Ultrasound Bioeffects for the Perinatologist
Authors
INTRODUCTION
In the past few decades, ultrasound has become the primary imaging procedure for an increasing number of conditions. Ultrasound imaging is an integral part of the practice of obstetrics, gynecology, radiology, cardiology, neurology and neurosurgery, pediatrics, gastroenterology, urology, angiology, surgery, internal medicine, and more recently emergency medicine as well as in musculoskeletal disorders. In obstetrics, ultrasound is increasingly used for applications such as the assessment of gestational age and the diagnosis of placenta previa, multiple gestations, fetal growth restriction, and many fetal structural abnormalities such as spina bifida and hydrocephalus, as well as screening for genetic (chromosomal and other) abnormalities. Diagnostic ultrasound has also proved useful in antenatal diagnostic procedures (chorionic villus sampling and amniocentesis) and interventions (medical and surgical) for fetal diseases.
There is no doubt that a spectacular improvement in ultrasound imaging quality contributed to the rapid increase in its use as a diagnostic tool. Furthermore, the introduction of the linear array real-time scanner in the 1970s led to a significant increase in the amount of ultrasound imaging performed during pregnancy. Important advances in real-time scanners include the introduction of duplex systems, which combine real-time imaging with pulsed (including color) Doppler ultrasound. These systems made possible the measurement of fetal and uteroplacental blood flow. Information on that flow has become a very important obstetric application of diagnostic ultrasound. Advances in computer technology have allowed three-dimensional reconstructions (3D) of acquired information and even "real-time" 3D, also known as 4D.
As a result of these advances, a rapidly increasing number of pregnant women have been exposed to ultrasound. Over two decades ago, about 40% of all pregnant women in the United States had undergone routine scanning.1 However, there are reasons to believe that today this number is significantly higher and may reach close to 100% in developed countries; in West Germany, virtually all babies are exposed to ultrasound in utero, often multiple times. Similarly, in the USA the vast majority of pregnant women under medical care will undergo at least one scan.
Since ultrasound is a form of energy, there is potential for effects in living tissues (bioeffects). The issue of whether there may be long-term adverse effects to the fetus from exposure to ultrasound needs to be raised.2 In other words, “How safe is ultrasound?” It is well established that under certain conditions ultrasound can lead to undesirable side-effects.3, 4, 5, 6, 7 Therefore, it is unrealistic to expect that the answer to this question can be a simple “yes” or “no”. Rather, the question to be answered should be, “Is diagnostic ultrasound, as presently used in clinical practice, safe for the fetus?”
The main goals of this chapter are to:
1. Summarize the available knowledge regarding whether ultrasound energy produces bioeffects at the levels currently used with commercial diagnostic equipment;
2. Describe how manipulation of instrument controls alters the emitted (and received) acoustic energy;
3. Educate the clinical users in ways to minimize fetal exposure, without sacrificing diagnostic quality.
It must be stressed at the outset of this review that, to date, no evidence has been found of harmful effects of ultrasound in humans at clinically used exposure levels. However, it must also be stressed that all available epidemiologic literature dates from before 1992, a time when maximal allowed acoustic output of diagnostic systems for fetal use was increased by a factor of almost 8 (from 94 mW/cm2 to 720 mW/cm2).8 New applications of and new advances in diagnostic imaging regularly prompt discussions on its safety and prudent use. Common to these discussions is that they call for avoidance of unnecessary exposure.7, 9, 10, 11 A further concerning issue is exposure of fetuses during the first trimester, a time of maximal susceptibility to damaging influences, because of new screening protocols, including relatively prolonged exposure to spectral Doppler.12 Also of concern is that although there is no reason to believe that there are risks related to ultrasonic exposure, there are many inexpensive ultrasonic scanners throughout the world. In addition, the knowledge of clinical users of ultrasound on bioeffects and safety is, generally, very limited.13 Therefore, there is an ongoing need to disseminate the knowledge of interactions between ultrasound energy and biologic tissue, and to identify the potential conditions for bioeffects.7, 10, 14
Any modern literature search on ultrasonically induced bioeffects will result in a list containing hundreds of papers and reports on the topic. Because the literature on this subject is enormous, only those papers that are considered to be relevant for this review are discussed here. In particular, attention is focused on data pertaining to mammalian tissue. Those readers who are interested in reviewing all data available are referred to excellent textbooks15, 16 or to several comprehensive reports.3, 4, 5, 6, 7, 14, 17, 18, 19, 20, 21, 22, 23
An informed discussion on bioeffects requires a basic understanding of the physical parameters involved and their relation to the observed effect. More specifically, the relation between the magnitude of the effect and the magnitude of the characteristic parameters of the ultrasound agent should be known. In addition, it is important to determine whether the effect will give rise to any concern. Otherwise, the significance of the experimental outcome can be misinterpreted easily and may lead to meaningless conclusions regarding the outcome's relevance to clinical practice.
For the reasons above and to judge the significance of the findings reported in the literature properly, it is appropriate to start with a brief review of the relevant ultrasound parameters and the physical mechanisms of interaction between ultrasound and biologic tissue. This is followed by a summary of the range of acoustic output levels produced by the currently used clinical diagnostic machines and a succinct discussion of recent developments in the regulatory area. Briefly, these developments, secondary to an authorized increase in acoustic output, allowed manufacturers to introduce a real-time on-screen display involving thermal index and mechanical index (TI and MI, respectively). These indices inform the user of the potential for the two most relevant ultrasonically induced bioeffects: heating and cavitation. Understanding the implications of such an on-system display is of vital importance for practitioners, including physicians, sonographers, and other allied health personnel applying ultrasound energy to patients. This is because the displayed information about the potential of bioeffects requires practitioners to make a direct decision as to the risks and benefits associated with the given clinical examination.7, 9, 10, 11, 14, 15, 16, 17, 18, 19, 20, 24
Next, a survey of the literature on biologic effects is given. As already noted, the biologic experiments that are discussed were carefully selected and limited to those that bear the closest resemblance to clinical exposure conditions. Special attention is given to human epidemiology studies; limitations and deficiencies of the current research on ultrasonically induced bioeffects are also discussed.
Since this chapter is primarily intended for clinicians, practical aspects of scanning are considered with particular attention to changes in acoustic output as a result of manipulation of the machine controls. Simple rules are suggested to keep the exposure to a minimum compatible with image quality adequate for diagnostic purposes.23, 25
The conclusions focus on a critical summary of the current evidence on risks associated with ultrasonic exposure in diagnostic clinical examinations, including several relevant statements on in vivo mammalian bioeffects and safety by the American Institute of Ultrasound in Medicine (AIUM) Bioeffects Committee,20 as well as safety statements from some international bodies.26, 27, 28
INTERACTION BETWEEN ULTRASOUND AND BIOLOGIC TISSUE – PHYSICAL MECHANISMS
It is widely accepted that there are two basic mechanisms, namely, thermal and nonthermal, by which ultrasound is known to affect biologic materials.3, 12, 13, 14, 17, 18, 19, 20 Nonthermal mechanisms include cavitational and noncavitational effects, which are associated with certain mechanical aspects of the acoustic or ultrasonic field. These aspects can be described in terms of second-order phenomena, such as radiation pressure, radiation force, radiation torque, and acoustic streaming, and are comprehensively reviewed in other sources.19, 20
Because the data on second-order phenomena were obtained primarily by studying in vitro systems, and because their biologic significance is not immediately clear, attention is focused on thermal and cavitational phenomena.
Thermal mechanism
The thermal mechanism is associated with the absorption of acoustic energy by tissue and the generation of heat. It appears to be the best understood, and analytic models have been developed to predict the possible temperature elevation in tissue.18 These models relate acoustic energy to the associated temperature increase, provided that absorption coefficients for the tissues considered are known. A brief description of the physical and physiologic variables that play a role in the generation of this temperature elevation follows.
As an ultrasound beam traverses tissue layers, the rate of energy deposition is determined by the factors defined by the operational characteristics of the imaging system and the physical parameters of the tissue being imaged. The system's operating characteristics are functions of the mode (imaging and/or Doppler) being used, as well as of the focal characteristics of the transducer and its frequency. As an example of the way these characteristics influence energy transfer, one can note the difference in energy distribution between scanned modes such as B-mode and color-Doppler and unscanned modes such as pulsed-wave (PW) Doppler. More specifically, B-mode energy is distributed over a large volume, whereas in unscanned modes, such as PW Doppler, the acoustic energy is aimed along a single line. Similarly, a highly focused transducer has the potential for a highly concentrated energy deposition, whereas weakly focused transducers tend to spread the energy over larger volumes. Whether energy is deposited in a given tissue volume is determined by that tissue's absorption characteristics, which may vary significantly depending on the organ considered. For example, there is almost no absorption in liquids such as amniotic fluid, blood, and urine. However, an adult bone absorbs about 60–80% of the acoustic energy impinging on it.18 With fetal bone, there is a wide variation in absorptive behavior, depending on the degree of ossification. The clinical situation of greatest interest as far as thermal effects are concerned is a fetus in utero with an ossified bone structure and a mother with a thin abdominal wall. In such a case there is little attenuation of acoustic energy because of the thin layer of intervening maternal tissue, yet there is a high degree of absorption associated with the fetal bone.18
Local generation of heat per second in a certain tissue volume can be calculated, based on the tissue absorption coefficient (in most tissues this coefficient is proportional to the frequency) and the average intensity per time unit.
Another important determinant of local heating involves the degree of attenuation in tissue layers in front of the point of interest. An increased amount of attenuation in the overlying tissues decreases the energy available for conversion into heat. Thus, the use of fetal Doppler through a thick abdominal wall is less likely to cause a significant temperature increase than are examinations involving patients with thin abdominal walls.
Although the above discussion has concentrated on heat sources, there are at least two mechanisms of heat loss. Blood perfusion is an efficient mechanism for heat removal. The degree of blood perfusion varies between the tissue types: among the best perfused organs are the kidneys, heart, and brain, whereas bone and resting muscle are among the least perfused.22 Another cooling mechanism is due to heat conduction. The degree of thermal conductivity is relatively uniform among the tissues and is fairly close to that of water, with the exception of bone, which is highly conductive, and fat, which is a poor thermal conductor.22
There seems to be an agreement that an in situ temperature rise to or above 41°C is considered hazardous in fetal exposures because it may lead to undesired effects. A more detailed discussion of the recent AIUM recommendations concerning possible thermal mechanism-related bioeffects is given in the closing section of this chapter.17, 21
Experimental studies indicate that intact mammalian systems (in vivo) do not show a significant rise in temperature when exposed to pulsed imaging equipment.3, 12, 17 However, peripheral vessel pulsed and continuous-wave (CW) Doppler equipment, when used for a relatively long time (1–10 min), may be an exception.17, 24 Therefore, Doppler should be used with care, especially during applications in which Doppler is used for the study of blood velocities in the umbilical cord and studies of the first trimester fetus.29, 30, 31
Cavitational mechanism
The term cavitation refers to phenomena associated with the vibration and motion dynamics of small gaseous bodies (bubbles) when exposed to an ultrasound field.12, 17, 20 If the bubble does not collapse during the ultrasound exposure, the condition is referred to as stable cavitation, in contrast to inertial (collapse) cavitation (formerly known as transient cavitation5), during which the vibration amplitude of the bubble wall increases so much that the bubble implodes. This implosion generates highly localized shock waves and is also associated with extremely high local temperatures (up to 10,000K).32 In addition to the temperature elevation, the implosion may result in the generation of free radicals such as hydroxyl radicals and hydrogen. These radicals are very active and may lead to some undesired biologic changes, such as spontaneous biochemical reactions within the tissue.33
In this context it is worthwhile to point out that lung hemorrhage has been observed in mice after exposure to ultrasound waves with relatively low peak pressures of 1–2 MPa. (The pascal is the SI unit for pressure. It is a measure of perpendicular force per unit area. One megapascal (MPA) is equivalent to 9.87 atmospheres. It is used in acoustics and other fields to define the pressure exercised by a sound wave on the insonated tissue.) More specifically, cavitation-related events were reported as a mechanism leading to lesions in the lung tissue of adult mice.34 The hemorrhage of lung tissue in mice occurred as a result of exposure to 1-MHz, 10-μs pulses of about 1 MPa pressure amplitude. Also, the lung tissue damage was observed in the lungs of monkeys exposed to 3.7 MPa using a commercial diagnostic ultrasound imaging device operating at the maximum output in combined pulsed and color Doppler mode.35 This is of interest because monkey represents a biologic model closely resembling human. This suggests that the alveoli may act as cavitation nuclei and that caution should be exercised in cases in which the ultrasound interacts with lung tissue. Although only a limited number of primates were used in the study, these results are important because lung hemorrhage was observed at clinically relevant exposure conditions. Also, although these results have not yet been independently confirmed, this study suggests an increased potential risk when frequent cardiac examinations are performed in neonates. It is appropriate to point out here that certain tissues may be more prone to cavitation-like events than others. Thus, no kidney hemorrhage in mice was observed at peak positive pressures of 9–10 MPa and negative peak pressure amplitudes of 4–5 MPa at frequencies of 1.2 and 3.8 MHz.36 Thus, it appears that in kidney tissue there are no nuclei to sustain a gas body.
