility. Hum Reprod 6: 676, 1991. |
|
Palermo G, Joris H. Devroey P et al: Induction of acrosome reaction in human spermatozoa used for subzonal insemination. Hum Reprod 7: 248, 1992 |
|
Ng SC, Bongso TA, Liow SL et al: Microinsemination sperm transfer (MIST) and its application to male subfertility: Current strategies to improve results. Ann Acad Med Singapore 21: 561, 1992 |
|
Cohen J, Malter HE, Talansky BE, Grifo J: Micromanipulation of Human Gametes and Embryos, p 152. New York, Raven Press, 1992 |
|
Edward RG, Van Steirteghem AC: Intracytoplasmic sperm injections (ICSI) and human fertilization: Does calcium hold the key to success? Hum Reprod 8: 988, 1993 |
|
Tesarik J, Sousa M, Testart J: Human oocyte activation after intracytoplasmic sperm injection. Hum Reprod 9: 511, 1994 |
|
Lanzendorf SE, Slusser MS, Maloney MK et al: A preclinical evaluation of pronuclear formation by microinjection of human spermatozoa into human oocytes. Fertil Steril 49: 835, 1988 |
|
Palermo G, Joris H, Devroey P et al: Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 240: 17, 1993 |
|
Van Steirteghem A, Liu J, Nagy Z et al: Use of assisted fertilization. Hum Reprod 8: 1784, 1993 |
|
Fujii Y, Motoyama H, Hiraguchi K et al: A simple method for recovering the motile spermatozoa from extremely low quality sperm samples. Hum Reprod 12: 1218, 1997 |
|
Chen S, Ho H, Chen H et al: Intracytoplasmic sperm injection (ICSI) for severe semen abnormalities: Dissecting the tail of spermatozoa at the tip. Hum Reprod 11: 2640, 1996 |
|
Casper RF, Meriano JS, Jarvi KA et al: The hypo-osmotic swelling test for selection of viable sperm for intracytoplasmic sperm injection in men with complete asthenozoospermia. Fertil Steril 65: 972, 1996 |
|
Kim ED, Lamb DJ, Lipshultz LI: Intracytoplasmic sperm injection for treatment of the infertile male. Texas Med 93: 50, 1997 |
|
Palermo GD, Cohen J, Rosenwaks Z: Intracytoplasmic sperm injection: A powerful tool to overcome fertilization failure. Fertil Steril 65: 899, 1996 |
|
Ubaldi F, Nagy Z, Liu J et al: A survey of four years of experience with intracytoplasmic sperm injection. Acta Europaea Fertilitatis 26: 7, 1995 |
|
Baker HWG, Liu DY, Bourne H et al: Diagnosis of sperm defects in selection of patients for assisted fertilization. Hum Reprod 8: 1779, 1993 |
|
Persson JW, Peters GB, Saunders DM: Is ICSI associated with risks of genetic disease? Implications for counseling, practice and research. Human Reproduction 11: 921, 1996 |
|
Bonduelle M, Legein J, Buysse A: Prospective follow-up study of 423 children born after intracytoplasmic sperm injection. Hum Reprod 11: 1558, 1996 |
|
Abuzeid MI, Sasy MA, Salem HH: Intracytoplasmic sperm injection for treatment of non-obstructive azoospermia. Gynecol Endocrinol 11: 335, 1997 |
|
De Jonge CJ, Pierce J: Intracytoplasmic sperm injection—What kind of reproduction is being assisted? Hum Reprod 10: 2518, 1995 |