Surgical Treatment of Endometrial Carcinoma
Authors
INTRODUCTION
Cancer of the corpus is the most common malignancy in the female pelvis. According to the American Cancer Society, approximately 45,500 women in the United States were projected to develop uterine cancer in 2011, with some 8120 dying of their disease.1 In contrast, some 21,990 ovarian cancers and 12,710 cervical cancers were predicted to be diagnosed in 2011. Although this is a large number, it should be remembered that, in contrast, 23,480 breast carcinomas were forecast.
Breast, colon, and ovarian cancers are frequently seen in women with endometrial cancer. Certainly, if the woman has any of these cancers, one must be cognizant of this relation so that proper screening and evaluation can be performed, not only at initial diagnosis, but also in subsequent follow-ups as well. Women with a family history of endometrial cancer appear to be at increased risk of having this malignancy. Women with conditions that produce continuous, unopposed estrogen, such as Stein-Leventhal syndrome and granulosa-thecal cell tumors, appear to have an increased risk of endometrial cancer. An association between the use of tamoxifen in patients with breast cancer and endometrial cancer has been suggested, a recent review of the NSABP study comparing tamoxifen to raloxifene noted a significantly increased number of both atypical hyperplasia and endometrial cancer in the tamoxifen patients; however, increased surveillance in these patients is recommended.2
PROGNOSTIC FACTORS
The International Federation of Gynecology and Obstetrics (FIGO) recognizes two prognostic factors in its most recent staging (Table 1). For many years, grade and depth of invasion have been recognized as important prognostic factors. In 1988, FIGO decided that endometrial cancer should be surgically staged, thereby increasing our ability to determine the true extent of disease.3 Before that time, clinical staging had been used, with replete data in the literature detailing the large number of patients whose disease process was considerably different than the clinical stage. More than 25% of stage I patients had disease outside the uterus. In stage II, approximately half had a different stage; in many instances, they actually had only disease in the uterine fundus, which had tremendous treatment implications. With the new staging, treatment can be better defined. Knowing the depth of invasion and grade of tumor, even in stage I, allows more precise determination of prognosis and treatment. The 1998 staging was modified in 2009 as noted in Table 1.
Table 1. Carcinoma of the endometrium
| Stage | Findings |
| IA | Tumor confined to the uterus, no or <1/2 (<50%) myometrial invasion |
| IB | Tumor confined to the uterus, >1/2 (>50%) myometrial invasion |
| II | Cervical stromal invasion, but not beyond the uterus |
| IIIA | Tumor invades serosa or adnexa |
| IIIB | Vaginal and/or parametrial involvement |
| IIIC1 | Pelvic node involvement |
| IIIC2 | Paraaortic involvement |
| IVA | Tumor invasion bladder and/or bowel mucosa |
| IVB | Distant metastases including abnormal metastases and/or inguinal lymph |
MUtch D. The new FIGO staging for carcinoma of the endometrium IJGO 2009, 105:109
Phenotypic Status
Classically, obesity, hypertension, and diabetes have described the typical patient with endometrial cancer. Today, most studies suggest that hypertension and diabetes, when corrected for weight and age, are not necessarily risk factors. Obesity, nulliparity, and late menopause are more suggestive of this type of patient. If these conditions are present in excess, it is said that the risk of adenocarcinoma increases fivefold. This patient usually has a well-differentiated lesion with superficial invasive disease and an excellent prognosis, and is treated with simple hysterectomy and bilateral salpingo-oophorectomy. Some investigators have designated this patient as type I. In contrast, the type II patient usually is thin, parous, and black; has a poorly differentiated, poor histotype deeply invasive cancer with extrauterine disease, requiring more aggressive treatment; and has a resultant poor prognosis. In the former patient, unopposed estrogen may be the etiology, but this does not seem to be a factor in the latter patient.
The American Cancer Society (ACS) Task Force on Screening for Endometrial Cancer evaluated risk factors to identify women who might benefit from some type of screening. Multiple risk factors commonly said to be associated with endometrial cancer were reviewed in depth. Women with hereditary nonpolyposis colon cancer (HNPCC) account for only 2–10% of all female colon cancers, yet approximately 5% of all endometrial cancers occur in women with this risk factor. These women have 22–50% lifetime risk of having endometrial cancer develop, and the disease occurs at a younger age, approximately 15 years younger than in women without HNPCC. The highest risk of HNPCC carriers developing endometrial cancer occurs between 40 and 60 years of age, with the absolute risk being greater than 1% per year. Only HNPCC was thought to be significantly related to warrant consideration for screening. The ACS recommends annual screening with endometrial biopsy should be offered to this group of women beginning at age 35. Patients should be informed about potential benefits, risks, and limitations of testing for early endometrial cancer detection.4 Otherwise routine screening is not recommended.