The bioeffects observed in the study by Tarrantal and Canfield37 and the study by Carstensen and colleagues38 are also relevant to the situation in which the fetus undergoes prolonged exposure to ultrasound in an early stage of pregnancy. Therefore, the ultrasound examination time should be minimized, consistent with the requested diagnostic information.
Inertial cavitation leading to severe tissue hemorrhage also has been experimentally observed during therapeutic treatment of the kidney using an extracorporeal shock-wave lithotriptor.39
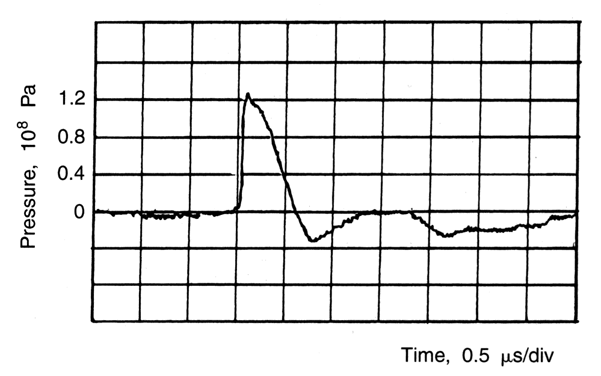
Although of only peripheral interest to the present discussion, it should be recognized that a sufficiently high negative peak amplitude of the ultrasonic wave can cause severe damage to the tissue. A typical pulse generated by a lithotriptor is shown in Figure 1. The negative pressure amplitude of the typical lithotriptor pulse (see Fig. 1) may be of the same order of magnitude as the corresponding pressure amplitude of the pulses used in diagnostic ultrasound (see Acoustic output levels).
During stable cavitation, the vibration amplitude of the bubble wall may set up microflow (microstreaming). The theory of microstreaming is well developed and is well described by Nyborg.40 The theory predicts that the microstreaming can generate shear stress that acts on adjacent bodies such as cell membranes.5, 41, 42 If the shear stress is sufficiently large, it may cause breakage of the cell membrane.41, 42 It may be argued that cell membrane breakage can also happen during exercise such as jogging. Nevertheless, the breakage, even if unimportant from a biologic point of view, occurs because of the body's response to an external agent, and therefore it should not be totally dismissed. Although phenomena resembling stable cavitation have been reported in in vivo experiments, it appears that they occurred under exposure conditions not directly relevant to diagnostic imaging.34
ULTRASOUND FIELD PARAMETERS
As already mentioned, interpreting the data on the safety of ultrasound requires some knowledge of the basic terminology of key ultrasound field parameters, such as acoustic pressure or intensity. Thus, before the review of bioeffects observed, it is essential to briefly clarify the nomenclature used in ultrasonic exposimetry and to introduce acoustic field parameters relevant to ultrasound bioeffects.43
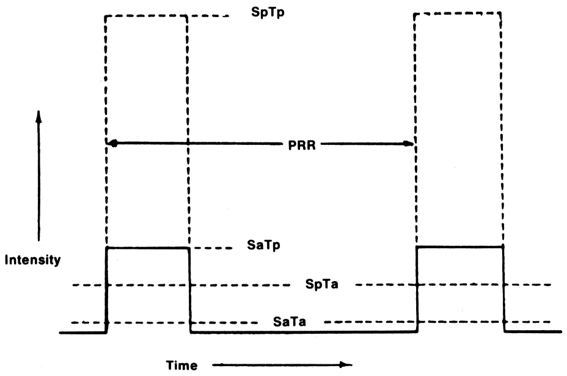
Depending on the time and location of the measurement, several parameters can be described in relation to time or space: temporal peak intensity (TP, the greatest intensity), average intensity over time, i.e., including “silent” time between pulses (TA, temporal-average intensity), maximal intensity at a particular location (SP, spatial-peak intensity) as well as average-spatial intensity (SA). By combining time and space, six intensities can be described: spatial average-temporal average (ISATA), spatial average-pulse average (ISAPA), spatial average-temporal peak (ISATP), spatial peak-temporal average (ISPTA), spatial peak-pulse average (ISPPA), and spatial peak-temporal average (ISPTA). The most practical, and commonly referred to, is the ISPTA.
Rigorous definitions of these parameters can be found in several publications.43, 44
It should be pointed out that a combination of spatial and temporal intensities is needed to relate an observed bioeffect to ultrasound field parameters, e.g., the spatial peak, temporal average (SpTa) intensity. Also, it should be noted that although bioeffects are conventionally related to the acoustic intensity or I (units: W/cm2), the field parameter currently gaining attention is acoustic pressure P (units: pascals or Pa; 105 Pa = 1 bar ~ 1 atmosphere), because it is often the primary parameter measured. Moreover, knowledge of peak negative pressure amplitude is needed to determine the value of the MI (see The output display standard section below).
In addition to these intensities, the beam pattern distribution, total acoustic power (W), pulse repetition rate (PRR), and imaging frequency are needed to adequately determine ultrasound field parameters.
Complete ultrasound dosimetry also requires information on exposure time, including dwell time. The dwell time is defined as the time during which the ultrasound beam (more specifically its focal zone) remains at the same site of the body in usual clinical practice. It should immediately be noted and remembered that no epidemiological data available include dwell time, nor do the TI and MI take time into account.
Each expression of intensity mentioned above serves a different purpose.43 Briefly, the ISpPa is a measure of the ultrasound energy associated with a single pulse, and Im intensity characterizes the maximum instantaneous energy in the period of the pulse duration. The ISpTa corresponds to the energy averaged over a period of time and is proportional to the PRF. Although a typical imaging system has a PRF of the order of 1 kHz, a PRF as high as 20 kHz may be used in blood flow velocity measurements using Doppler devices. The rationale for determining peak negative and peak positive pressure amplitudes and the intensity parameters is their potential for producing bioeffects. A comprehensive discussion of this issue is given in the article by Nyborg and Wu.43
ACOUSTIC OUTPUT LEVELS
Once the definitions of the different acoustic intensities are known, the data available on the acoustic output of ultrasonic equipment for imaging and Doppler applications can be summarized. The acoustic output levels of the diagnostic devices quoted here were compiled from published data and are listed in Table 1, Table 2, and Table 3.45 The Doppler instruments measured included CW Doppler units for cardiovascular investigations, fetal monitors, stand-alone pulsed Doppler equipment, color Doppler, and duplex scanners working in Doppler mode, all in the frequency range of 2–8 MHz. Measurements were carried out in water and revealed that pulsed Doppler equipment could generate SpTa intensities that exceeded 100 mW/cm2, with a maximum of 4520 mW/cm2.45 (The maximum SpPa intensity for pulsed Doppler equipment was about 770 W/cm2.45) One should remember that the maximal ISPTA allowed since 1992 by the FDA for fetal use is 720mW/cm2.
Table 1. Highest values of acoustic parameters encountered at the output of pulsed Doppler equipment (water values)
P+ | |
P− | 3.8 [MPa] |
ISpPa | 771 [W/cm2] |
ISpTa | 4.52 [W/cm2] |
Power | 410 [mW] |
Mechanical index (MI) | 1.1 |
P, peak pressure amplitude.
Data from multiple sources.20, 45
Table 2. Highest values of acoustic parameters encountered at the output of continuous-wave Doppler equipment (water values)
PV and cardiac |
|
ISpTa | 850 [mW/cm2] |
Peak pressure | 160 [kPa] |
Fetal heart |
|
ISpTa | 33 [mW/cm2] |
Peak pressure | 31 [kPa] |
(Data from Bioeffects and Safety of Diagnostic Ultrasound. Laurel, MD, American Institute of Ultrasound in Medicine, 1993)
Table 3. Highest values of acoustic parameters encountered at the output of diagnostic pulse-echo equipment (water values)20, 45
P+ | 8.8 [MPa] |
P− | 4.3 [MPa] |
ISpPa | 933 [W/cm2] |
ISpTa | 440 [mW/cm2] (stationary) |
ISpTa | 177 [mW/cm2] (scanning) |
Power | 350 [mW] (B-scan) |
Power | 17 [mW] (M-mode) |
Mechanical index (MI) | 1.0 |
(Data from Bioeffects and Safety of Diagnostic Ultrasound. Laurel, MD, American Institute of Ultrasound in Medicine, 1993)
The output of some CW cardiovascular Doppler devices produced intensity levels of the order of 850 mW/cm2, whereas fetal monitoring equipment intensities were of the order of 30 mW/cm2. Table 1 and Table 2 compile the highest intensities measured at the output of pulsed and CW Doppler equipment, respectively. Table 3 summarizes maximum output levels encountered at the output of clinical pulsed echo equipment.
THE OUTPUT DISPLAY STANDARD
Before a review of the clinical evidence for ultrasonically induced bioeffects can take place, it is important to briefly discuss the clinical implications of the standard which was implemented in the USA around 1992–1994: the Standard for Real-Time Display of Thermal and Mechanical Indices on Diagnostic Ultrasound Equipment, generally known as the Output Display Standard or ODS.7, 20, 44 Secondary to end-users desire for better imaging and as a result of discussions that involved the FDA, the AIUM, and the National Electrical Manufacturers Association (NEMA), in 1994 the FDA revised its guidance on diagnostic ultrasound 510(k) submissions to allow the use of the MI in place of the ISpPa in determining substantial equivalence of devices. This revision assumes that on-system displays20 of numerical indices, including MI and TI, will inform the user about the potential for either thermal or nonthermal bioeffects associated with the actual examination settings of the imaging system. This enables the clinician to increase acoustic power output beyond the existing FDA guidelines when clinically warranted. Before the 1994 FDA revision, such an increase was not possible. The maximum available acoustic output was limited by the manufacturer's software, which would not allow the output to exceed FDA guidelines for maximum exposure. It must be stressed that with the implementation of the ODS, diagnostic ultrasound systems can have a higher output limit. With the higher limits comes the potential for increased risk to the patient, so the clinician must make a careful risk/benefit analysis. Therefore, the purpose of the ODS is to help the clinician implement the ALARA (as low as reasonably achievable) principle and minimize the potential for bioeffects. A very important aspect of the implementation of the ODS was education of the end-users about bioeffects of ultrasound and safety-related issues.