Differentiation
For more than a century, it has been known that tumor differentiation is an important prognostic factor in endometrial cancer.5 This has been substantiated in many studies (Table 2). Patients with well-differentiated cancers have an excellent prognosis with hysterectomy as their only treatment. Patients with poorly differentiated tumors tend to have more deeply invasive cancers and a greater tendency to have extrauterine disease, require more intense therapy, and have a poor prognosis. In several large studies, it would appear that approximately one-third of all adenocarcinomas are well differentiated, with approximately one-quarter being poorly differentiated.
Table 2. Five-year survival of stage I carcinoma of the endometrium*
| Grade | Surgical | Clinical |
| G1 | 93% | 60% |
| G2 | 90% | 50% |
| G3 | 79% | 29% |
*Survival rates based on 5219 patients.
(Pecorelli S: Int J Gynecol Obstet 2006;95:S121)
Depth of Myometrial Invasion
As depth of invasion increases, prognosis worsens (Table 3). This factor may be even more predictive of prognosis than differentiation of tumor. Depth of invasion appears to be an indicator of tumor volume, which is a most important prognostic factor in any cancer. In a large study carried out by the Gynecologic Oncology Group (GOG), approximately 15% of patients with clinical stage I cancer had disease limited to the endometrium and 40% had significant (middle or deep third) myometrial invasion.5 There appears to be a correlation between grade and depth of invasion. In general, as depth of invasion increases, there is a greater likelihood that the disease process is poorly differentiated. More than one-third of grade 3 lesions tend to be deeply invasive; only 7% are limited to the endometrium. In contrast, only 10% of grade 1 lesions are deeply invasive. It appears that patients with grade 3 disease limited to the endometrium have a better prognosis than do patients with grade 1, deeply invasive disease.
Table 3. Relationship between depth of myometrial invasion and 5-year survival rate (stage I)
| Stage | Surgical stage |
| IA | 91% |
| IB | 91% |
| IC | 85% |
(Pecorelli S: Int J Gynecol Obstet 2006;95:S117)
Extent of Disease
Proper evaluation, both surgical and pathologic, must be performed to evaluate the patient's cancer fully. Without adequate evaluation at the time of surgery, surgical staging is no better than clinical staging, with its inherent problems already discussed. Proper treatment can be applied only when the extent of disease is known. Spread of disease from the corpus to the endocervix not only changes the stage, but can also affect survival (Table 4). If the disease extends into the endocervical canal, the incidence of pelvic lymph node metastases increases considerably. In fact, there are considerably more patients with metastases to the pelvic nodes in a corpus et colli than there are patients with primary cervical cancer with disease limited to the cervix. Approximately 10% of stage I endometrial cancers metastasize to the adnexa. Occasionally, this is appreciated because of the enlarged adnexa noted on pelvic examination, but in many instances, discovery is at the time of surgery. Histologic confirmation is required for a patient to be placed into stage III disease based on adnexal metastasis. Obviously, treatment must be adequate for this extrauterine disease.
Table 4. Five-year survival in endometrial cancer*
| Stage | Surgical | Clinical |
| I | 92% | 81% |
| II | 82% | 72% |
| III | 68% | 57% |
| IV | 30% | — |
*Survival rates based on 6085 patients.
(Pecorelli S: Int J Gynecol Obstet 2006;95:S140)
Intraperitoneal disease, although unusual, can occur when disease clinically is thought to be limited to the uterus. Complete evaluation of the intraperitoneal space is, therefore, imperative. In the large GOG study, 6% of patients with clinical stage I disease were found to have intraperitoneal disease.