In the following pages, the thermal and mechanical indices are defined and the potential for added diagnostic and clinical benefits is briefly discussed. Additional comments on the indices are given in the Conclusions section. A more comprehensive treatment of the different tissue models (including homogeneous tissue, soft tissue, and bone tissue) can be found elsewhere.7, 20, 24, 32
The thermal index (TI) provides some indication of potential temperature increase and the mechanical index (MI) provides indication of potential for non-thermal (i.e., mechanical) effects.32, 43, 46 The TI is the ratio of total acoustic power to the acoustic power estimated to be required to increase tissue temperature by a maximum of 1oC. It is an estimate of the maximal temperature rise at a given exposure. There are three variants: TI for soft tissue (TIS), to be used mostly in early pregnancy when ossification is minimal; TI for bones (TIB), to be used when the ultrasound beam impinges on bone, at or near the beam focus, such as late second and third trimesters of pregnancy; and TI for transcranial studies (TIC) when the transducer is essentially against bone, mostly for examinations in adult patients. These indices were required to be displayed if equal to or over 0.4. It needs to be made very clear that TI does not represent an actual or an assumed temperature increase. It bears some correlation with temperature rise in degrees Celsius but in no way allows an estimate or a guess as to what that temperature change actually is in the tissue. Because of the inherent uncertainties in the TI and because exposure time is not a factor in its equation. Several authors have suggested modifications or frank changes in the way thermal effects can be assessed.47, 48, 49
The MI has been developed as an indicator of the potential for nonthermal damage or cavitation-like phenomena related to B-mode operation. MI is inversely proportional to the center transducer frequency, i.e., the higher the frequency, the lower the risk of mechanical effects. It is important to realize that the MI is not based on actual in situ measurements. It is a theoretical formulation of the ratio of the pressure to the square root of the ultrasound frequency (hence, the higher the frequency, the lower the risk of mechanical effect). Both the TI and MI can and should be followed as an indication of change in output during the clinical examination. A clear extension of the above statements is that education of the end user is a major part in the implementation of the indices. Furthermore, several assumptions were made which have resulted in some questions on the clinical value of these indices. Possibly the most significant (from a clinical aspect) is the choice of the homogeneous attenuation path model (defined as the H3 model), with an attenuation coefficient of 0.3 dB/cm/MHz. The reason to employ models of this nature is the impossibility, for obvious reasons, to perform these measurements in pregnant women. This coefficient may be an overestimation of the attenuation in many clinical scenarios, a situation which would underestimate the actual exposure. In NCRP report number 140,46 there is an entire chapter (Chapter 9), indicating conditions where both indices may be inaccurate, e.g., long fluid path (full bladder, amniotic fluid, ascites or hydrocephalus) or path through increased amounts of soft tissue such as in obese patients. Because of these uncertainties, the accuracy of the TI and MI may be within a factor of 2 or even 6. For example, an onscreen TI of 1 may correspond to an actual value of 0.5 or 2 if the error factor is 2, but possibly 0.33 or 6, if the error factor is 6 (as previously stated, these are not actual temperature indications). A further disturbing and confusing element is that outputs reported by manufacturers are not necessarily equivalent to those calculated in the laboratory.50
Regarding the second important aspect of the ODS implementation, namely education, it appears this was not accomplished. Several surveys have clearly shown a lack of knowledge among clinicians in Europe,51 the United States,52 and Asia,53 and that training of residents in obstetrics and gynecology in the United States is grossly lacking in this regard.54
BIOEFFECTS
In general, the bioeffects literature can be divided into two classes: one based on the epidemiology of the large number of ultrasound examinations performed both clinically and as laboratory experiments, and another in which a causal relation between bioeffects and the applied acoustic energy is developed. Both classes are discussed below.
Effects of ultrasound on mammalian tissues in vivo
Simple in vitro models, such as cells or tissue cultures, are often used in the search for biologic effects to gain an understanding of the possible mechanisms of interaction between ultrasound and biologic tissue. The results of the research on biologic effects in nonmammalian species, such as insects, amphibians, and avians, indicate that studies on these relatively simple organisms are helpful in understanding the mechanisms of interaction between ultrasound and biologic systems.18 However, from a clinical standpoint, studies of mammalian species are of more relevance. The examples chosen for the present discussion pertain mainly to in vivo insonation of mammalian tissue and include exposure conditions at clinically relevant frequencies. Most of the bioeffects studies were performed on small rodents, such as mice or rats. Although these animals constitute a fairly inexpensive model for in vivo bioeffects studies, the extrapolation of experimental results to humans is not immediately obvious. A very comprehensive review of the effects of ultrasound on mammalian development was prepared by Sikov.19 He evaluated bioeffects depending on gestational age and thus attempted to extract information on the relation between exposure parameters and stage of development at exposure.
Because this approach seems useful for drawing some conclusions about the adverse effects of diagnostic ultrasound in prenatal practice, it was adapted in the following discussion of bioeffects findings.
EARLY STAGE
Several researchers studied the influence of ultrasound exposure in the period before implantation. In the CW studies, Takeuchi and co-workers34 used pregnant rats exposed to a 2.5-MHz ultrasound field on the second and third day of gestation, at spatial average intensities of 150 mW/cm2. No increase in prenatal mortality was found. Similarly, no increase in the rate of postnatal malformation was found after 20-minute exposures. These results are not surprising in view of the fact that absorption in the embryonic tissue is lower than that of the surrounding tissue. Therefore, it is likely that more heat was generated in the vicinity of the embryo than in the embryo tissue itself.
Stolzenberg and associates35 exposed pregnant mice to 2-MHz of CW ultrasound in the first 3 days of gestation. A 1-inch (about 25 mm) planar source transducer was used, and the spatial average intensity was determined to be 1 W/cm2. A decreased uninterrupted pregnancy rate was noted after exposure for 5 minutes on the third day and after exposure for 200 seconds on day zero. Also, a reduction in fetal weight after delivery was observed at thresholds corresponding to exposure for 100 and 200 seconds on day zero and 200 and 400 seconds on the first day. In another series of studies,36 ultrasound exposure led to damage of maternal tissue, which was reflected in increased mortality, decreased weight gain, and paralysis of the pups.
Thus, it appears that in vivo exposure to CW ultrasound at spatial average intensities below 1 W/cm2 does not affect embryos at the early stage of gestation. However, limited data suggest that levels of ultrasound of 1 W/cm2 may lead to undesirable changes in maternal tissue.
The application of ultrasound in guided oocyte aspiration for in vitro fertilization and embryo transfer is rapidly growing. Therefore, the recent studies aimed at determining the interaction between ultrasound exposure and successful fertilization are included in this discussion.39, 41 In general, the clinically available data on ultrasound exposure of oocytes during meiosis are confusing. Although some researchers reported a deleterious effect on the fertility of patients undergoing artificial insemination (they claimed a reduction in the cumulative rate of pregnancy),42 others claimed an increase in the success rate, allowing ultrasound monitoring of follicular growth.55
An attempt to clarify this ambiguity was described by Mahadevan and colleagues.56 Their study sought to determine how oocytes obtained under ultrasound guidance affected the pregnancy rate. The results obtained at 3.5 MHz suggest that exposure of human oocytes to ultrasonic waves during the different phases of meiosis does not significantly influence the developmental potential of the in vitro fertilized embryos. Unfortunately, except for ultrasound frequency, these researchers did not give any of the relevant exposure parameters discussed earlier.
ORGANOGENESIS
McClain and associates57 exposed rats to 10 mW/cm2 CW Doppler ultrasound for up to 2 hours at frequencies of 2.25 and 2.5 MHz. The fetuses were examined on day 20, and no consistent increase in mortality was observed, nor did the authors detect any other abnormalities.
Stolzenberg and co-workers35 reported a decrease in the uninterrupted pregnancy rate of mice exposed between days 6 and 8 to a spatial average intensity of 1 W/cm2. They reported statistically significant fetal weight reductions for exposures longer than 140 seconds. More evidence of the possibility of ultrasonically producing embryolethal effects during organogenesis has been described.58, 59, 60, 61 Sikov and colleagues58, 59, 60, 61 exposed an exteriorized rat uterus to three frequencies (0.8, 2, and 3.2 MHz) at day 9 and evaluated the offspring at day 20. The exposure was performed at different intensity levels, with exposure times at 5 or 15 minutes. No effect on fetal weight was observed, even at spatial average intensities as high as 30 W/cm2, but prenatal mortality at 15–20 W/cm2 (spatial average) clearly increased with increasing exposure time. The cause of this was ascribed to a thermal mechanism. A recent controversial study looked at neuronal migration in rat pups after maternal exposure to ultrasound.62 Neurons of the cerebral neocortex in many animals (including humans) are generated during fetal life in the brain proliferative zones and then migrate to their final destinations by following an inside-to-outside sequence. In Ang's experiment neurons generated at embryonic day 16 and destined for the superficial cortical layers were chemically labeled in over 335 rats. A small but statistically significant number of neurons failed to acquire their proper position and remained scattered within inappropriate cortical layers and/or in the subjacent white matter when exposed to ultrasound for a total of 30 min or longer during the period of their migration. The magnitude of dispersion of labeled neurons was variable but increased with duration of exposure to ultrasound (although not linearly, with the most extended exposure yielding less effect than the one immediately lower). It is not clear whether a relatively small misplacement, in a relatively small number of cells that retain their origin cell class is of any clinical significance. It is also important to note that there are several major differences between the experimental setup of Ang et al. and the clinical use of ultrasound in humans.63 Most noticeable was the exposure duration, up to 7 hours in Ang’s setup and the fact that scans were performed over a period of several days. Furthermore, embryos received whole-brain exposure to the beam, which is rare in humans. Brains of mice are much smaller than those in humans, and develop over days. This should not completely deter from the study which encourages caution. Another study which demonstrates potential harmful effects of ultrasound (when spectral Doppler is used), showed that even relatively short insonation of chick embryos to clinically relevant Doppler resulted in short- and medium-memory loss and reduced ability to learn.64
FETAL BONE HEATING
Exposure conditions in which the ultrasound energy impinges on a bone embedded in tissue with relatively little absorption or attenuation have been investigated. This situation is particularly relevant in obstetrics, because the developing fetus is exposed to diagnostic ultrasound. Carstensen and associates65 insonified exposed mouse skulls after thermocouples were implanted. Adult and young mice were used. With a focal intensity of 1.5 W/cm2, a steady temperature rise of 5.6 ± 0.3°C was observed in old mice. The temperature rose to about 75% of the final value in the first 15 seconds. Comparison of these results with the theoretic predictions obtained from an appropriate tissue model24 indicated an agreement to within 20%; however, the theoretic transient response indicated the possibility of a considerably faster rate of temperature increase – 75% was predicted to be achieved within 5 seconds of exposure. These results indicate that there may be a reason for concern, especially with the rate of temperature increase. Also, at this rate of heating, the exposure time should be limited to about 1 minute.24 If the temperature increase had been, for example 7°C, the exposure time necessary for a bioeffect to occur would have been 15 seconds. It cannot be ruled out that during an actual examination, the acoustic beam might be held stationary for such a period. Ultrasound-induced temperature elevations in fetal brains of time-mated guinea pigs were measured during in vitro/ex utero insonation. The greatest temperature rise occurred in brain tissue close to bone and correlated with both gestational age and progression in bone development.66 The skull bone becomes progressively thicker and denser with gestational age. The temporal average intensity is about 4 times the in situ intensity currently permitted by the FDA for diagnostic use. The mean peak temperature elevation of greater than 5°C in some fetuses was subsequently confirmed in an independent study using identical ultrasound conditions.67 The ultrasound exposure was also found to produce an increase of 2.6°C in the mid brain when the bony cranium was removed from some fetuses. Most of the heating (80% of the mean maximum temperature increase) occurred within 40 seconds. The rate of heating is relevant to the safety of pulsed Doppler examinations in which the dwell time may be an important factor. The results demonstrate that the amount of the ultrasound-induced temperature increase that occurs in the fetal brain near bone is directly related to the amount of mineralization deposited in the bone, that is, the fetal age. The implications of this finding are that the worst-case heating will occur later in pregnancy (from the second trimester) when more bone is present, as opposed to earlier in pregnancy (during embryonic development) where there is no bone.68 For a more detailed discussion of these experiments, see Abramowicz et al., 2008.21
POSTNATAL DEVELOPMENT
Equally important in determining the safety of diagnostic ultrasound is knowing the influence of prenatal ultrasound exposure on postnatal development. In a carefully devised study,69 pregnant rodents were exposed to 500 mW/cm2 spatial average intensity at 2-MHz ultrasound for 1–3 min, or to 1 W/cm2 spatial average intensity for 40–60 seconds. No fetal mortality was observed; however, a weight decrease in the pups delivered was observed after exposure to 0.5 W/cm2 for 180 seconds.
In another study, the effects of prenatal ultrasound exposure on adult offspring behavior in the Wistar rat were investigated.70 The animals were exposed on days 15 through 17 and on 19 of gestation to a 5-MHz, 1-kHz pulse repetition rate and intensities (ISpTp) of 500 and 1500 W/cm2. The total exposure time was 35 minutes. Two hundred seventy-eight offspring were subjected on postnatal day 60 to two of four well-established behavioral tests. The animals were tested in random order within sexes. The results showed no consistent dose-related alterations in adult behavior due to prenatal fetal exposure.
Vorhees and co-workers71 used a unique approach to study the possible teratogenic effects of ultrasound exposure. They eliminated the need for anesthesia (and therefore the possible complications caused by forced restraint) by training the rats to remain immobile during the ultrasound exposure. The animals were exposed to 0.1, 2, or 30.0 W/cm2 (ISpTa), 3 MHz CW, for about 15 min/day on days 4 through 19 of gestation. No dose-dependent changes in various maternal parameters, the incidence of malformation, or fetal weight were observed. In a follow-up study, Vorhees and colleagues72 exposed rats to these levels of ultrasound for 10 min on gestational days 4 through 20. Although they did not observe exposure-related alterations in maternal parameters, offspring survival and growth, or neonatal psychophysiologic parameters, changes did occur in offspring adult behavior, in locomotor activity, and in two measures of the multiple-T water maze test performance at the highest dosage level.
In the studies aimed at determining the effects of ultrasound exposure on uteroplacental function, ter Haar and co-workers73 found that a spatial peak intensity of 2 W/cm2 at 3 MHz caused an exteriorized uterus to increase in frequency of contraction, which lasted for about 4 minutes after the ultrasound was turned off. They reported no increase in temperature and thus ascribed the increased rate of contractions to an unspecified nonthermal mechanism.
Placental function during ultrasound exposure was investigated by Kelman and associates.74, 75, 76 In their experiments they insonified the placenta of a guinea pig with 2.5 MHz of ultrasound at different intensity levels and in both pulsed and CW modes. Functional changes were observed after 10 minutes of exposure at SpTp intensities of 30 W/cm2 in pulsed mode (pulse length, 10 μs; PRR, 1 kHz) and SpTa intensities of 28–46 W/cm2 in the CW mode.
Again, it appears that at sufficiently high intensity levels, ultrasound can affect vitelline blood flow and lead to increased uterine contractility and changes in placental function. However, the exposure levels far exceed those present in the clinical environment. Murrils and associates77 found that fetal Doppler monitoring did not influence fetal activity, which would be an indication of ultrasound effects on the perinatal nervous system.
Bioeffects related to hyperthermia
The evidence above indicates that there is a teratogenic effect of heat on the prenatal development of mammalian tissues. Experiments with cultured rat embryos stressed the importance of ambient temperature; no bioeffects were observed when embryos were exposed to pulsed ultrasound for 15 minutes at ISpTa levels of 1.2 W/cm2.78 However, the same experiment repeated with the temperature elevated 1.5°C above normal (40°C) resulted in retardation of head growth. Although the outcome of this experiment cannot immediately be extrapolated to humans, it indicates that ultrasound exposure of a febrile pregnant patient under certain conditions might constitute an increased risk for potential bioeffects.
There are relatively few papers containing diagnostically relevant information on this subject; therefore, it is appropriate to draw attention here to an important survey.79 This survey established that no thermal bioeffects were observed at temperature elevations of 39°C, regardless of how long the ultrasound exposure lasts. However, for each increasing degree of temperature elevation, to stay within safety limits, the duration of ultrasound examination must be reduced by a factor of four. More specifically, the review indicated that the maximum safe duration for a temperature of 43°C is 1 minute, and for 42°C it is 4 minutes. Similarly, at 41°C the exposure time may be increased to 16 minutes, and at 40°C the duration of examination may be as long as 64 minutes. Based on the data available, the survey concluded that if the maximum temperature rise during the ultrasound exposure is kept less than 2°C, any biologic effect (in a febrile patient) is highly unlikely. It is possible to express the relation between the exposure time required for a bioeffect to occur and the associated TI, namely, t = 4 < (6 − TI). Thus, for a TI of 6, the exposure time should be limited to 1 minute. Similarly, a TI of 4 means that the exposure time should be less than 16 minutes (see Appendix).
Findings indicating that the ultrasound imaging transducer may act as a substantial heat source are also of interest.80 The temperature at a clinically operated Doppler transducer was reported to increase by 10°C when the Doppler was applied to skin with a standard coupling gel. Although tissue heating from the transducer is most likely limited to the tissue volume in the immediate vicinity of the transducer, this effect should be kept in mind for ultrasound examinations in which an endocavity (e.g., endovaginal) transducer is used, or when performing contact scanning of the neonatal brain or in ophthalmologic applications.
Bioeffects related to cavitation
A few relevant papers reporting lung hemorrhage in mice and in monkeys caused by cavitation-like phenomena have already been discussed under Cavitational mechanism.37, 81 These papers describe significant biologic effects associated with gas body activation. The effects were produced in vivo in mammalian lung tissue in small rodents (mice) and in primates (monkeys). The bioeffects were observed at pressure amplitudes and pulse durations representative of those currently encountered at the output of diagnostic ultrasound equipment, particularly when used in pulsed Doppler mode.22 As previously noted, the results described in those papers are important for the present discussion because they led to a modification of the AIUM statement on “In Vivo Mammalian Bioeffects”. The statement, which was renamed “Non-Human Mammalian In Vivo Biological Effects”, emphasizes the importance of the MI.20
Dalecki and colleagues82 published data indicating that, in general, threshold pressures increase with increasing frequency. The thresholds for hemorrhage in the intestine of adult mice ranged from about 1 MPa at 1 MHz to 3 MPa at 3 MHz (10-μs pulse duration, 100-Hz repetition rate).
To date there is no evidence that flowing cardiovascular blood will permit cavitation in vivo. Although there is some indication that microscopic gas bodies may exist in biologic tissue, there is no evidence that exposure of these bodies to the ultrasound levels similar to those used in clinical diagnostic practice will cause any significant bioeffects. However, it is conceivable that vibration of those bodies may introduce cell membrane stresses that can lead to extravasation of blood in lung tissue.5 This may indicate a need for appropriate modification during cardiovascular examinations and transesophageal echocardiography.
The above findings indicate that the body regions containing gas volumes are very sensitive to the potential side-effects of ultrasound exposure and that biologic effects can be introduced by using a commercially available diagnostic imaging system in tissues containing gas volumes. Carefully designed studies are needed to assess any potential hazards with conditions that are typical for diagnostic ultrasound. Such hazards may also be associated with ultrasonic contrast agents injected into the vascular system.83 A further concern is that ultrasound contrast agents may alter tissue properties and potentially increase bioeffects due to the insonation.84
To summarize, the thresholds for confirmed cavitation-induced biologic effects in mammalian tissues in the diagnostic frequency range of 2–8 MHz are above 1 MPa.20 All of the biologic effects that have been confirmed under diagnostically relevant exposure conditions involve tissues that are known to contain microscopic gas bodies. For tissues not containing gas (such as the kidney), no damage was observed at peak positive pressures of the order of 10 MPa.38 Lung hemorrhage has been observed in neonatal and adult mice, rabbits, monkeys, and neonatal swine with diagnostically relevant ultrasound pressures ranging from 1 to 3 MPa. No damage was observed in the lungs of adult swine at pressures of the order of 5 MPa (see Appendix).37, 81
Epidemiologic studies
The evidence of ultrasonically induced bioeffects in humans is perhaps the most important information from the clinician's point of view. As pointed out by Ziskin and Petitti,2 “No matter how many laboratory experiments show a lack of effect from diagnostic ultrasound, it will always be necessary to study directly its effect in human populations before any definitive statement regarding risk can be made.”
There are extensive reviews of the literature on the epidemiology of human exposure to ultrasound.1, 3, 6 As would be expected, the major thrust of these reviews is the assessment of the possible side-effects of ultrasound exposure in utero.85 This is mainly because most pregnant women are exposed to ultrasound examination in the early stages of pregnancy and because any harmful effects to the fetus may last for years to come. A review on epidemiological data was recently published.86
In general, few data pertaining to human ultrasound exposure are available.87 Some of the published data are reviewed briefly here. The experiments of O'Brien,88 who observed that in utero exposure to ultrasound resulted in reduced birth weight in mice, prompted similar studies in humans. Bakketeig and associates,89 in their randomized, controlled trial, reported that they were unable to find any effect of ultrasound on birth weight in humans. A similar conclusion was drawn from the earlier studies on the relation between ultrasound exposure and birthweight.90
Although no fetal structural anomalies were observed in the studies performed by Stark and co-workers90 and Bakketeig and colleagues,89 Stark and associates reported somewhat disturbing findings in their retrospective follow-up examinations of 425 children exposed to ultrasound in utero and 381 unexposed children. They reported an increased risk of dyslexia; however, their study suffers from the fact that the subjects were not selected at random. No evidence has been found that ultrasound exposure in utero may lead to an increased rate of childhood cancer.91, 92 Likewise, no relation between in utero exposure and children with hearing deficiencies has been determined.90 Several publications have seemingly demonstrated an increase in nonright handedness among infants and young adult who were insonated in utero.93, 94, 95 The first report of a possible link between prenatal exposure to ultrasound and subsequent nonright handedness in the insonated children was published in 1993 by Salvesen et al.93 The acoustic outputs of the ultrasound instrumentation used are thought to have been around 1mW/cm2. The association was barely significant at the 5% level, and the authors mention the possibility that these are chance findings. When examining their data further they described that the association was restricted to males.94 A second group of researchers, this time in Sweden and with Salvesen as a coauthor published similar findings of a statistically significant association between ultrasound exposure in utero and nonright handedness in males.95 The authors somewhat stretched their findings to describe nonright handedness as brain damage but with no valid mechanistic explanation of the findings. Therefore, although there may be a small increase in the incidence of nonright handedness in male infants, there is not enough evidence to infer a direct effect on brain structure or function or even that nonright handedness is an adverse effect. Detailed analysis of these findings have been published.96, 97 Several recent extensions or further analysis of these studies tend to demonstrate that the effect of ultrasound on handedness, if at all present, is very weak.98, 99
Deficiencies of the bioeffects studies
The data presented earlier indicate that the exposure of pregnant rodents to CW ultrasound at sufficiently high intensities during organogenesis can lead to undesirable biologic changes in the developing fetus. Also, evidence has emerged indicating that the body regions containing gas volumes may be particularly prone to potential side-effects when exposed to sufficiently high pressure amplitudes.37, 81 Although the data were carefully selected to include only those studies that can be readily related to diagnostically relevant exposure conditions, it should be stressed that in most cases the bioeffects were observed at exposure conditions that were different from those typical of clinical diagnostic practice. Before 1990, most of the bioeffects observed were ascribed to the thermal mechanism of interaction between the ultrasound and biologic tissue, but it has now been demonstrated that exposure to ultrasound produced by commercially available diagnostic imaging equipment can produce nonthermal, cavitation-like bioeffects in the lung tissue of primates.37
Many bioeffects studies describe the effects of whole-body heating, which most likely acts through a mechanism that is not immediately comparable to ultrasound-induced hyperthermia. Few studies have been specifically designed to determine threshold values for abnormal embryonic or fetal development.79 On the basis of the thermal criterion, a diagnostic exposure that produces a maximum temperature rise of 1.5°C above normal physiologic levels can be used without reservation in clinical examinations (see Appendix). However, a diagnostic exposure that produces an in situ temperature elevation to or above 41°C for 15 min should be considered hazardous to embryonic or fetal development. Any increase in the tissue temperature will act as a potentiating factor. Therefore, ultrasound examination in a febrile patient may constitute an additional embryonic and fetal risk.5
The reviews on human exposure clearly indicate that epidemiologic studies and surveys yield no evidence of adverse effects from diagnostic ultrasound, but they also point out several serious deficiencies of all the data available. These deficiencies are carefully discussed by Ziskin and Petitti.2
Ziskin and Petitti point out that the available results of epidemiologic studies are based on about 500,000 patient examinations that were collected from 179 users of clinical ultrasound. However, the results allow only a fairly cautious statement to be made, namely, that the users overwhelmingly believe that their experience with diagnostic ultrasound had been safe.