Lymph Node Involvement
For many years, it was thought that endometrial cancer did not go to the lymph nodes, particularly the pelvic nodes. During the 1960s and even before, there were data suggesting that pelvic lymph nodes could be involved with metastases, even with early-stage disease. These data were based on patients who had been primarily treated with radical hysterectomy and pelvic lymphadenectomy. In the early 1970s, an increased amount of information became available to substantiate the fact that patients with clinical stage I cancers had a significant chance of having metastases to the pelvic lymph nodes. In the large GOG study, 9% of patients with clinical stage I had metastases to the pelvic lymph nodes. Almost without exception, the metastases were microscopic and could not be determined on gross evaluation. Palpation of the lymph nodes in both pelvic and paraaortic areas is extremely unreliable in endometrial cancer. The incidence of lymph node metastases is directly related to grade and depth of invasion (Tables 5 and 6). Only 3% of patients with well-differentiated cancers have pelvic node involvement; 18% of patients with poorly differentiated tumors have lymph node metastases. Only 1% of patients with disease limited to the endometrium disease have pelvic node metastases, whereas 25% of patients with deep invasion have pelvic nodes involved. It appears that depth of invasion may be more predictive of pelvic lymph node metastases than grade.5
Table 5. Grade versus positive pelvic and aortic nodes
| Grade (n) | Pelvic n (%) | Aortic n (%) |
| G1 (180) | 5 (3) | 3 (2) |
| G2 (288) | 25 (9) | 14 (5) |
| G3 (153) | 28 (18) | 17 (11) |
(Modified from Creasman WT, Morrow CP, Bundy L et al: Surgical pathological spread patterns of endometrial cancer. Cancer 1987;60:2035.)
Table 6. Maximal invasion and node metastasis
| Maximal invasion (n) | Pelvic n (%) | Aortic n (%) |
| Endometrium only (87) | 1 (1) | 1 (1) |
| Superficial muscle (279) | 15 (5) | 8 (3) |
| Intermediate muscle (116) | 7 (6) | 1 (1) |
| Deep muscle (139) | 35 (25) | 24 (17) |
(Modified from Creasman WT, Morrow CP, Bundy L et al: Surgical pathological spread patterns of endometrial cancer. Cancer 1987;60:2035.)
For many years, it was stated that if lymph node metastasis occurred in endometrial cancer, it was to the paraaortic nodes. There were few data to substantiate that impression until large cooperative group studies indicated that approximately 5% of patients with clinical stage I cancer had metastases to the aortic nodes.5 Although lymph node metastases can be present without pelvic nodes involved, two-thirds of patients with metastases to the para-aortic nodes also have pelvic nodes involved. Nodal involvement is directly related to differentiation of the tumor and depth of invasion. In the GOG study, 11% of patients with clinical stage I cancer had metastases to the pelvic, para-aortic, or both lymph node groups.
Peritoneal Cytology
Malignant effusions are a poor prognostic sign. Even when ascitic fluid is not present in the peritoneal cavity, cytologic evaluation can be performed. It appears that the presence of malignant cells in the peritoneal cytology is extremely important in evaluating the extent of disease and therefore prognosis. If no ascitic fluid is present when the peritoneal cavity is entered, 100 ml of saline can be injected into the pelvic cavity. The saline is allowed to admix in the cul-de-sac and then is withdrawn and sent to the cytologic laboratory.
The possible prognostic role of malignant peritoneal cytology in endometrial cancer has come under considerable discussion during the past several years. Most of these studies suggest that in 10–20% of cases thought to be clinical stage I (pre-1988 FIGO stage), malignant cells were present in the peritoneal cytology. On multivariate analysis, this finding has been shown to be highly predictive of other poor prognostic factors, such as intraperitoneal disease and metastases to the lymph nodes.5 Most studies in the literature suggest that the finding of malignant peritoneal cytology is itself a poor prognostic factor. Is malignant peritoneal cytology a poor prognostic factor if there is no other evidence of disease outside the uterus? Several studies suggest it is important; others have not found this relation.
SURGICAL MANAGEMENT
The extent of disease in a patient with adenocarcinoma of the endometrium can be evaluated only with primary surgery. The patient can then be treated in a highly individualized manner instead of being treated with standard therapy, as occurs with all patients. Concern about not giving preoperative irradiation can be alleviated when one realizes that postoperative irradiation is as effective, if not more so, than preoperative irradiation.
Experience has indicated that the proper surgical procedure for evaluating these factors should be peritoneal cytology, a total abdominal hysterectomy, bilateral salpingo-oophorectomy, and pelvic and para-aortic lymphadenectomy. Immediately when the peritoneal cavity is opened, cytology should be obtained as noted previously. The usual extrafascial total abdominal hysterectomy and bilateral salpingo-oophorectomy are then performed. Para-aortic nodes are removed by retracting the small intestines into the upper abdomen and incising the peritoneum over the upper common iliac arteries and lower aorta. The main vessels are outlined and the tissue overlying the vena cava and aorta is removed, beginning at the bifurcation and extending cephalad to the retroperitoneal duodenum. Hemostasis can be managed easily with hemoclips. Lymph node dissection in the pelvis is accomplished by entering the retroperitoneal space in routine fashion. The lower common and external iliac arteries are outlined, as is the hypogastric artery. Any enlarged nodes identified should be removed separately for histologic evaluation. The lymphoid tissue from the lower common iliac and external iliac artery down to the inguinal ligament is removed, as well as the tissue from the obturator fossa superior to the obturator nerve. Using this technique, approximately 25–30 lymph nodes will be available for evaluation. All tissue removed is histologically evaluated for differentiation of the tumor, size of the tumor, depth of myometrial invasion, location of the tumor in the uterus, adnexal involvement, peritoneal cytology, and involvement of the pelvic or para-aortic lymph nodes. Specific, individualized radiation therapy or chemotherapy is then given, depending on the extent of disease.6
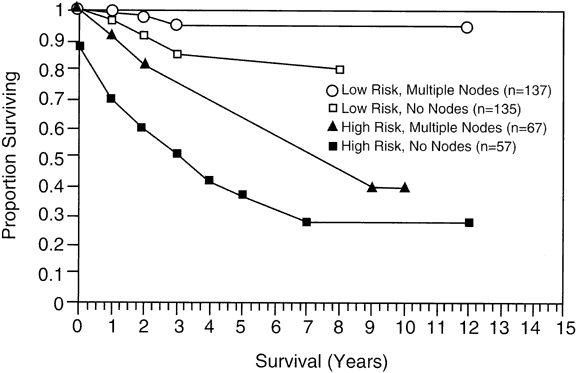
For several years, opponents of surgical staging suggested that lymphadenectomies were diagnostic only and therefore only rearranged the staging of patients with adenocarcinoma of the endometrium. Proponents argued that if that were the only accomplishment, then more accurate staging was achieved and better therapy could be applied. Data now suggest that lymphadenectomy appears to be therapeutic as well as diagnostic. Investigators at the University of Alabama at Birmingham found improved survival if a lymphadenectomy was performed, both in low-risk and high-risk patients (Fig. 1).7
Analysis from Birmingham evaluated 670 patients with endometrial cancer who underwent full surgical staging. There were 334 patients with stage IB, of which 99% did not receive postoperative radiation therapy. There were 16 recurrences, of which 10 occurred in the pelvis. Of these, 10 were salvaged with a mean follow-up of 23 months. There were 84 patients with stage IC, of which 57 did not receive adjuvant therapy. There were seven recurrences with four being salvaged with additional therapy. The authors thought that conservative therapy appears to be a reasonable alternative to adjuvant radiation therapy.
Chan and associates have reviewed the large SEER database in regards to survival and extent of lymphadenectomy. An independent risk factor was the extent of the lymphadenectomy. When only one lymph node was removed and it contained metastasis, survival was 51% compared to one positive node in which more than 20 lymph nodes were removed where survival was 83%. If more than five lymph nodes were positive but only six to ten nodes were removed, survival was 21%. In a similar patient with more than 20 lymph nodes removed, survival was 61%. This suggests that occult disease may be removed if the lymphadenectomy is more extensive and therefore may be therapeutic as well as diagnostic.8
Recently there has been increased discussion concerning who needs lymphadenectomies and what is the extent of the lymph node dissection. All agree that “low risk” patients do not need lymphadenectomy. These patients usually include G1 and 2 with superficial invasion. How that is best determined before a possible lymphadenectomy continues to be discussed.