Ziskin and Petitti also note that all clinical studies on the possible harmful effects of ultrasound exposure to the fetus produced negative results. However, the acoustic outputs of the diagnostic instruments used were generally not known. In addition, the sample sizes (the number of patients included in the studies) were limited to a few hundred. In their careful assessment of the limitations of the epidemiologic studies, Ziskin and Petitti focus on the lack of information or simply inadequate ultrasound dosimetry. Also, the reasons for the examinations were not clearly stated, and sparse information is available on the number of examinations and the gestational age at the time of exposure. In addition, most studies were performed with pulsed ultrasound. As shown in Table 1, Doppler ultrasound results, in general, in higher values of ISpTa. Ziskin and Petitti suggest that the existing data on exposure of the fetus in utero are inadequate to conclude that diagnostic ultrasound is safe at all combinations of dose and period of exposure for both imaging and Doppler devices.
Another point addressed by these researchers is the importance of statistical considerations, including sample size and statistical level of significance. Because a more thorough treatment of statistics is beyond the scope of this chapter, it can be simply stated that the larger the sample, the higher the statistical level of significance that can be achieved. The usefulness of small samples is extremely limited; studies that use small samples usually have inconclusive results.
In summary, the human studies that have been performed do not preclude the possibility that adverse effects may be found under certain conditions. The limited data available indicate that no relation has been found between prenatal exposure to ultrasound and subsequent postnatal changes in children, but statistical considerations show that minor chemical and behavioral changes, long-term delayed effects, and certain genetic effects could easily escape detection.2 There is still a need for a well-designed, randomized clinical study addressing the risk for the fetus exposed in utero.
PRACTICAL ASPECTS: HOW TO KEEP IT SAFE
While a relatively large body of literature exists on theoretical aspects of ultrasound bioeffects as well as laboratory evidence, very little exists on human fetal exposure. This is a subject of major interest to the end-user who performs the actual clinical exams. Some information exists on outputs of different instruments.100 This and other information are, generally, quickly outdated, since commercial machines are constantly introduced to the market. From a clinical standpoint, there is no easy way to verify the actual output of the instrument in use. Instruments from various manufacturers act differently. In addition, each attached transducer will generate a specific output, further complicated by which mode is being applied.101 When comparing modes, the ISPTA increases from B-mode (34 mW/cm2, average) to M-mode to color Doppler to spectral Doppler (1180 mW/cm2). Average values of the temporal averaged intensity are 1 W/cm2 in Doppler mode but can reach 10 W/cm2.101 Therefore special precaution is needed when using Doppler in obstetrics, particularly in early gestation. Intensities for color Doppler are higher than B-mode but much lower than spectral Doppler. This is mainly due to the mode of operation: color is obtained by sequences of pulses, scanned through the area of interest (“the color box”). It is important to keep in mind that intensity measurements described in manufacturers’ manuals, have been derived in laboratory conditions and that real-life conditions may be different.102 Furthermore, one of the most important issues to realize and remember at all times during an exam is that many machine controls can greatly alter the output. For instance, the degree of temperature elevation is proportional to the product of the amplitude of the sound wave by the pulse length and the PRF. It thus becomes immediately evident why any increase in any of these characteristics can add to the risk of elevating the tissue temperature. Three important parameters are under end-user control: (1) the operating or scanning mode, including transducer choice, (2) the system setup and output control, and (3) the dwell time.
- Scanning mode: this refers to B-mode, M-mode, color Doppler, and spectral (pulsed wave) Doppler, from the lowest to the highest intensities. High pulse repetition frequencies are used in pulsed Doppler techniques. Therefore greater temporal average intensities and powers are generated than in B- or M-mode, and hence greater heating potential. In spectral Doppler, the beam needs to be held in a relatively constant position over the vessel of interest, which may induce a further increase in temporal average intensity. This is somewhat mitigated by movements of the examiner's hand, the fetus, and the mother (breathing and body movements). Transducer choice is of great consequence since it will determine frequency, penetration, resolution, and field of view, all of which can also alter output.
- System setup: starting or default output power determines intensity but a more subtle element is fine tuning performed by the examiner to optimize the image. This influences output but with no visible effect (except if one closely follows TI and/or MI displays). Controls that regularize output include focal depth, with highest power usually at deeper focus but, occasionally on some machines, highest in the near field), increasing frame rate, limiting the field of view, for instance by high-resolution magnification or certain zooms. In Doppler mode, output is modified by changing sample volume and/or velocity range (all done to optimize received signals). Receiver gain often has similar effects to the above controls on the recorded image but none on the output of the outgoing beam and is, therefore, completely safe to manipulate. In addition, over the years, output of instruments has increased.101 There is also a push, among some scientists, to completely abolish the previously recommended output limits.103
- Dwell time is the actual insonation time and is directly under control of the examiner. Dwell time is not taken into account in the calculation of the safety indices, nor is it, in general, reported in clinical or experimental studies. Time is very important because it takes only one pulse to induce cavitation, and about a minute to raise insonated tissue temperature to its peak, less in the vicinity of bone. Directly associated with dwell time is examiner knowledge and experience. It is safe to assume that the more experienced the examiner, the less scanning time will be needed.
The end-user of clinical ultrasound is interested in knowing how to keep the exam safe. In terms of clinical exposure, a general recommendation is that diagnostic ultrasound should be used only when indicated and exposure should be kept as low as possible to obtain diagnostic images and exposure time should be kept as short as possible.104 This forms the ALARA (as low as reasonably achievable) principle.
Recommendations have been published, based, more or less, on scientific data. The 1999 statement of the British Medical Ultrasound Society (BMUS) declares: “For equipment for which the safety indices are displayed over their full range of values, the TI should always be less than 0.5 and the MI should always be less than 0.3. When the safety indices are not displayed, Tmax should be less than 1°C and MImax should be less than 0.3. Frequent exposure of the same subject is to be avoided”.105 They have very strict recommendations for maximum allowed exposure time, depending on the TI (see Table 4, modified from BMUS Statement).106
Table 4. British Medical Ultrasound Society recommendations for ultrasound exposure time
| TI | Maximum exposure time (min) |
| 0.7 | 60 |
| 1.0 | 30 |
| 1.5 | 15 |
| 2.0 | 4 |
| 2.5 | 1 |
The World Federation of Ultrasound in Medicine and Biology (WFUMB) offers some scientific rationalization, stating that diagnostic exposure resulting in a temperature rise of no more than 1.5°C above normal physiological levels (37°C) may be used clinically without reservation on thermal grounds.107 Furthermore, diagnostic exposure that elevates embryonic and fetal in situ temperature above 41°C (4°C above normal temperature) for 5 minutes or more should be considered potentially hazardous.107 Of note, extra caution may be needed in febrile pregnant women to avoid unnecessary additional embryonic and fetal risk from ultrasound examinations. In general, precautions are much milder regarding mechanical phenomena, which, in the absence of gas nuclei (as is the case in fetal lungs and bowels and assuming no use of contrast agents) are probably negligible.
If one wishes to consider MI and TI as adequate indicators of safety or risk of bioeffects, it is important to determine how their values vary in routine clinical exams. This has been performed by only one group of researchers, for the first,108 second and third trimesters,109 routine Doppler studies,110 nuchal translucency screening111 as well as three to four dimensional scanning.112 It is worth noting that with routine use, admittedly by knowledgeable examiners, values of TI and MI remained very low (generally under 0.5) except in Doppler mode where TI could reach values of 3 with the particular instruments used in these studies. Because of differences in scanning protocols, gestational age ranges, and variations in maternal body habitus, it is very difficult to provide simple recommendations.25
CONCLUSIONS
In general, the literature describing clinically relevant exposure conditions is rather limited, and few results, if any (even of experiments in vivo), can be immediately applied or extrapolated to discussions of clinical safety. The reason for this is the large number of variables that must be considered, which makes bioeffects experiments difficult to design and perform free from artifacts. Also, the number of subjects exposed to the ultrasound field is often limited, so the data obtained cannot be considered statistically significant.2, 3 It is worth reiterating that lung hemorrhage was produced in the lung of a macaque monkey whose chest was exposed to the maximum output level produced by a commercially available diagnostic scanner.37 The mechanism of injury was determined to be a nonthermal one; the tissue damage appeared to be caused by gas volumes existing in the lung tissue. Although these findings were not confirmed by an independent laboratory, they indicate that caution should be used in diagnostic examinations related to echocardiographic and esophageal applications.
Results of experiments performed at exposure levels and durations higher than those used in diagnostic examinations are difficult to extrapolate to human examination conditions. In addition, reproducibility of the results presents a further limitation: often the bioeffects observed cannot be confirmed by an independent laboratory. It is conceivable that more uniform requirements regarding the determination of acoustic output parameters would facilitate comparison of the experiments between different laboratories.
The current evidence on ultrasonically induced bioeffects in the laboratory setting (with the possible exception of the results published in the article by Tarrantal and Canfield37 and those pointed out in the article by Barnett and colleagues5) is not immediately applicable to the clinical situation. However, it is important to keep in mind that the uncertainties experienced in determining the intensity levels and the associated exposure conditions do not permit a categorical statement to be made regarding the unconditional safety of diagnostic ultrasound. It is also important to reiterate that the available data from both empirical and epidemiologic studies indicate that there is no verified evidence of adverse effects in patients caused by exposure to diagnostic levels of ultrasound (see Appendix).
Although these findings are consistent with almost five decades of clinical experience, it is also important to realize that the acoustic exposure levels in the available data may not be representative of the full range of current fetal exposure (see Table 1, Table 2, and Table 3). As already indicated, the acoustic outputs of diagnostic instruments have been increasing,20, 45 and laboratory studies indicate that tissue damage can occur at the exposure levels produced by commercially available equipment.5 Furthermore, the only epidemiological information available is on subjects who were scanned before 1992, a time when the acoustic output of instrumentation was allowed to increase for fetal use by a factor of almost 8.8 The increased use of ultrasound in early pregnancy and, more specifically the use of pulsed Doppler should be approached with some caution.113
Acoustic output measurements indicate that the peak rarefactional pressure amplitudes of some Doppler systems can exceed 4 MPa. Although such pressure amplitudes appear to be sufficient to cause adverse effects,37 so far no evidence has been presented indicating any pulmonary extravasation after ultrasound exposure. However, these data indicate that appropriate caution should be exerted during neonatal examination, because even small amounts of extravasation can be of concern. In practice, this would require operators to follow the ALARA principle and to use the minimum transmitting acoustic power necessary to obtain adequate diagnostic data. The continuously updated display of the TI and MI should be of significant assistance here. As already mentioned, these indices provide information on the likelihood of an adverse biologic effect occurring from the ultrasound examination that is currently performed in clinical practice.7 The TI provides real-time information indicating the worst-case temperature rise at the actual operating conditions of the imaging equipment. The MI indicates the potential for cavitation phenomena to occur. If the predefined set value is exceeded, indicating the potential for harm, the clinician will be able to make an informed decision regarding the benefit/risk ratio of the given ultrasound examination. The decision may be influenced by factors that are not covered in the indices. These factors include consideration of the imaging site perfusion, patient obesity (which may severely restrict penetration depth), and a possible reduction in the exposure time by minimizing the duration of examination. A continuously updated on-screen display of the MI and TI is incorporated in recent designs of diagnostic ultrasound imaging equipment. It is appropriate to note that an output display is not required if the transducer and the system are not capable of exceeding an MI or TI of 1. However, if the transducer and system are capable of exceeding an MI or TI of 1, then the system must display values as low as 0.4 to assist the operator to implement the ALARA principle (see Appendix).7 It should be noted that the MI, which is defined as a derated (0.3 dB/cm/MHz) peak rarefactional pressure in MPa (ρr.3) divided by the square root of the center frequency in MHz, is not a perfect indicator. Although it simplifies the description of thresholds for bioeffects in tissues containing stabilized gas bodies, it may underestimate conditions in situ. Another limitation of the MI is that it describes conditions only at the focus. This may not be the primary point of interest, for instance in cardiological examinations. In this case, the most common way for the lung to be exposed during a diagnostic procedure is where the transducer is applied directly to the chest of the patient. Here, the focal region of the transducer is not within the lung tissue. An in-depth discussion of which index is most appropriate for a given examination, as well as a careful discussion of the limitations involved in implementing the ALARA principle with the use of these indices, is given in the article titled Medical Ultrasound Safety (also see Appendix).7
Overall, the clinical data are reassuring in that no established adverse effects on human patients exposed to diagnostic ultrasound examinations have been reported. However, the deficiencies of the data available and the tendency to increase the acoustic output power indicate the need for carefully planned experiments to provide safety information relevant to medical practice. Knowledge of the relevant ultrasound field parameters, such as the maximum peak pressure (with regard to both space and time), working or center frequency, pulse waveform, beam profile, and pulse repetition frequency, is essential for proper comparison of the experimental results. This knowledge, along with the actual exposure time, should be included in papers reporting on the biologic effects of ultrasound.114, 115
Analysis of the acoustic output data given in the previous sections indicates that the diagnostic procedure that introduces the greatest concern for thermal bioeffects is the use of PW Doppler in a fetal examination. Cavitation or a gas body-related mechanism does not appear to be associated with B-scan imaging of the developing fetus.
This chapter concludes with a brief review of official AIUM statements (see Appendix). These statements were prepared by the AIUM Bioeffects Committee, whose expertise in the field is widely recognized in the ultrasound community throughout the world. The statements summarize the current knowledge on the physical mechanisms of interaction between ultrasound and biologic tissue and are based on both empirical and clinical data. The empirical data were scrutinized to detect possible dose–response relations regardless of the underlying mechanisms.
The data in the literature allow a fairly comprehensive statement on “Non-Human In Vivo Biologic Effects”, which covers both thermal and nonthermal mechanisms and provides guidelines to estimate potentially hazardous pressure amplitudes or intensity levels and exposure times.
In conclusion, diagnostic ultrasound has an excellent safety record. The clinical data are reassuring in that there is no report of damage to human patients from diagnostic ultrasound. However, it must be emphasized that neither theoretic calculations nor experimental results can yield unambiguous and definite evidence that fully guarantees the safety of ultrasound diagnostics, particularly in regards to delayed, subtle effects. Because diagnostic ultrasound is an extremely powerful tool in the hands of experienced physicians and sonographers, the final decision regarding the risks and benefits can be made only by the individual responsible for applying the ultrasound to the patient.
APPENDIX: The American Institute of Ultrasound in Medicine (AIUM) Official Statements
Official Statement
Approved
March 2007
PRUDENT USE AND CLINICAL SAFETY
Diagnostic ultrasound has been in use since the late 1950s. Given its known benefits and recognized efficacy for medical diagnosis, including use during human pregnancy, the American Institute of Ultrasound in Medicine herein addresses the clinical safety of such use:
No independently confirmed adverse effects caused by exposure from present diagnostic ultrasound instruments have been reported in human patients in the absence of contrast agents. Biological effects (such as localized pulmonary bleeding) have been reported in mammalian systems at diagnostically relevant exposures but the clinical significance of such effects is not yet known. Ultrasound should be used by qualified health professionals to provide medical benefit to the patient.
Official Statement
Approved
March 2007
PRUDENT USE IN OBSTETRICS
The AIUM advocates the responsible use of diagnostic ultrasound and strongly discourages the non-medical use of ultrasound for entertainment purposes. The use of ultrasound without a medical indication to view the fetus, obtain a picture of the fetus or determine the fetal gender is inappropriate and contrary to responsible medical practice. Ultrasound should be used by qualified health professionals to provide medical benefit to the patient.
Official Statement
Approved
March 2007
IN VITRO BIOLOGICAL EFFECTS
It is often difficult to evaluate reports of ultrasonically induced in vitro biological effects with respect to their clinical significance. The predominant physical and biological interactions and mechanisms involved in an in vitro effect may not pertain to the in vivo situation. Nevertheless, an in vitro effect must be regarded as a real biological effect.
Results from in vitro experiments suggest new endpoints and serve as a basis for design of in vivo experiments. In vitro studies provide the capability to control experimental variables that may not be controllable in vivo and thus offer a means to explore and evaluate specific mechanisms and test hypotheses. Although they may have limited applicability to in vivo biological effects, such studies can disclose fundamental intercellular or intracellular effects of ultrasound.
While it is valid for authors to place their results in context and to suggest further relevant investigations, reports which do more than that should be viewed with caution.
Official Statement
Approved
March 2007
SAFETY IN TRAINING AND RESEARCH
Diagnostic ultrasound has been in use since the late 1950s. There are no confirmed adverse biological effects on patients resulting from this usage. Although no hazard has been identified that would preclude the prudent and conservative use of diagnostic ultrasound in education and research, experience from normal diagnostic practice may or may not be relevant to extended exposure times and altered exposure conditions. It is therefore considered appropriate to make the following recommendation: When examinations are carried out for purposes of training or research, the subject should be informed of the anticipated exposure conditions and how these compare with normal diagnostic practice.
Official Statement
Approved
March 2008
Users of ultrasound systems who intend to report on bioeffects studies are encouraged to seek the assistance of the manufacturers of their systems (or specialists in acoustic output measurement) to ensure adequate calibration and sufficiently accurate estimates or measurement of pertinent Acoustic Output Parameters. Data necessary to characterize a system are described in "Guidelines for Journal of Ultrasound in Medicine Authors and Reviewers on Measurement and Reporting of Acoustic Output and Exposure", J Ultrasound Med 2005; 24: 1171–1179. There is no obligation to reveal the nature of the study or [examination] results to obtain the data needed for calibration. Users should be aware that neither the Output Display Indices (TI and MI) nor the "worst-case" output data provided in manufacturers' Users' Manuals are sufficient to characterize the acoustic output of ultrasound systems under the specific conditions and settings selected by a user for a given study or examination.
Official Statement
Approved
November 2008
MAMMALIAN IN VIVO ULTRASONIC BIOLOGICAL EFFECTS
Information from experiments utilizing laboratory mammals has contributed significantly to our understanding of ultrasonically induced biological effects and the mechanisms that are most likely responsible. The following statement summarizes observations relative to specific ultrasound parameters and indices. The history and rationale for this statement are provided in Bioeffects and Safety of Diagnostic Ultrasound (AIUM, 1993).
In the low megahertz frequency range there have been no independently confirmed adverse biological effects in mammalian tissues exposed in vivo under experimental ultrasound conditions, as follows.
- When a thermal mechanism is involved, these conditions are unfocused-beam intensities* below 100 mW/cm2, focused-beam ** intensities below 1 W/cm2, or thermal index values less than 2. Furthermore, such effects have not been reported for higher values of thermal index when it is less than 6 −(log10t/0.6) where t is exposure time ranging from 1 to 250 minutes, including off-time for pulsed exposure.
- When a nonthermal mechanism is involved,*** in tissues that contain well-defined gas bodies, these conditions are in situ peak rarefactional pressures below approximately 0.3 MPa or mechanical index values less than approximately 0.3. Furthermore, for other tissues no such effects have been reported.
**Quarter-power (−6 dB) beam width smaller than four wavelengths or 4 mm, whichever is less at the exposure frequency.
***For diagnostically relevant ultrasound exposures.
Official Statement
Approved
November 2008
Contrast agents carry some potential for nonthermal bioeffects when ultrasound interacts with the gas bodies. The mechanism for such effects is related to the physical phenomenon of acoustic cavitation. Several published reports describe adverse bioeffects in mammalian tissue in vivo resulting from exposure to diagnostic ultrasound with gas body contrast agents in the circulation. Induction of premature ventricular contractions by triggered contrast echocardiography in humans has been reported for a noncommercial agent and in laboratory animals for commercial agents. Microvascular leakage, killing of cardiomyocytes, and glomerular capillary hemorrhage, among other bioeffects, have been reported in animal studies. Two medical ultrasound societies have examined this potential risk of bioeffects in diagnostic ultrasound with contrast agents and provide extensive reviews of the topic: the World Federation for Ultrasound in Medicine and Biology (WFUMB) Contrast Agent Safety Symposium (WFUMB, 2007) and the American Institute of Ultrasound in Medicine 2005 Bioeffects Consensus Conference (Miller et al, 2008). Based on review of these reports and of recent literature, the Bioeffects Committee issues the following statement:
Statement on Bioeffects of Diagnostic Ultrasound with Gas Body Contrast Agents
Induction of premature ventricular contractions, microvascular leakage with petechiae, glomerular capillary hemorrhage, and local cell killing in mammalian tissue in vivo have been reported and independently confirmed for diagnostic ultrasound exposure with a mechanical index (MI) above about 0.4 and a gas body contrast agent present in the circulation.
Although the medical significance of such microscale bioeffects is uncertain, minimizing the potential for such effects represents prudent use of diagnostic ultrasound. In general, for imaging with contrast agents at an MI above 0.4, practitioners should use the minimal agent dose, MI, and examination time consistent with efficacious acquisition of diagnostic information. In addition, the echocardiogram should be monitored during high-MI contrast cardiac-gated perfusion echocardiography, particularly in patients with a history of myocardial infarction or unstable cardiovascular disease. Furthermore, physicians and sonographers should follow all guidance provided in the package inserts of these drugs, including precautions, warnings and contraindications.
References
1. Miller DL, Averkiou MA, Brayman AA, et al. Bioeffects considerations for diagnostic ultrasound contrast agents. J Ultrasound Med 2008; 27:611–632.
2. WFUMB. Symposium on Safety of Ultrasound in Medicine: Ultrasound Contrast Agents. Safety of ultrasound contrast agents. Ultrasound Med Biol 2007; 33:171–234.
Official Statement
Approved
March 2010
CONCLUSIONS REGARDING EPIDEMIOLOGY FOR OBSTETRIC ULTRASOUND
Based on the epidemiologic data available and on current knowledge of interactive mechanisms, there is insufficient justification to warrant conclusion of a causal relationship between diagnostic ultrasound and recognized adverse effects in humans. Some studies have reported effects of exposure to diagnostic ultrasound during pregnancy, such as low birth weight, delayed speech, dyslexia and non-right-handedness. Other studies have not demonstrated such effects. The epidemiologic evidence is based on exposure conditions prior to 1992, the year in which acoustic limits of ultrasound machines were substantially increased for fetal/obstetric applications.
Official Statement
Approved
March 2009
CONCLUSIONS REGARDING HEAT
- Excessive temperature increase can result in toxic effects in mammalian systems. The biological effects observed depend on many factors, such as the exposure duration, the type of tissue exposed, its cellular proliferation rate, and its potential for regeneration. Age and stage of development are important factors when considering fetal and neonatal safety. Temperature increases of several degrees Celsius above the normal core range can occur naturally; there have been no significant biological effects observed resulting from such temperature increases except when they were sustained for extended time periods.
- For exposure durations up to 50 hours, there have been no significant biological effects observed due to temperature increases less than or equal to 2°C above normal.
- For temperature increases greater than 2°C above normal, there have been no significant biological effects observed due to temperature increases less than or equal to
![]()
where t is the exposure duration ranging from 1 to 250 minutes. For example, for temperature increases of 4°C and 6°C, the corresponding limits for the exposure duration t are 16 min and 1 min, respectively. - In general, adult tissues are more tolerant of temperature increases than fetal and neonatal tissues. Therefore, higher temperatures and/or longer exposure durations would be required for thermal damage.
- For exposure durations up to 50 hours, there have been no significant biological effects observed due to temperature increases less than or equal to 2°C above normal.
- The temperature increase during exposure of tissues to diagnostic ultrasound fields is dependent upon (a) output characteristics of the acoustic source such as frequency, source dimensions, scan rate, power, pulse repetition frequency, pulse duration, transducer self heating, exposure time and wave shape and (b) tissue properties such as attenuation, absorption, speed of sound, acoustic impedance, perfusion, thermal conductivity, thermal diffusivity, anatomical structure and nonlinearity parameter.
- For similar exposure conditions, the expected temperature increase in bone is significantly greater than in soft tissues. For this reason, conditions where an acoustic beam impinges on ossifying fetal bone deserve special attention due to its close proximity to other developing tissues.