For sometime the question has been whether the lymphadenectomy is only diagnostic or whether it may also be therapeutic. Two prospective randomized studies have suggested the lymphadenectomy is not therapeutic. The ASTEC study randomized 1408 women with endometrial cancer to receive a hysterectomy and bilateral salpingo-oophorectomy with or without a lymphadenectomy.9 Overall survival was the primary outcome and at a median follow-up of 37 months there was no difference in overall recurrence-free survival. This was an intent-to-treat protocol and unfortunately compliance was a major problem. In the no node group, 5% had nodes removed and almost 30% of these patients had nodal metastasis; 8% of the lymphadenectomy group did not have nodes removed and over one-third had nine or fewer nodes removed. Paraaortic node removal was not part of the protocol; however, some did have nodes removed. Patients were randomized to a second protocol evaluating postoerative radiation therapy. Surgical staging was not used in the second randomization and again a major compliance problem excited. The validity of this study has been questioned because of the compliance issues and second randomization.
The second randomized study was from Italy.10 This study randomized 514 patients to hysterectomy, bilateral salpingo-oophorectomy with or without pelvic lymphadenectomy. Paraaortic nodes were not removed and removal of 20 lymph nodes was an eligibility requirement. Although pelvic lymphadenectomy improved surgical staging it did not improve disease-free or overall survival. Adjuvant therapy was given at the discretion of the treating physician.
The SEPAL study from Japan was a retrospective cohort evaluation.11 At one hospital a hysterectomy, bilateral salpingo-oophorectomy and pelvic lymphadenectomy was done and at another hospital an additional paraaortic lymphadenectomy was done. Lymphadenectomy was extensive with a median of 34 nodes removed in pelvic only patients and 59 pelvic and 29 paraaortic nodes in the other group. Cox regression analysis for all patients noted the survival was significantly longer in the pelvic and paraaortic patients compared with pelvic nodes only. These findings remained even after correcting for adjuvant therapy.
It would appear that lymphadenectomy may have a significant impact on survival if both pelvic and paraaortic nodes are removed.
In the 2006 FIGO Annual Report, approximately 94% of all reported patients with endometrial cancer were surgically staged. When 5-year survivals were compared between surgical and clinical staging, a considerable difference is noted. This suggests that knowing the exact extent of disease is important and that clinical staging has a large margin of error regarding identifying spread of disease. For surgical staging, patients with stage I had a 5-year survival of 93%, 90%, and 79% for grades 1, 2, and 3, respectively, compared to 60%, 50%, and 29% for clinical staging, respectively.
It appears that evaluation of the prognostic factors may identify patients who are at high risk of dying of the disease. The ability to evaluate these items can be obtained only by the primary surgical approach. After extant disease has been identified, therapy as needed can be directed to the involved area. It is hoped that individualized therapy will improve survival. It is important to identify patients who historically may have been treated with radiation therapy. With surgical staging and knowing the exact extent of disease, many of these patients are no longer thought to require adjuvant therapy. Implications regarding cost and morbidity may be tremendous.
REFERENCES
Jemal A, Siegel R, Ward E et al: Cancer statistics, 2008. CA Cancer J Clin. 2008; 58:71-96 |
|
Vogel VG, et al Update of NSABP study of tamoxifen and ralixifene ( STAR) P-2 trial. Can Prev Res 2010; 3(6): 696 |
|
Pecorelli S (ed): Annual report on the results of treatment in gynecological cancer (Vol 24). J Epid Biostat 6: 45, 2001 |
|
American Cancer Society guidelines for early detection of cancer. CA Cancer J Clin. 51:38, 2001 |
|
Creasman WT, Morrow CP, Bundy L et al: Surgical pathological spread patterns of endometrial cancer. Cancer 60: 2035, 1987 |
|
DiSaia PJ, Creasman WT: Clinical Gynecologic Oncology, p 156. St. Louis: CV Mosby, 1997 |
|
Kilgore LC, Patridge EE, Alvarez RD et al: Adenocarcinoma of the endometrium: Survival comparison of patients with and without pelvic node sampling. Gynecol Oncol 56: 26, 1995 |
|
Chan JK, Cheung MK, Huh WK et al: Therapeutic role of lymph node resection in endometrioid corpus cancer: a studyof 12,333 patients. Cancer. 2006 Oct 15;107(8):1823-30. |
|
ASTEC study group, Kitchener H, Swart AM, Qian Q, et al: Efficacy of systematic pelvic lymphadenectomy in endometrial cancer. Lancet 2009; 373: 125-36 |
|
Panici PB, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs no lymphadenectomy in early stage endometrial carcinoma. J Natl Cancer Inst 2008; 100: 1707-16 |
|
Todo Y, Kato H, Kaneuchi M, et al: Survival effect of paraaortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis Lancet 2010; 375: 1165-72 |