- Calculations of the maximum temperature increase resulting from ultrasound exposure in vivo should not be assumed to be exact because of the uncertainties and approximations associated with the thermal, acoustic and structural characteristics of the tissues involved. However, experimental evidence shows that calculations are capable of predicting measured values within a factor of two. Thus, it appears reasonable to use calculations to obtain safety guidelines for clinical exposures where temperature measurements are not feasible. To provide a display of real-time estimates of tissue temperature increases as part of a diagnostic system, simplifying approximations are used to yield values called Thermal Indices. Under most clinically relevant conditions, the soft-tissue thermal index, TIS, and the bone thermal index, TIB, either overestimate or closely approximate the best available estimate of the maximum temperature increase (ΔTmax). For example, if TIS = 2, then ΔTmax
![]() 2°C.
2°C. - The current FDA regulatory limit for ISPTA.3 is 720 mW/cm2. For this, and lesser intensities, the best available estimate of the maximum temperature increase in the conceptus can exceed 2°C.
- The soft-tissue thermal index, TIS, and the bone thermal index, TIB, are useful for estimating the temperature increase in vivo. For this purpose, these thermal indices are superior to any single ultrasonic field quantity such as the derated spatial-peak, temporal-average intensity, ISPTA.3. That is, TIS and TIB track changes in the maximum temperature increases, ΔTmax, thus allowing for implementation of the ALARA principle, whereas ISPTA.3 does not. For example,
- At a constant value of ISPTA.3, TIS increases with increasing frequency and with increasing source diameter.
- At a constant value of ISPTA.3, TIB increases with increasing focal beam diameter.
Official Statement
- At a constant value of ISPTA.3, TIS increases with increasing frequency and with increasing source diameter.
Approved April 2011
- Pulsed Doppler (spectral, power and color flow imaging) ultrasound should not be used routinely.
- Pulsed Doppler ultrasound may be used for clinical indications such as to refine risks for trisomies.
- When performing Doppler ultrasound, the displayed Thermal Index (TI) should be less than or equal to 1.0 and exposure time should be kept as short as possible (usually no longer than 5-10 minutes) and not exceed 60 minutes.
- When using Doppler ultrasound for research, teaching and training purposes, the displayed TI should be less than or equal to 1.0 and exposure time should be kept as short as possible (usually no longer than 5-10 minutes) and not exceed 60 minutes. Informed consent should be obtained.
- In educational settings, discussion of first trimester pulsed or color Doppler should be accompanied by information on safety and bioeffects (e.g. TI, exposure times, and how to reduce the output power).
- When scanning maternal uterine arteries in the first trimester, there are unlikely to be any fetal safety implications as long as the embryo/fetus lies outside the Doppler ultrasound beam.
(Reprinted with permission of AIUM, 14750 Sweitzer Lane, Suite 100, Laurel, MD 20707–5906)
REFERENCES
Diagnostic Ultrasound Imaging in Pregnancy: Report of a Consensus Development Conference, Bethesda, MD, February 6–8. NIH publication No. 84–667. Bethesda, MD, Department of Health and Human Services, 1984 |
|
Ziskin MC, Petitti DB: Epidemiology of human exposure to ultrasound: A critical review. Ultrasound Med Biol 14: 91, 1988 |
|
Biological Effects of Ultrasound: Mechanisms and Clinical Implications. NCRP Report No. 74. Bethesda, MD, National Council on Radiation Protection and Measurements, 1984 |
|
Thomenius KE, Lewin PA: Ultrasound bioeffects 1991: An update. Ultrasound Quarterly 9: 11, 1991 |
|
Barnett SE, ter Haar GR, Ziskin MC et al: Current status of research on biophysical effects of ultrasound. Ultrasound Med Biol 20: 205, 1994 |
|
Exposure Criteria for Medical Diagnostic Ultrasound. NCRP Publication Report No. 113. Bethesda, MD, National Council on Radiation Protection and Measurements, 1992 |
|
Medical Ultrasound Safety, pp 1 – 40. Laurel, MD, American Institute of Ultrasound in Medicine, 1994 |
|
Miller MW, Brayman AA, Abramowicz JS: Obstetric ultrasonography: a biophysical consideration of patient safety--the"rules" have changed. Am J Obstet Gynecol 179: 241, 1998 |
|
Nyborg WL: Optimization of exposure conditions for medical ultrasound. Ultrasound Med Biol 11: 245, 1985 |
|
Wells PNT: The prudent use of diagnostic ultrasound. Ultrasound Med Biol 13: 391, 1987 |
|
Ziskin MC: The prudent use of diagnostic ultrasound. J Ultrasound Med 6: 415, 1987 |
|
Ndumbe FM, Navti O, Chilaka VN et al: Prenatal diagnosis in the first trimester of pregnancy. Obstet Gynecol Surv 63: 317, 2008 |
|
Sheiner E, Abramowicz JS: Clinical end users worldwide show poor knowledge regarding safety issues of ultrasound during pregnancy. J Ultrasound Med 27: 499, 2008 |
|
Brendt RL: The effects of embryonic and fetal exposure to X-ray, microwaves, and ultrasound. Clin Perinatol 13: 615, 1986 |
|
Nyborg WL, Ziskin MC: Biological Effects of Ultrasound. New York, Churchill-Livingstone, 1985 |
|
Williams AR: Ultrasound: Biological Effects and Potential Hazards. London, Academic Press, 1983 |
|
Stratmeyer ME, Stewart HF: An Overview of Ultrasound: Theory Measurements, Medical Applications and Biological Effects. Washington, DC, US Department of Health and Human Services, Publication No. (FDA) 82–8290, 1982 |
|
Sikov MR: Effect of ultrasound on development. I. Introduction and studies in inframammalian species. J Ultrasound Med 5: 577, 1986 |
|
Sikov MR: Effect of ultrasound on development. II. Studies in mammalian species and overview. J Ultrasound Med 5: 651, 1986 |
|
Bioeffects and Safety of Diagnostic Ultrasound, pp 1 – 40. Laurel, MD, American Institute of Ultrasound in Medicine, 1993 |
|
Abramowicz JS, Barnett SB, Duck FA et al: Fetal thermal effects of diagnostic ultrasound. J Ultrasound Med 27: 541, 2008 |
|
Stratmeyer ME, Greenleaf JF, Dalecki D et al: Fetal ultrasound: mechanical effects. J Ultrasound Med 27: 597, 2008 |
|
Abramowicz JS, Sheiner E:Ultrasound bioeffects and safety: what the practitioner should know, in Fleischer AC, Manning FA, Jeanty P and Romero R (eds): Sonography in Obstetrics and Gynecology, Principles and Practice, Seventh Edition, 2009, in press |
|
Thomenius KE: Estimation of the potential for bioeffects. In Ziskin MC, Lewin PA (eds): Ultrasonic Exposimetry, pp 371 – 407. Boca Raton, FL, CRC Press, 1993 |
|
Nelson TR, Fowlkes JB, Abramowicz JS: Ultrasound biosafety: considerations for the practicing sonographer/sonologist. J Ultrasound Med, 2008, in press |
|
http:/www.bmus.org/ultras-safety/us-safety04.asp |
|
Rott H: Clinical Safety Statement for Diagnostic Ultrasound. European Committee for Medical Ultrasound Safety (ECMUS). Eur J Ultrasound 8: 67, 1998 |
|
Abramowicz JS, Kossoff G, Marsal K et al: Safety Statement, 2000 (reconfirmed 2003). International Society of Ultrasound in Obstetrics and Gynecology (ISUOG). Ultrasound Obstet Gynecol 21: 100, 2003 |
|
Teixeira LS, Leite J, Viegas MJ et al: Ductus venosus Doppler velocimetry in the first trimester: a new finding. Ultrasound Obstet Gynecol 31: 261–5, 2008 |
|
Marques Carvalho SR, Mendes MC, Poli Neto OB et al: First trimester fetal echocardiography. Gynecol Obstet Invest 65: 162–8, 2008 |
|
Wloch A, Rozmus-Warcholinska W, Czuba B et al: Doppler study of the embryonic heart in normal pregnant women. J Matern Fetal Neonatal Med 20: 533–9, 2007 |
|
Abbott JG: Rationale and derivation of MI and TI--a review. Ultrasound Med Biol 25: 431, 1999 |
|
Wu J, Nyborg WL: Ultrasound, cavitation bubbles and their interaction with cells. Adv Drug Deliv Rev 60: 1103–16, 2008 |
|
Takeuchi H, Nakasawa T, Kumakui K et al: Experimental studies on ultrasonic Doppler methods in obstetrics. Acta Obstet Gynaecol Japan 17: 11, 1970 |
|
Stolzenberg SJ, Torbit CA, Edmonds PD: Effects of ultrasound on the mouse exposed at different stages of gestation: Acute studies. Radiat Environ Biophys 17: 245, 1980 |
|
Stolzenberg SJ, Edmonds PD, Torbit CA: Toxic effects of ultrasound in mice: Damage to central and autonomic nervous systems. Toxicol Appl Pharmacol 53: 432, 1980 |
|
Tarrantal AF, Canfield DR: Ultrasound induced lung hemorrhage in the monkey. Ultrasound Med Biol 20 (1): 65, 1994 |
|
Carstensen EL, Hartman CL, Child SZ et al: Test from kidney hemorrhage following exposure to intense, pulsed ultrasound. Ultrasound Med Biol 16: 681, 1990 |
|
Nilsson N, Hansson R, Hamberger L et al: Collection of human oocytes by the use of sonography. J Fertil Steril 39: 603, 1983 |
|
Nyborg WL: Acoustic streaming. In Mason W (ed): Physical Acoustics, pp 265 – 285. New York, Academic Press, 1964 |
|
Gleicher N, Friberg J, Fullan NR et al: Egg retrieval for in-vitro fertilization by sonographically controlled vaginal culdocentesis. Lancet 2: 508, 1983 |
|
Demoulin A, Bologne R, Hustin J et al: Is ultrasound monitoring of follicular growth harmless? Ann NY Acad Sci 442: 146, 1985 |
|
Nyborg WL, Wu J: Relevant parameters with rationale. In Ziskin MC, Lewin PA (eds): Ultrasonic Exposimetry, pp 85 – 112. Boca Raton, FL, CRC Press, 1993 |
|
510(k) Guide for Measuring and Reporting the Acoustic Output of Diagnostic Ultrasound Medical Devices. Washington, DC, Center for Devices and Radiological Health, FDA 1985; revised in 1989 - 1992 and 1994 |
|
Duck FA, Martin K: Exposure values for medical devices. In Ziskin MC, Lewin PA (eds): Ultrasonic Exposimetry, pp 315 – 344. Boca Raton, FL, CRC Press, 1993 |
|
NCRP. (National Council on Radiation Protection and Measurements). Exposure Criteria for Medical Diagnostic Ultrasound: II. Criteria Based on All Known Mechanisms. Report No. 140. Bethesda, MD, 2002 |
|
Karagoz I, Kartal MK: A new safety parameter for diagnostic ultrasound thermal bioeffects: safe usetime. J Acoust Soc Am. 2009 Jun;125(6):3601-10. |
|
Ziskin MC: The thermal dose index. J Ultrasound Med. 2010 Oct;29(10):1475-9. |
|
Bigelow TA, Church CC, Sandstrom K et al: The thermal index: its strengths, weaknesses, and proposed improvements. J Ultrasound Med. 2011 May;30(5):714-34. |
|
Jago JR, Henderson J, Whittingham TA et al: A comparison of AIUM/NEMA thermal indices with calculated temperature rises for a simple third-trimester pregnancy tissue model. American Institute of Ultrasound in Medicine/National Electrical Manufacturers Association. Ultrasound Med Biol 25: 623–8, 1999 |
|
Marsal K: The output display standard: has it missed its target? Ultrasound Obstet Gynecol. 2005 Mar;25(3):211-4. |
|
Sheiner E, Shoham-Vardi I, Abramowicz JS: What do clinical users know regarding safety of ultrasound during pregnancy? J Ultrasound Med. 2007 Mar;26(3):319-25; quiz 326-7. |
|
Akhtar W, Arain MA, Ali A et al: Ultrasound biosafety during pregnancy: what do operators know in the developingworld?: national survey findings from pakistan. J Ultrasound Med. 2011 Jul;30(7):981-5. |
|
Houston LE, Allsworth J, Macones GA: Ultrasound is safe... right?: resident and maternal-fetal medicine fellowknowledge regarding obstetric ultrasound safety. J Ultrasound Med. 2011 Jan;30(1):21-7. |
|
Kerin JF: Determination of the optimal timing of insemination in women. In Richardson D, Joyce D, Symonds M (eds): Frozen Human Semen, pp 105 – 132. London, Royal College of Obstetrics and Gynaecology, 1979 |
|
Mahadevan K, Chandler D, Wiseman A et al: Evidence for absence of deleterious effects of ultrasound on human oocytes. J In Vitro Fert Embryo Transfer 4 (5): 277, 1987 |
|
McClain RM, Hoar RM, Saltzman MB: Teratologic study of rats exposed to ultrasound. Am J Obstet Gynecol 114: 39, 1972 |
|
Sikov MR, Hildebrand BP: Effects of ultrasound on the prenatal development of the rat. I. 3.2 MHz continuous wave at 9 days of gestation. J Clin Ultrasound 4: 357, 1976 |
|
Sikov MR, Hildebrand BP: Embryotoxicity of ultrasound exposure at nine days of gestation in the rat. In White D, Braun RE (eds): Ultrasound in Medicine. New York, Plenum Press, 1977 |
|
Sikov MR, Hildebrand BP: Effects of prenatal exposure to ultrasound. In Persaut TVN (ed): Advances in the Study of Birth Defects, Vol 2, p 267. Lancaster, England, MTP Press, 1979 |
|
Sikov MR, Collins HD, Carr DB: Intensity response relationships following exposure of the 9-day rat embryo to 0.8 MHz CW ultrasound. J Ultrasound Med 2: 41, 1983 |
|
Ang ES Jr, Gluncic V, Duque A et al: Prenatal exposure to ultrasound waves impacts neuronal migration in mice. Proc Natl Acad Sci U S A 22: 12903, 2006 |
|
Abramowicz JS: Prenatal exposure to ultrasound waves: is there a risk? Ultrasound Obstet Gynecol 29: 363, 2007 |
|
Schneider-Kolsky ME, Ayobi Z, Lombardo P et al: Ultrasound exposure of the foetal chick brain: effects on learning and memory. Int J Dev Neurosci. 2009 Nov;27(7):677-83. Epub 2009 Aug 5. |
|
Carstensen EL, Child SZ, Nyborg WL: Ultrasonic heating of the skull. J Acoust Soc Am 87: 1310, 1990 |
|
Bosward KL, Barnett SB, Wood AK et al: Heating of guinea-pig fetal brain during exposure to pulsed ultrasound. Ultrasound Med Biol 19: 415–24, 1993 |
|
Horder MM, Barnett SB, Vella GJ et al: Ultrasound-induced temperature increase in the guinea-pig fetal brain in vitro. Ultrasound Med Biol 24: 697–704, 1998 |
|
Horder MM, Barnett SB, Vella GJ et al: Ultrasound-induced temperature increase in guinea-pig fetal brain in utero: Ultrasound Med Biol 24: 1501–10, 1998 |
|
Stolzenberg SJ, Torbit CA, Pryor GT et al: Toxicity of ultrasound in mice: Neonatal studies. Radiat Environ Biophys 18: 37, 1980 |
|
Jensh R, Lewin PA, Poczobutt MT et al: Effects of prenatal ultrasound exposure on adult offspring behavior in the Wistar rat. Experimental Biology and Medicine 210: 171, 1995 |
|
Vorhees CV, Acuff-Smith KD, Weisenburger WP et al: A teratologic evaluation of continuous wave daily ultrasound exposure in unanesthetized pregnant rats. Teratology 44: 667, 1994 |
|
Vorhees CV, Acuff-Smith KD, Schilling MA et al: Behavioral teratogenic evaluation of continuous wave ultrasound exposure in unanesthetized rats. Teratology 50: 238, 1994 |
|
ter Haar G, Dyson M, Talben D: Ultrasonically induced contractions in the mouse uterine smooth muscle in vivo. Ultrasonics 16: 275, 1978 |
|
Kelman BJ, Sikov MR, Pappas RA et al: Effect of ultrasound exposure on the function of the perfused guinea pig placenta. J Ultrasound Med 2: 42, 1983 |
|
Kelman BJ, Pappas RA, Sikov MR: Effects of ultrasound on placental function. J Ultrasound Med 2: 218, 1986 |
|
Sikov MR, Kelman BJ: Effect of ultrasound on maternal blood flow and transplacental movements of alpha-aminoisobutyric acid (AIB). Fed Proc 42: 1129, 1983 |
|
Murrils AJ, Barrington P, Harris PD et al: Influence of Doppler ultrasound on fetal activity. Br Med J 286: 1009, 1983 |
|
Barnett SB, Walsh DA, Angles JM: Novel approach to evaluate interaction of pulsed ultrasound with embryonic development. Ultrasonics 28: 166, 1990 |
|
Miller MW, Ziskin MC: Biological consequences of hyperthermia. Ultrasound Med Biol 15: 707, 1989 |
|
Calvert J, Duck F, Clift S et al: Surface heating by transvaginal transducers. Ultrasound Obstet Gynecol 29: 427–32, 2007 |
|
Child SZ, Hartman CL, Schery LA, Carstensen EL: Lung damage from exposure to pulsed ultrasound. Ultrasound Med Biol 16: 817, 1990 |
|
Dalecki D, Raeman CH, Child SZ et al: Thresholds for intestinal hemorrhage in mice from exposure to pulsed ultrasound. Ultrasound Med Biol 21: 1067, 1995 |
|
Miller DL, Averkiou MA, Brayman AA et al: Bioeffects considerations for diagnostic ultrasound contrast agents. J Ultrasound Med 27: 611–32, 2008 |
|
Umemura S, Yoshizawa S, Sasaki K et al: Acceleration of ultrasonic tissue heating by microbubble agent. J Acoust Soc Am 123: 3215, 2008 |
|
Marsal K: Exposure to ultrasound in utero: epidemiology and relevance of neuronal migrationstudies. Ultrasound Med Biol. 2010 Aug;36(8):1221-3. Epub 2010 May 5. |
|
Abramowicz JS, Fowlkes JB, Skelly AC et al: Conclusions regarding epidemiology for obstetric ultrasound. J Ultrasound Med 27: 637–44, 2008 |
|
Abramowicz JS, Folwkes BJ, Stratmeyer ME, Ziskin MC:Bioeffects and safety of fetal ultrasound exposure: Why do we need epidemiology? in Sheiner E (ed.): Textbook of Perinatal Epidemology, Nova Publishers, 2010 |
|
O'Brien WC: Dose dependent effect of ultrasound on fetal weight in mice. J Ultrasound Med 2: 1, 1983 |
|
Bakketeig LS, Eik-Nes SH, Jakobsen G et al: A randomized controlled trial of ultrasonographic screening in pregnancy. Lancet 2: 207, 1984 |
|
Stark CR, Orleans M, Haverkamp AD et al: Short and long-term risks after exposure to diagnostic ultrasound in utero. Obstet Gynecol 63: 194, 1984 |
|
Canwright RA, McKinney PA, Hopton PA et al: Ultrasound examination in pregnancy and childhood cancer. Lancet 2: 999, 1984 |
|
Kinier WLM, Waterhouse JA: Obstetric ultrasound and children's malignancies. Lancet 2: 997, 1984 |
|
Salvesen KA, Vatten LJ, Eik-Ness SH, Bakketeig LS. Routine ultrasonography in utero and subsequent handedness and neurological development. BMJ 307: 159–164, 1993 |
|
Salvesen KA, Eik-Ness SH, Vatten LJ et al. Routine ultrasound scanning in pregnancy. Authors’ reply. BMJ 307: 1562, 1993 |
|
Kieler H, Axelsson O, Haglund B et al. Routine ultrasound screening in pregnancy and the children’s subsequent handedness. Early Hum Dev 50: 233–245, 1998 |
|
Abramowicz JS, Kossoff G, Marsal K et al: Literature review by the ISUOG Bioeffects and Safety Committee. Ultrasound Obstet Gynecol 19: 318–9, 2002 |
|
Salvesen KA: Ultrasound and left-handedness: a sinister association? Ultrasound Obstet Gynecol 19: 217–21, 2002 |
|
Heikkila K, Vuoksimaa E, Oksava K et al: Handedness in the helsinki ultrasound trial. Ultrasound Obstet Gynecol. 2011 Jun;37(6):638-42. doi: 10.1002/uog.8962. |
|
Salvesen KA: Ultrasound in pregnancy and non-right handedness: meta-analysis of randomized trials. Ultrasound Obstet Gynecol. 2011 May 16. doi: 10.1002/uog.9055. |
|
Henderson J, Willson K, Jago JR et al: A survey of the acoustic outputs of diagnostic ultrasound equipment in current clinical use. Ultrasound Med Biol 21: 699, 1995 |
|
Duck FA, Henderson J. Acoustic output of modern instruments: is it increasing? In: Barnett SB, Kossoff G, eds. Safety of Diagnostic Ultrasound. New York, London: The Parthenon Publishing Group, 1998 |
|
Jago JR, Henderson J, Whittingham TA, Willson K. How reliable are manufacturer's reported acoustic data? Ultrasound Med Biol 25: 135, 1995 |
|
O'Brien WD Jr, Abbott JG, Stratmeyer ME et al: Acoustic output upper limits proposition: should upper limits be retained? J Ultrasound Med 21: 1335–41, 2002 |
|
Stratmeyer ME, Christman CL: Biological effects of ultrasound. Women Health 7: 65–81, 1982 |
|
Thermal teratology. European Committee for Medical Ultrasound Safety (ECMUS). Eur J Ultrasound 9: 281–283, 1999 |
|
Safety Group of the British Medical Ultrasound Society: Guidelines for the safe use of diagnostic ultrasound equipment. Ultrasound, 2010;18:52-59 |
|
WFUMB. WFUMB Symposium on Safety of Ultrasound in Medicine: Conclusions and Recommendations on Thermal and Non-Thermal Mechanisms for Biological Effects of Ultrasound (Barnett SB, ed.) Ultrasound Med Biol 24: S1–S55, 1998 |
|
Sheiner E, Shoham-Vardi I, Hussey MJ et al: First-trimester sonography: is the fetus exposed to high levels of acoustic energy? J Clin Ultrasound 35: 245–9, 2007 |
|
Sheiner E, Freeman J, Abramowicz JS: Acoustic output as measured by mechanical and thermal indices during routine obstetric ultrasound examinations. J Ultrasound Med 24: 1665–70, 2005 |
|
Sheiner E, Shoham-Vardi I, Pombar X et al: An increased thermal index can be achieved when performing Doppler studies in obstetric sonography. J Ultrasound Med 26: 71–6, 2007 |
|
Sheiner E, Abramowicz JS: Acoustic output as measured by thermal and mechanical indices during fetal nuchaltranslucency ultrasound examinations. Fetal Diagn Ther. 2009;25(1):8-10. Epub 2008 Dec 15. |
|
Sheiner E, Hackmon R, Shoham-Vardi I et al: A comparison between acoustic output indices in 2D and 3D/4D ultrasound in obstetrics. Ultrasound Obstet Gynecol 29: 326–8, 2007 |
|
Abramowicz JS: Fetal Doppler: how to keep it safe? Clin Obstet Gynecol. 2010 Dec;53(4):842-50. |
|
Edmonds PD, Abramowicz JS, Carson PL, Carstensen EL, Sandstrom KL: Guidelines for Journal of Ultrasound in Medicine Authors and Reviewers on Measurement and Reporting of Acoustic Output and Exposure. J Ultrasound Med 24, 1171–1179, 2005 |
|
ter Haar G, Shaw A, Pye S et al: Guidance on reporting ultrasound exposure conditions for bio-effects studies. Ultrasound Med Biol. 2011 Feb;37(2):177-83. |